Artificial Intelligence in Oral Cancer: A Comprehensive Scoping Review of Diagnostic and Prognostic Applications
Abstract
1. Introduction
- Discussion on the AI technologies implemented for oral cancer detection and diagnosis.
- The AI analysis of early discovery and its assessment result in an increase in diagnostic sensitivity.
- Using AI applications to predict oral cancer risk and prognosis.
- Identify the elements that are deficient and influence them towards areas of research that need to be probed in the future.
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
- Artificial Intelligence;
- Machine Learning;
- Deep Learning;
- Neural Networks;
- Oral Cancer;
- Oral Squamous Cell Carcinoma;
- Diagnosis;
- Screening;
- Prognosis;
- Imaging;
- Histopathology.
- Population: Individuals diagnosed with oral cancer or at risk of its development.
- Interventions: The utilization of artificial intelligence, particularly machine learning methodologies such as deep neural networks (DNNs), has been associated with the detection of oral cancer and the screening of oral precancerous lesions.
- Results: Articles showcasing the precision, responsiveness, and selectivity, prognostic significance, and clinical outcomes of artificial intelligence (AI) implementations in oral cancer. The types of investigations include cohort studies, case–control studies, experimental clinical trials, and reviews/meta-analysis.
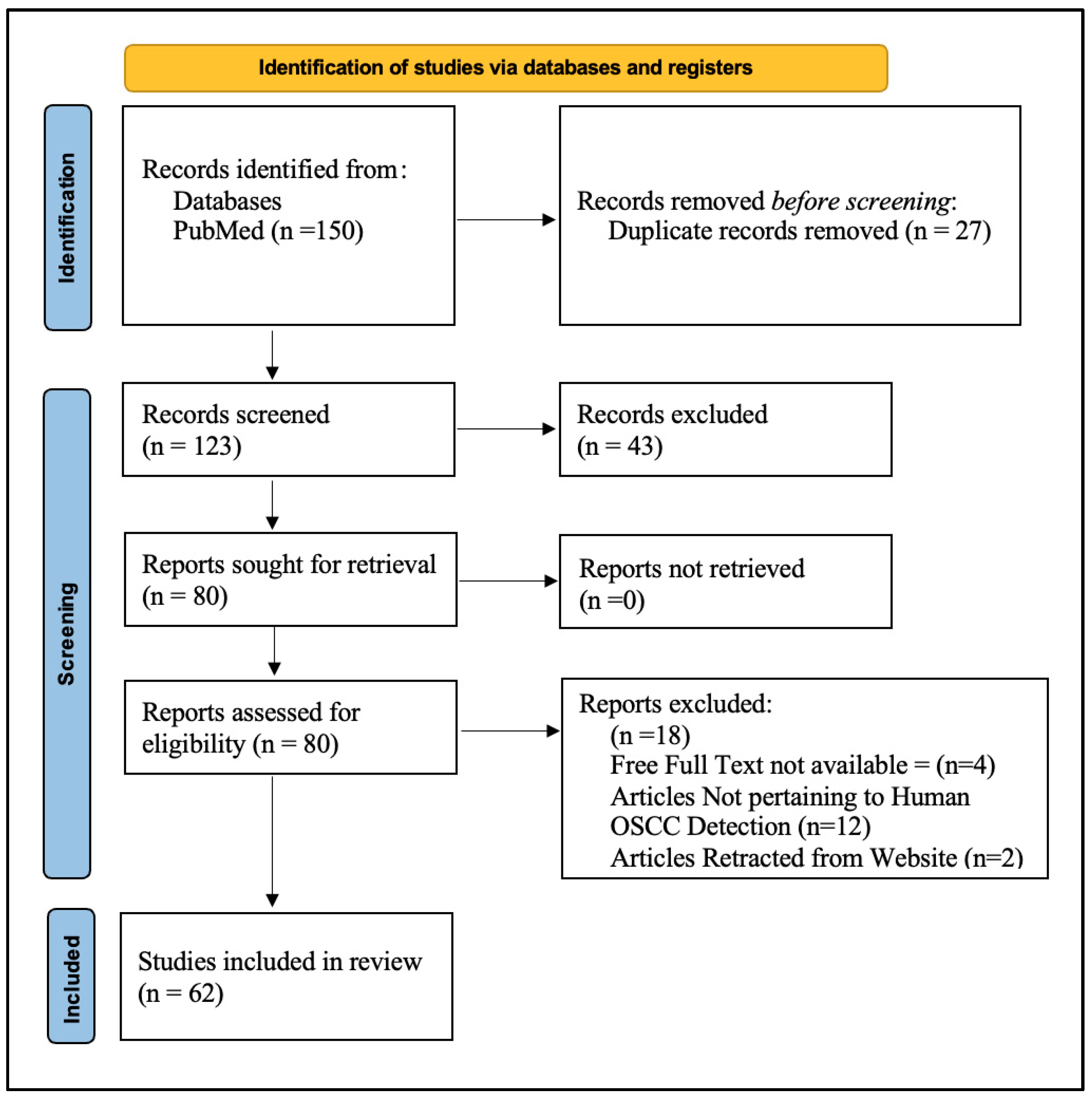
2.3. Study Selection
2.4. Data Extraction
- Features of the study (author, year, and location);
- AI techniques used;
- Results that were reported, such as the sensitivity, specificity, and accuracy of the diagnosis.
2.5. Data Synthesis
3. Results
3.1. Applications
- Diagnostic Accuracy: AI can improve diagnostic accuracy and facilitate individualized treatment plans, according to Nishath Sayed Abdul et al. [25]. Despite its potential, acceptance for clinical usage is still heavily influenced by ethical and regulatory issues.
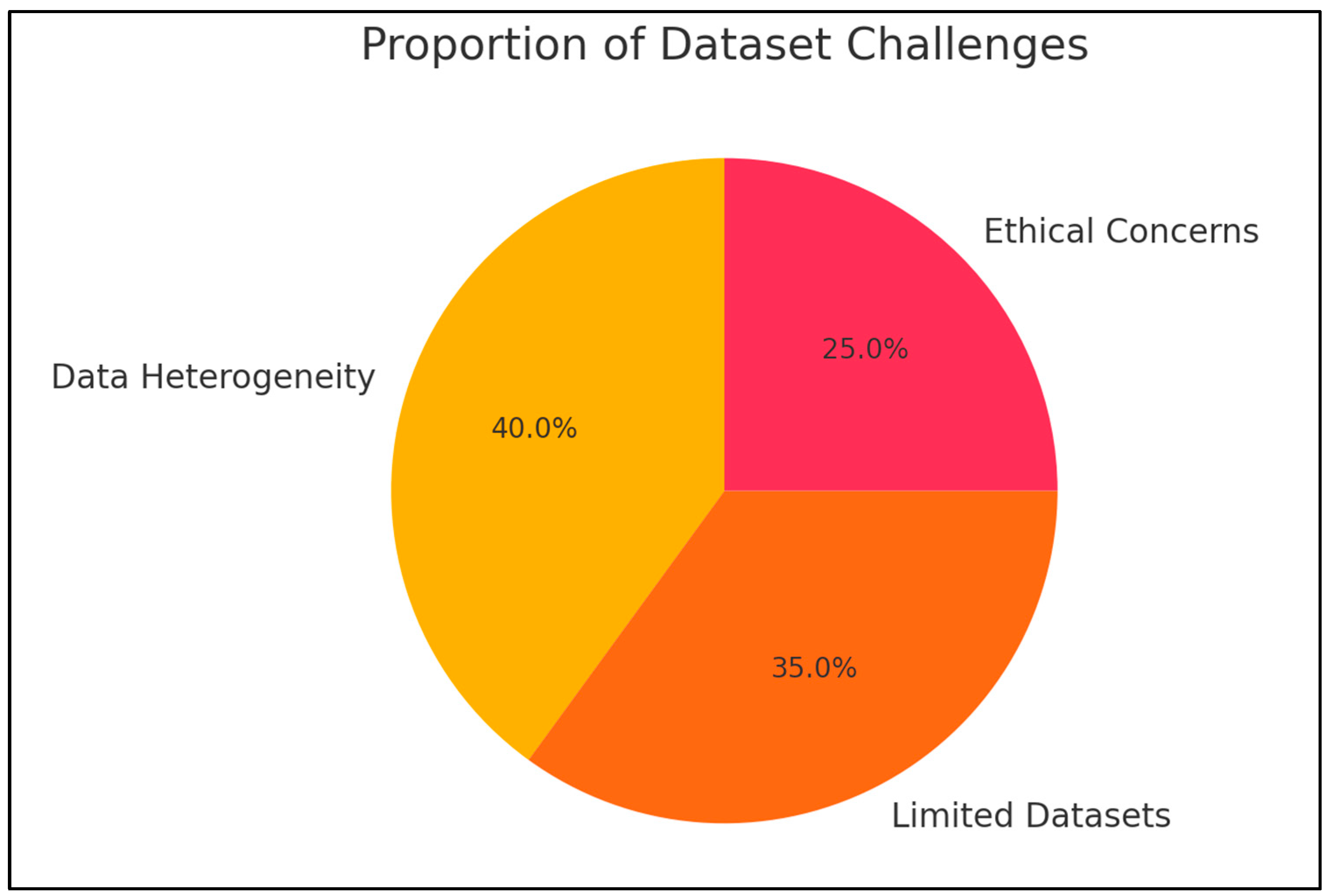
- Datasets and Training: A challenge to implementing AI is the absence of reliable datasets. In order to support AI-assisted systems for diagnosing oral squamous cell carcinoma and other potentially malignant illnesses using machine and deep learning, Maria Clara Falcão Ribeiro-de-Assis et al. [26] presented the NDB-UFES dataset. Similar to this, Sara Bassani et al. [27] examined 13 studies that examined the detection of oral cancer and found that while AI algorithms showed promise, the majority used conventional histology. However, wider applicability was hampered by the scarcity of big training datasets.
- Algorithm Performance: The effectiveness of various AI models has been the subject of numerous studies. Artificial neural networks (ANNs) fared noticeably better than conventional forecasting techniques in high-risk groups, according to Shruthi Hegde et al. [28]. However, after reviewing supervised machine learning techniques, Natheer Al-Rawi et al. [30] came to the conclusion that, when compared to other AI techniques, deep convolutional neural networks (DCNNs) were superior in early detection.
- Sophisticated AI Models: For feature extraction and classification, Rana Alabdan et al. [31] presented a sophisticated model that combines DCNN and Inception modules. In terms of diagnostic accuracy, this model fared better than previous deep learning frameworks when combined with the moth flame optimization (MFO) technique.
- Special Considerations: Anjali Pillai et al. [29] could not find any significant variations in survival rates between patient groupings; nevertheless, because of possible immunological dysregulation, patients with autoimmune conditions had somewhat lower survival rates. This emphasizes how crucial it is to take patient-specific aspects into account when implementing AI-driven solutions in healthcare settings.
3.2. Challenges
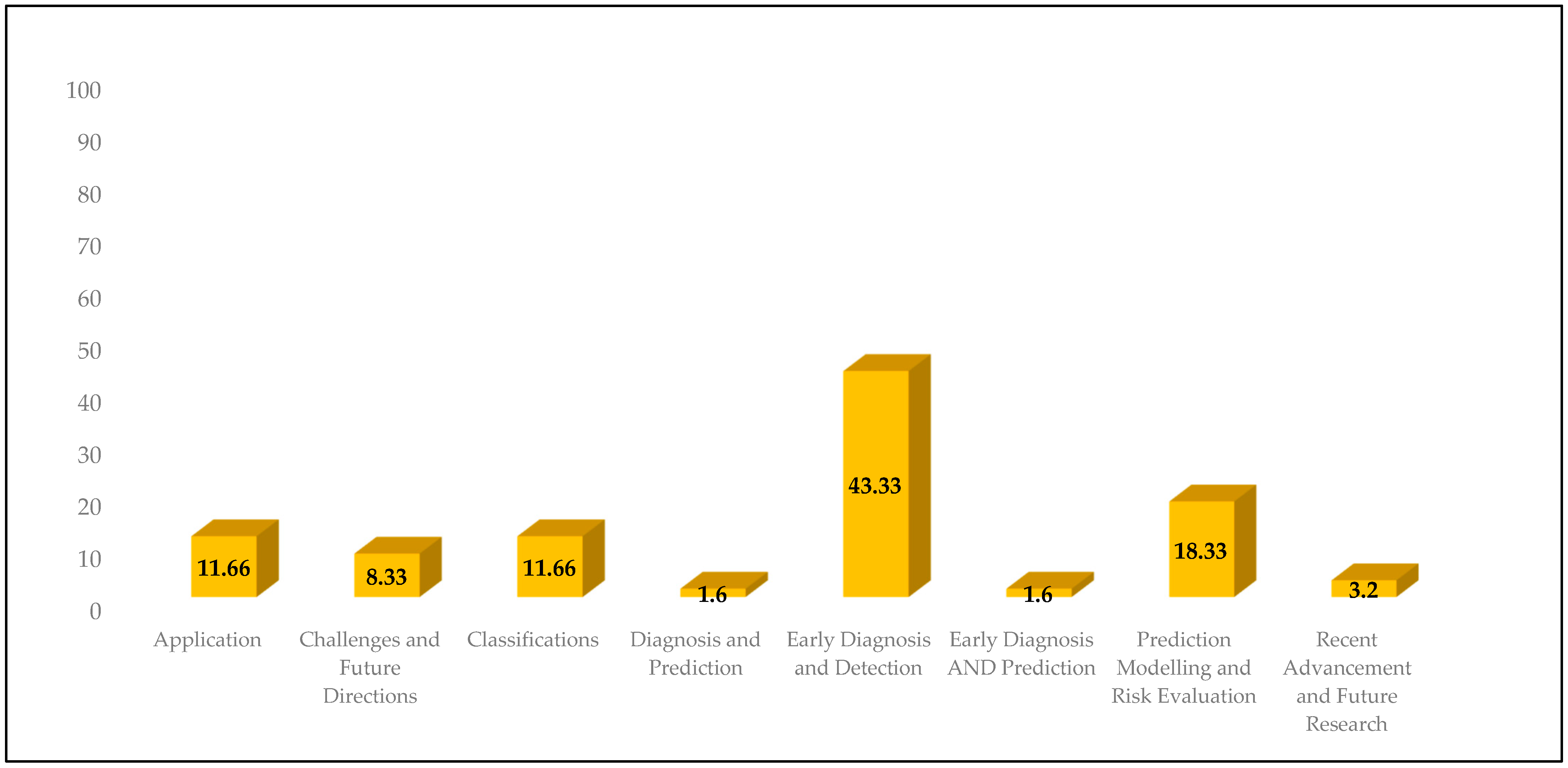
3.3. Classifications
3.4. Diagnosis and Prediction
3.5. Early Diagnosis and Detection
3.6. Early Diagnosis and Prediction
3.7. Prediction Modelling and Risk Evaluation
3.8. Recent Advancement and Future Research
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sankaranarayanan, R.; Ramadas, K.; Amarasinghe, H.; Subramanian, S.; Johnson, N. Oral cancer: Prevention, early detection, and treatment. In Cancer: Disease Control Priorities, 3rd ed.; The World Bank: Washington, DC, USA, 2015; Volume 3, pp. 85–99. [Google Scholar]
- Oral Cavity & Oropharyngeal Cancer Key Statistics 2021. Cancer.org. Available online: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html (accessed on 4 September 2024).
- Borse, V.; Konwar, A.N.; Buragohain, P. Oral cancer diagnosis and perspectives in India. Sens. Int. 2020, 1, 100046. [Google Scholar] [CrossRef] [PubMed]
- Rajaguru, H.; Prabhakar, S.K. Performance comparison of oral cancer classification with Gaussian mixture measures and multi layer perceptron. In IFMBE Proceedings; Springer: Singapore, 2017; pp. 123–129. [Google Scholar]
- GLOBOCAN 2020: New Global Cancer Data|UICC. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 11 August 2024).
- Krishna, S.; Lavanya, J.; Kavya, G.; Prasamya, N.; Swapna. Oral cancer diagnosis using deep learning for early detection. In Proceedings of the 2022 International Conference on Electronics and Renewable Systems (ICEARS), Tuticorin, India, 16–18 March 2022; IEEE: New York, NY, USA, 2022. [Google Scholar]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Vasconcellos Le Campion, A.C.O.; Ribeiro, C.M.B.; Luiz, R.R.; da Silva Júnior, F.F.; Barros, H.C.S.; dos Santos, K.d.C.B.; Ferreira, S.J.; Gonçalves, L.S.; Ferreira, S.M.S. Low survival rates of oral and oropharyngeal squamous cell carcinoma. Int. J. Dent. 2017, 2017, 5815493. [Google Scholar] [CrossRef]
- Sharma, N.; Om, H. Extracting significant patterns for oral cancer detection using apriori algorithm. Intell. Inf. Manag. 2014, 06, 30–37. [Google Scholar] [CrossRef][Green Version]
- Song, B.; Sunny, S.; Uthoff, R.D.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Anbarani, A.; Wilder-Smith, P.; Kuriakose, M.A.; et al. Automatic classification of dual-modalilty, smartphone-based oral dysplasia and malignancy images using deep learning. Biomed. Opt. Express 2018, 9, 5318–5329. [Google Scholar] [CrossRef]
- Das, N.; Hussain, E.; Mahanta, L.B. Automated classification of cells into multiple classes in epithelial tissue of oral squamous cell carcinoma using transfer learning and convolutional neural network. Neural Netw. 2020, 128, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Faes, L.; Kale, A.U.; Wagner, S.K.; Fu, D.J.; Bruynseels, A.; Mahendrian, T.; Moraes, G.; Shamdas, M.; Kern, C.; et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit Health 2019, 1, e271–e297. [Google Scholar] [CrossRef]
- Yang, H.; Jo, E.; Kim, H.J.; Cha, I.-H.; Jung, Y.-S.; Nam, W.; Kim, J.-Y.; Kim, J.-K.; Kim, Y.H.; Oh, T.G.; et al. Deep learning for automated detection of cyst and tumors of the jaw in panoramic radiographs. J. Clin. Med. 2020, 9, 1839. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Aubreville, M.; Knipfer, C.; Oetter, N.; Jaremenko, C.; Rodner, E.; Denzler, J.; Bohr, C.; Neumann, H.; Stelzle, F.; Maier, A. Automatic classification of cancerous tissue in laserendomicroscopy images of the oral cavity using deep learning. Sci Rep. 2017, 7, 11979. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kaur, M.; Manhas, J. Tissue level based deep learning framework for early detection of dysplasia in oral squamous epithelium. J. Multimed. Inf. Syst. 2019, 6, 81–86. [Google Scholar] [CrossRef]
- Ilhan, B.; Lin, K.; Guneri, P.; Wilder-Smith, P. Improving oral cancer outcomes with imaging and artificial intelligence. J. Dent. Res. 2020, 99, 241–248. [Google Scholar] [CrossRef]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine learning in medicine: A practical introduction. BMC Med. Res. Methodol. 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine learning in medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Cuocolo, R.; Caruso, M.; Perillo, T.; Ugga, L.; Petretta, M. Machine Learning in oncology: A clinical appraisal. Cancer Lett. 2020, 481, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Khanagar, S.B.; Naik, S.; Al Kheraif, A.A.; Vishwanathaiah, S.; Maganur, P.C.; Alhazmi, Y.; Mushtaq, S.; Sarode, S.C.; Sarode, G.S.; Zanza, A.; et al. Application and performance of artificial intelligence technology in oral cancer diagnosis and prediction of prognosis: A systematic review. Diagnostics 2021, 11, 1004. [Google Scholar] [CrossRef]
- Vinay, V. Artificial Intelligence in Oral Cancer: A Comprehensive Scoping Review of Diagnostic and Prognostic Applications. figshare. Preprint 2024. [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- García-Pola, M.; Pons-Fuster, E.; Suárez-Fernández, C.; Seoane-Romero, J.; Romero-Méndez, A.; López-Jornet, P. Role of artificial intelligence in the early diagnosis of oral cancer. A scoping review. Cancers 2021, 13, 4600. [Google Scholar] [CrossRef]
- Abdul, N.S.; Shivakumar, G.C.; Sangappa, S.B.; Di Blasio, M.; Crimi, S.; Cicciù, M.; Minervini, G. Applications of artificial intelligence in the field of oral and maxillofacial pathology: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 122. [Google Scholar] [CrossRef]
- Ribeiro-de-Assis, M.C.F.; Soares, J.P.; de Lima, L.M.; de Barros, L.A.P.; Grão-Velloso, T.R.; Krohling, R.A.; Camisasca, D.R. NDB-UFES: An oral cancer and leukoplakia dataset composed of histopathological images and patient data. Data Brief 2023, 48, 109128. [Google Scholar] [CrossRef]
- Bassani, S.; Santonicco, N.; Eccher, A.; Scarpa, A.; Vianini, M.; Brunelli, M.; Bisi, N.; Nocini, R.; Sacchetto, L.; Munari, E.; et al. Artificial intelligence in head and neck cancer diagnosis. J. Pathol. Inform. 2022, 13, 100153. [Google Scholar] [CrossRef]
- Hegde, S.; Ajila, V.; Zhu, W.; Zeng, C. Artificial intelligence in early diagnosis and prevention of oral cancer. Asia-Pacific J. Oncol. Nurs. 2022, 9, 100133. [Google Scholar] [CrossRef]
- Pillai, A.; Valero, C.; Zanoni, D.; Navas, K.; Morris, Q.; Ganly, I.; Patel, S.G. Prognostic impact of autoimmune disease in oral cavity squamous cell carcinoma. J. Surg. Oncol. 2022, 126, 1183–1190. [Google Scholar] [CrossRef]
- Al-Rawi, N.; Sultan, A.; Rajai, B.; Shuaeeb, H.; Alnajjar, M.; Alketbi, M.; Mohammad, Y.; Shetty, S.R.; Mashrah, M.A. The effectiveness of artificial intelligence in detection of oral cancer. Int. Dent. J. 2022, 72, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Alabdan, R.; Alruban, A.; Hilal, A.M.; Motwakel, A. Artificial-intelligence-based decision making for oral potentially malignant disorder diagnosis in Internet of Medical Things environment. Healthcare 2022, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Kumar, A.; Srinivasan, K. A current review of machine learning and deep learning models in oral cancer diagnosis: Recent technologies, open challenges, and future research directions. Diagnostics 2023, 13, 1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, M.; Li, R.; Bai, J. Current advances in noninvasive methods for the diagnosis of oral squamous cell carcinoma: A review. Eur. J. Med. Res. 2023, 28, 53. [Google Scholar] [CrossRef] [PubMed]
- Satish, K.S.; Saravanan, K.S.; Augustine, D.; Saraswathy, G.R.; Sowmya, S.V.; Khan, S.S.; Haragannavar, V.C.; Chakraborty, S.; Dsouza, P.L.; N, K.H.; et al. Leveraging technology-driven strategies to untangle omics big data: Circumventing roadblocks in clinical facets of oral cancer. Front. Oncol. 2023, 13, 1183766. [Google Scholar] [CrossRef]
- Su, Y.-F.; Chen, Y.-J.; Tsai, F.-T.; Li, W.-C.; Hsu, M.-L.; Wang, D.-H.; Yang, C.-C. Current insights into oral cancer diagnostics. Diagnostics 2021, 11, 1287. [Google Scholar] [CrossRef]
- Haj-Hosseini, N.; Lindblad, J.; Hasséus, B.; Kumar, V.V.; Subramaniam, N.; Hirsch, J.-M. Early detection of oral potentially malignant disorders: A review on prospective screening methods with regard to global challenges. J. Maxillofac. Oral Surg. 2024, 23, 23–32. [Google Scholar] [CrossRef]
- Arumuganainar, D. Extra tree classifier predicts an interactome hub gene as HSPB1 in oral cancer: A bioinformatics analysis. Cureus 2024, 16, e59863. [Google Scholar] [CrossRef]
- Sukegawa, S.; Ono, S.; Tanaka, F.; Inoue, Y.; Hara, T.; Yoshii, K.; Nakano, K.; Takabatake, K.; Kawai, H.; Katsumitsu, S.; et al. Effectiveness of deep learning classifiers in histopathological diagnosis of oral squamous cell carcinoma by pathologists. Sci. Rep. 2023, 13, 11676. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.G.; Ingham, J.; Whitley, C.A.; Al Jedani, S.; Gunning, P.J.; Gardner, P.; Shaw, R.J.; Barrett, S.D.; Triantafyllou, A.; Risk, J.M.; et al. Metric-based analysis of FTIR data to discriminate tissue types in oral cancer. Analyst 2023, 148, 1948–1953. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, B.G.; Hwang, S.H. Efficacy of artificial intelligence-assisted discrimination of oral cancerous lesions from normal mucosa based on the oral mucosal image: A systematic review and meta-analysis. Cancers 2022, 14, 3499. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Kc, D.R.; Yang, R.Y.; Li, S.; Zhang, C.; Liang, R. Classification of mobile-based oral cancer images using the Vision Transformer and the Swin Transformer. Cancers 2024, 16, 987. [Google Scholar] [CrossRef] [PubMed]
- James, B.L.; Sunny, S.P.; Heidari, A.E.; Ramanjinappa, R.D.; Lam, T.; Tran, A.V.; Kankanala, S.; Sil, S.; Tiwari, V.; Patrick, S.; et al. Validation of a point-of-care optical coherence tomography device with machine learning algorithm for detection of oral potentially malignant and malignant lesions. Cancers 2021, 13, 3583. [Google Scholar] [CrossRef] [PubMed]
- Khanagar, S.B.; Alkadi, L.; Alghilan, M.A.; Kalagi, S.; Awawdeh, M.; Bijai, L.K.; Vishwanathaiah, S.; Aldhebaib, A.; Singh, O.G. Application and performance of artificial intelligence (AI) in oral cancer diagnosis and prediction using histopathological images: A systematic review. Biomedicines 2023, 11, 1612. [Google Scholar] [CrossRef]
- Li, J.; Kot, W.Y.; McGrath, C.P.; Chan, B.W.A.; Ho, J.W.K.; Zheng, L.W. Diagnostic accuracy of artificial intelligence assisted clinical imaging in the detection of oral potentially malignant disorders and oral cancer: A systematic review and meta-analysis. Int. J. Surg. 2024, 110, 5034–5046. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, S.; Ramalingam, K.; Nair, P.; Patel, S.; Menon, P.A.; Malik, N.; Mhaske, S. Automated analysis of nuclear parameters in oral exfoliative cytology using machine learning. Cureus 2024, 16, e58744. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.N.; Chaudhary, M.; Patel, S.A.; Zade, P.R. Screening of oral squamous cell carcinoma through color intensity-based textural features. Cureus 2024, 16, e56682. [Google Scholar] [CrossRef]
- Zayed, S.O.; Abd-Rabou, R.Y.M.; Abdelhameed, G.M.; Abdelhamid, Y.; Khairy, K.; Abulnoor, B.A.; Ibrahim, S.H.; Khaled, H. The innovation of AI-based software in oral diseases: Clinical-histopathological correlation diagnostic accuracy primary study. BMC Oral Health 2024, 24, 598. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Sethy, P.K.; Dewangan, A.K.; Nanthaamornphong, A.; Behera, S.K.; Devi, B. Enhancing oral squamous cell carcinoma detection: A novel approach using improved EfficientNet architecture. BMC Oral Health 2024, 24, 601. [Google Scholar] [CrossRef] [PubMed]
- Struckmeier, A.-K.; Buchbender, M.; Agaimy, A.; Kesting, M. Diagnostic accuracy of contrast-enhanced computed tomography in assessing bone invasion in patients with oral squamous cell carcinoma. Clin. Oral Investig. 2024, 28, 314. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Song, C.; Wang, X.; Feng, Z.; Su, Y.; Wang, H. Integrated bioinformatics analysis of SEMA3C in tongue squamous cell carcinoma using machine-learning strategies. Cancer Cell Int. 2024, 24, 58. [Google Scholar] [CrossRef]
- Dinesh, Y.; Ramalingam, K.; Ramani, P.; Deepak, R.M. Machine learning in the detection of oral lesions with clinical intraoral images. Cureus 2023, 15, e44018. [Google Scholar] [CrossRef]
- Struckmeier, A.-K.; Yekta, E.; Agaimy, A.; Kopp, M.; Buchbender, M.; Moest, T.; Lutz, R.; Kesting, M. Diagnostic accuracy of contrast-enhanced computed tomography in assessing cervical lymph node status in patients with oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 17437–17450. [Google Scholar] [CrossRef]
- Ahmad, M.; Irfan, M.A.; Sadique, U.; Haq, I.U.; Jan, A.; Khattak, M.I.; Ghadi, Y.Y.; Aljuaid, H. Multi-method analysis of histopathological image for early diagnosis of Oral Squamous Cell Carcinoma using deep learning and hybrid techniques. Cancers 2023, 15, 5247. [Google Scholar] [CrossRef]
- Yang, Z.; Pan, H.; Shang, J.; Zhang, J.; Liang, Y. Deep-learning-based automated identification and visualization of oral cancer in optical coherence tomography images. Biomedicines 2023, 11, 802. [Google Scholar] [CrossRef]
- Oya, K.; Kokomoto, K.; Nozaki, K.; Toyosawa, S. Oral squamous cell carcinoma diagnosis in digitized histological images using convolutional neural network. J. Dent. Sci. 2023, 18, 322–329. [Google Scholar] [CrossRef]
- Xu, X.; Xi, L.; Wei, L.; Wu, L.; Xu, Y.; Liu, B.; Li, B.; Liu, K.; Hou, G.; Lin, H.; et al. Deep learning assisted contrast-enhanced CT-based diagnosis of cervical lymph node metastasis of oral cancer: A retrospective study of 1466 cases. Eur. Radiol. 2023, 33, 4303–4312. [Google Scholar] [CrossRef]
- Jing, F.; Zhang, J.; Cai, X.; Zhou, X.; Bai, J.; Zhang, H.; Li, T. Screening for biomarkers for progression from oral leukoplakia to oral squamous cell carcinoma and evaluation of diagnostic efficacy by multiple machine learning algorithms. Cancers 2022, 14, 5808. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-J.; Wang, Y.-C.; Hsueh, P.-C.; Wu, C.-C. Development and validation of machine learning-based risk prediction models of oral squamous cell carcinoma using salivary autoantibody biomarkers. BMC Oral Health 2022, 22, 534. [Google Scholar] [CrossRef]
- Adeoye, J.; Wan, C.C.J.; Zheng, L.-W.; Thomson, P.; Choi, S.-W.; Su, Y.-X. Machine learning-based genome-wide salivary DNA methylation analysis for identification of noninvasive biomarkers in oral cancer diagnosis. Cancers 2022, 14, 4935. [Google Scholar] [CrossRef]
- Deif, M.A.; Attar, H.; Amer, A.; Elhaty, I.A.; Khosravi, M.R.; Solyman, A.A.A. Diagnosis of oral squamous cell carcinoma using deep neural networks and binary Particle Swarm Optimization on histopathological images: An AIoMT approach. Comput. Intell. Neurosci. 2022, 2022, 6364102. [Google Scholar] [CrossRef] [PubMed]
- Birur, N.P.; Song, B.; Sunny, S.P.; Keerthi, G.; Mendonca, P.; Mukhia, N.; Li, S.; Patrick, S.; Shubha, G.; Subhashini, A.R.; et al. Field validation of deep learning based Point-of-Care device for early detection of oral malignant and potentially malignant disorders. Sci Rep. 2022, 12, 14283. [Google Scholar] [CrossRef] [PubMed]
- Fati, S.M.; Senan, E.M.; Javed, Y. Early diagnosis of oral squamous cell carcinoma based on histopathological images using deep and hybrid learning approaches. Diagnostics 2022, 12, 1899. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Yamashiro, T.; Heianna, J.; Nakasone, T.; Kobayashi, T.; Mishiro, S.; Hirahara, D.; Takaya, E.; Mimura, H.; Murayama, S.; et al. Deep learning for the preoperative diagnosis of metastatic cervical lymph nodes on contrast-enhanced computed ToMography in patients with oral squamous cell carcinoma. Cancers 2021, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Sunny, S.; Baby, A.; James, B.L.; Balaji, D.; Aparna, N.V.; Rana, M.H.; Gurpur, P.; Skandarajah, A.; D’Ambrosio, M.; Ramanjinappa, R.D.; et al. A smart tele-cytology point-of-care platform for oral cancer screening. PLoS ONE 2019, 14, e0224885. [Google Scholar] [CrossRef] [PubMed]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Wilder-Smith, P.; et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Huang, X.; Liu, J.; Qin, Y.; Wu, X.; Ye, S.; Du, S.; Liao, W. Application of three-dimensional reconstruction technology in dentistry: A narrative review. BMC Oral Health 2023, 23, 630. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Peng, X.; Li, B.; Han, Q.; Ren, B.; Li, M.; Li, L.; Li, Y.; Cheng, G.; et al. The clinical potential of oral Microbiota as a screening tool for oral squamous cell carcinomas. Front. Cell Infect. Microbiol. 2021, 11, 728933. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, X.; Narayanan, R.; Shankar, V.; Ethiraj, S.; Wang, X.; Duan, N.; Ni, Y.-H.; Hu, Q.; Zare, R.N. Oral squamous cell carcinoma diagnosed from saliva metabolic profiling. Proc. Natl. Acad. Sci. USA 2020, 117, 16167–16173. [Google Scholar] [CrossRef] [PubMed]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Gurushanth, K.; Wooten, K.; Gupta, V.; Platek, M.E.; et al. Small form factor, flexible, dual-modality handheld probe for smartphone-based, point-of-care oral and oropharyngeal cancer screening. J. Biomed. Opt. 2019, 24, 106003. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, X.; Fu, Y.; Liu, Y.; Zheng, L.; Liu, F.; Li, T.; Yin, X.; Qiao, X.; Xu, X. Identification of AUNIP as a candidate diagnostic and prognostic biomarker for oral squamous cell carcinoma. EBioMedicine 2019, 47, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Nikkuni, Y.; Nishiyama, H.; Hayashi, T. Prediction of histological grade of oral squamous cell carcinoma using machine learning models applied to 18F-FDG-PET radiomics. Biomedicines 2024, 12, 1411. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Hu, J.; Tang, P.; Xu, T.; He, L.; Zeng, Z.; Sheng, J. Application of CT and MRI images based on artificial intelligence to predict lymph node metastases in patients with oral squamous cell carcinoma: A subgroup meta-analysis. Front. Oncol. 2024, 14, 1395159. [Google Scholar] [CrossRef] [PubMed]
- Einhaus, J.; Gaudilliere, D.K.; Hedou, J.; Feyaerts, D.; Ozawa, M.G.; Sato, M.; Ganio, E.A.; Tsai, A.S.; Stelzer, I.A.; Bruckman, K.C.; et al. Spatial subsetting enables integrative modeling of oral squamous cell carcinoma multiplex imaging data. iScience 2023, 26, 108486. [Google Scholar] [CrossRef]
- Siddalingappa, R.; Kanagaraj, S. K-nearest-neighbor algorithm to predict the survival time and classification of various stages of oral cancer: A machine learning approach. F1000Research 2022, 11, 70. [Google Scholar] [CrossRef]
- Zhang, X.; Gleber-Netto, F.O.; Wang, S.; Martins-Chaves, R.R.; Gomez, R.S.; Vigneswaran, N.; Zhang, X.; Gleber-Netto, F.O.; Wang, S.; Martins-Chaves, R.R.; et al. Deep learning-based pathology image analysis predicts cancer progression risk in patients with oral leukoplakia. Cancer Med. 2023, 12, 7508–7518. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Malik, G.; Someshwar, S.; Le, H.T.T.; Polavarapu, R.; Chavali, L.N.; Melethadathil, N.; Sundararajan, V.S.; Valadi, J.; Kavi Kishor, P.B.; et al. Machine learning heuristics on gingivobuccal cancer gene datasets reveals key candidate attributes for prognosis. Genes 2022, 13, 2379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X. An immune-related gene signature can predict clinical outcomes and immunotherapeutic response in oral squamous cell carcinoma. Front. Genet. 2022, 13, 870133. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, J.W.; Kang, J.; Kwon, E.J.; Ha, M.; Kim, Y.K.; Lee, H.; Rhee, J.-K.; Kim, Y.H. Novel deep learning-based survival prediction for oral cancer by analyzing tumor-infiltrating lymphocyte profiles through CIBERSORT. Oncolmmunology 2021, 10, 1904573. [Google Scholar] [CrossRef]
- Camalan, S.; Mahmood, H.; Binol, H.; Araújo, A.L.D.; Santos-Silva, A.R.; Vargas, P.A.; Vargas, P.A.; Lopes, M.A.; Khurram, S.A.; Gurcan, M.N. Convolutional neural network-based clinical predictors of oral dysplasia: Class activation map analysis of deep learning results. Cancers 2021, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Hamana, K.; Uzawa, K.; Ogawara, K.; Shiiba, M.; Bukawa, H.; Yokoe, H.; Tanzawa, H. Monitoring of circulating tumour-associated DNA as a prognostic tool for oral squamous cell carcinoma. Br. J. Cancer 2005, 92, 2181–2184. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, Q.; Wu, Q.; Yao, M.; Chen, Y.; Zhou, H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: A systematic review and Bayesian network meta-analysis. BMC Oral Health 2019, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Panda, B.; Mishra, P.; Das, D.; Kumar, V.; Bhuyan, L. New Horizons and prospects in oral cancer detection. J. Pharm. Bioallied Sci. 2024, 16, S1072–S1076. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.T.N.; Faria, C.A.B.; Martins, N.F.; de Duda Júnior, L.G.S.; Azevêdo, A.B.F.; da Silva, W.R.; Sobral, A.P. Use of digital strategies in the diagnosis of oral squamous cell carcinoma: A scoping review. PeerJ 2024, 12, e17329. [Google Scholar] [CrossRef]
- Alhazmi, A.K.; Alhammadi, F.; Zain, A.A.; Kaed, E.; Ahmed, B. AI’s Role and Application in Education: Systematic Review. In Intelligent Sustainable Systems; Lecture Notes in Networks and Systems; Springer: Singapore, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Wang, H. Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical approach. J. Multidiscip. Healthc. 2023, 16, 1779–1791. [Google Scholar] [CrossRef]
- Sawhney, H.; Bhargava, D.; Kashwani, R.; Mishra, R. Artificial Intelligence as a Tool for Improving Oral Cancer Outcomes. Arch. Dent. Res. 2023, 14, 95–102. [Google Scholar] [CrossRef]
- Shan, T.; Tay, F.R.; Gu, L. Application of artificial intelligence in dentistry. J. Dent. Res. 2021, 100, 232–244. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wu, J. Early diagnosis of oral cancer using a hybrid arrangement of deep belief networkand combined group teaching algorithm. Sci. Rep. 2023, 13, 22073. [Google Scholar] [CrossRef]
- Singh, P.; Vijay, P.; Anjum, R.; Pardhe, N.D.; Jahan, A.; Khanam, W. Exploring the role of artificial intelligence in oral cancer diagnosis: Review. J. Oral Med. Oral Surg. Oral Pathol. Oral Radiol. 2024, 10, 154–156. [Google Scholar] [CrossRef]
- Mazurowski, M.A.; Buda, M.; Saha, A.; Bashir, M.R. Deep learning in radiology: An overview of the concepts and a survey of the state of the art with focus on MRI. J. Magn. Reson. Imaging 2018, 49, 939–954. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2018, 25, 44–56. [Google Scholar] [CrossRef]
- Karimian, G.; Petelos, E.; Evers, S.M.A.A. The ethical issues of the application of artificial intelligence in healthcare: A systematic scoping review. AI Ethics 2022, 2, 539–551. [Google Scholar] [CrossRef]
- Petti, S.; Johnson, N.; Scully, C.; Honour, P.; Atkinson, J.; Bagan, J.; Spain, M.; Carrozzo, R.; Küffer, F.; Odell, D.; et al. Issue information. Oral Dis. 2020, 27, 1–2. [Google Scholar] [CrossRef]
- Kováč, P.; Jackuliak, P.; Bražinová, A.; Varga, I.; Aláč, M.; Smatana, M.; Lovich, D.; Thurzo, A. Artificial Intelligence-Driven Facial Image Analysis for the Early Detection of Rare Diseases: Legal, Ethical, Forensic, and Cybersecurity Considerations. AI 2024, 5, 990–1010. [Google Scholar] [CrossRef]
- Kharche, A.; Mathur, A.; Mehta, V. AI-powered oral cancer detection: A breakthrough in dental diagnostics. Oral Oncol. Rep. 2024, 10, 100293. [Google Scholar] [CrossRef]
- Kavuluri, S.; Danwada, R. Artificial Intelligence and Machine Learning in Oral Cancer Detection: A Scoping Review of Modern Diagnostic Approaches and Future Frontiers in the Detection of Oral Cancer. J. Res. Med. Dent. Sci. 2024, 12, 1–3. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
| Sr. No | Themes Generated | Analysis Performed Using | Authors | Outcomes Reported |
|---|---|---|---|---|
| 1 | Application | Pathology | Abdul NS [25] | Artificial intelligence has proven beneficial in identifying tumors of the mouth cavity and forecasting the progression of oral and maxillofacial pathology. It can precisely identify people at elevated risk for oral cancer and the probability of disease recurrence. |
| 2 | Application | Histopathological Images | Ribeiro-de-Assis MCF [26] | The NDB-UFES dataset has been released to assist researchers in creating AI tools for the automated identification of oral potentially malignant diseases and oral squamous cell carcinoma. This dataset is designed for application in the domain of Artificial Intelligence, particularly in machine learning and deep learning. |
| 3 | Application | Histopathological Images | Bassani S [27] | Artificial intelligence techniques exhibit potential for enhancing diagnosis; nevertheless, the absence of extensive training datasets presents a significant problem that must be resolved. |
| 4 | Application | - | Hegde S [28] | This article examined the applications and benefits of artificial intelligence in oral cancer screening, diagnosis, prediction, treatment planning, and prognosis. The constraints and prospective advancements of AI in OC research were also examined. |
| 6 | Application | Immunological Assay | Pillai A [29] | The study revealed no significant differences in survival rates among groups; however, patients with autoimmune diseases exhibited marginally poorer results. This indicates that immunological dysregulation in these patients may influence their cancer outcomes. |
| 7 | Application | - | Al-Rawi N [30] | Supervised machine learning attained diverse degrees of accuracy, sensitivity, specificity, and AUC in the detection of oral cancer. No consensus exists about the optimal AI strategy for detection; however, deep learning, particularly deep convolutional neural networks, showed superior performance in early detection relative to supervised machine learning. |
| 8 | Application | Clinical Images | AlabdanR [31] | The research advocates for the implementation of the IDCNN model, which integrates the Inception module with DCNN, for feature extraction and classification purposes. The MFO approach helps enhance classification performance. The experimental findings indicate that the OIDCNN-OPMDD model surpasses other deep learning models. |
| 9 | Challenges and Future Directions | - | Dixit S [32] | This review discusses the potential use of (AI) in early detection and treatment of (OC). AI can accurately analyze large datasets from imaging modalities, and deep learning (DL) models have shown promise in overcoming challenges associated with early cancerous lesions. The review follows recommended guidelines and also discusses methods for reducing risk factors and preventing oral diseases. |
| 10 | Challenges and Future Directions | Biomarkers | Wang S [33] | Investigations are underway about liquid biopsy biomarkers for the early identification of oral squamous cell carcinoma (OSCC). The biomarkers encompass microbiome constituents, noncoding RNAs, extracellular vesicles, and circulating tumor DNA. This study examines the screening techniques for OSCC and presents novel approaches for illness diagnosis. |
| 11 | Challenges and Future Directions | Biomarkers | Satish KS [34] | This paper examines the application of omics data and machine learning methodologies in tackling the intricacies of oral cancer. It encompasses subjects like early detection, biomarker identification, therapeutic targets, drug resistance, precision medicine, and prognostic biomarkers. This review seeks to summarize the contemporary research on oral cancer utilizing omics, bioinformatics, and machine learning methodologies. |
| 12 | Challenges and Future Directions | - | Su Y-F [35] | This article examines contemporary diagnostic techniques for oral cancer and investigates prospective future technologies for enhanced diagnosis and therapy. The objective is to broaden diagnostic alternatives and improve the capacity to accurately identify and manage oral malignant lesions. |
| 13 | Challenges and Future Directions | - | Haj-Hosseini N [36] | The article discusses various technologies used for oral cavity screening, including sample-based methods and direct screening techniques. It emphasizes the importance of digitalization and automated AI-based analysis in making these technologies more accessible and reducing the need for highly trained specialists. |
| 14 | Classifications | Genetics | ArumuganainarD [37] | The additional tree classifier precisely identified interactomic hub genes, achieving 98% overall accuracy and 97% class accuracy. HSPB1 was discovered as a key gene using Cytohubba analysis. This classifier may enhance diagnostic and therapeutic approaches for oral cancer. |
| 15 | Classifications | Histopathological Images | SukegawaS [38] | The study revealed that the diagnostic accuracy of oral pathologists was markedly enhanced by using the findings of a deep learning model as adjunctive diagnoses. This indicates that integrating deep learning models can improve the diagnostic efficacy of pathologists in oral pathology. This research underscores the efficacy of employing robust deep learning models in oral pathology diagnosis. |
| 16 | Classifications | FTIR Data | Ellis BG [39] | The MLA has demonstrated effectiveness in detecting seven tissue types in complicated primary OSCC tumors. It effectively distinguished among three epithelial and four non-epithelial tissue types with elevated sensitivities and specificities. The study employed a limited sample size; however, the results will guide further, more extensive research endeavors. |
| 17 | Classifications | Radiography, Clinical Images | Kim J-S [40] | AI-assisted screening for oral precancerous lesions demonstrated a diagnostic odds ratio (DOR) of 121.66. Subgroup study indicated that optical coherence tomography (OCT) had superior accuracy and negative predictive value relative to photographic pictures and autofluorescence. The detection of oral malignant lesions by AI may serve as a non-invasive and expedient diagnostic instrument for early identification. |
| 18 | Classifications | Clinical Images | Song B [41] | The study compares the effectiveness of two transformer architectures, Vision Transformer (ViT) and Swin Transformer, for oral cancer image classification. The pre-trained Swin Transformer achieved 88.7% accuracy, outperforming ViT, VGG19, and ResNet50 models. The results suggest that transformer-based architectures have potential for advancing oral cancer image analysis. |
| 19 | Classifications | Point of Care Device | James BL [42] | This research demonstrated that an automated image processing technique could precisely differentiate dysplastic-OPML from malignant lesions utilizing OCT images. They investigated the application of artificial neural network methodologies to detect high-grade dysplasia. The research indicates that OCT may serve as an effective instrument for the screening and monitoring of oral cancer in resource-limited environments. |
| 21 | Classifications | Laserendomicroscopy Images | Aubreville M [15] | A novel technique for identifying CLE pictures of oral cavity lesions surpasses existing state-of-the-art methods. The approach attained an area under the curve (AUC) of 0.96 and a mean accuracy of 88.3%, demonstrating elevated sensitivity and specificity. The research utilized 7894 pictures from individuals identified with (OSCC). |
| 22 | Diagnosis and Prediction | - | Khanagar SB [43] | Artificial intelligence models have demonstrated encouraging outcomes in the diagnosis of oral cancer, differentiating between normal and malignant regions, forecasting patient survival, and assessing disease severity. These models have attained elevated levels of accuracy, sensitivity, and specificity, surpassing conventional clinical techniques. The application of AI in pathology can significantly improve diagnosis results and minimize errors. Regulatory authorities and governments must prioritize the endorsement and commercialization of these AI solutions for clinical application. |
| 23 | Early Diagnosis and Detection | - | Li J [44] | AI-driven screening utilizing clinical photography has demonstrated exceptional diagnostic efficacy in identifying oral mucosal neoplastic lesions and oral potentially malignant disorders (OPMDs). It possesses elevated sensitivity, specificity, and negative predictive value. The implementation of AI in general practice can improve diagnostic proficiency without requiring costly imaging technology. |
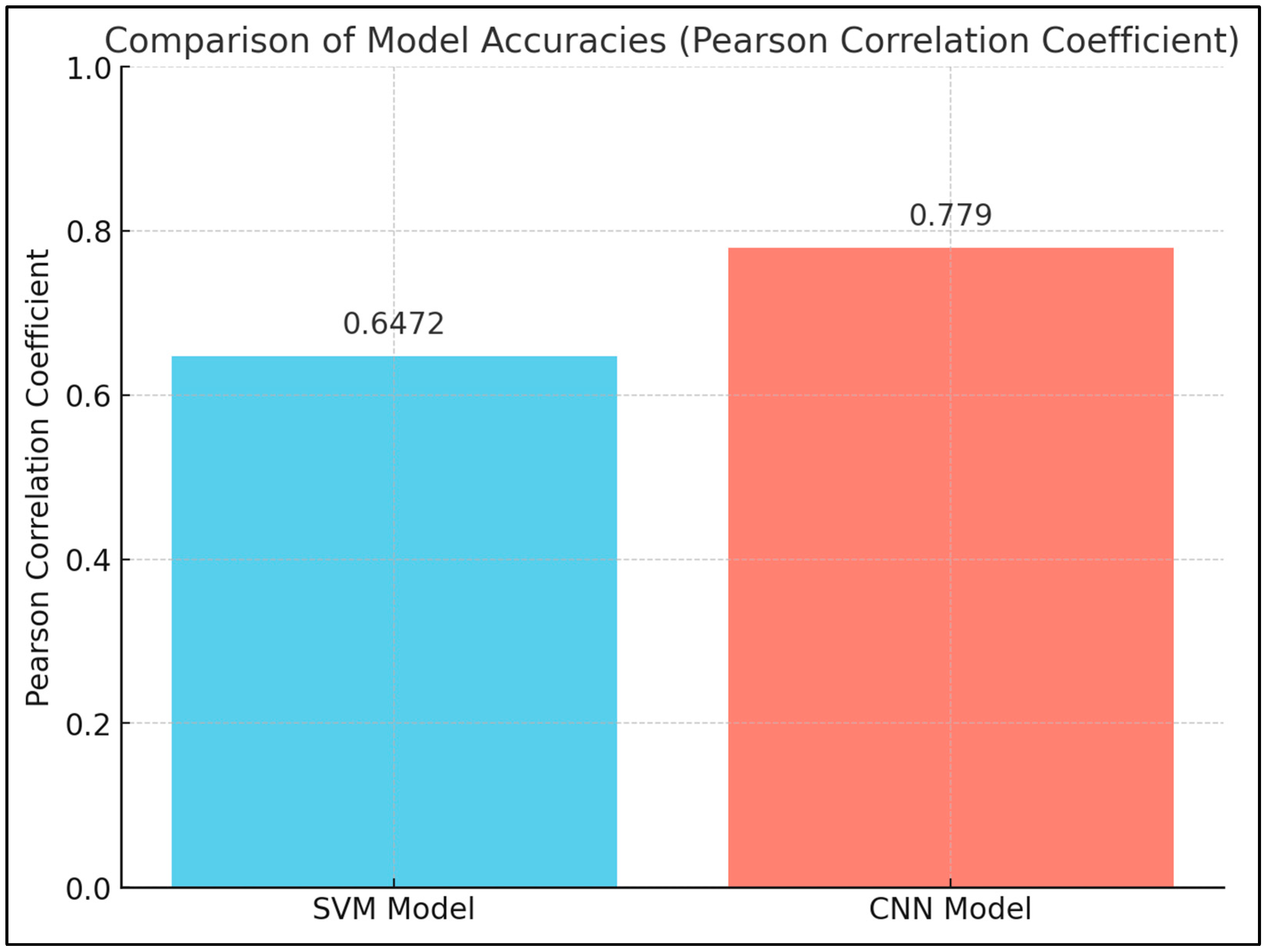
| 24 | Early Diagnosis and Detection | Nuclear Parameters | Mhaske S [45] | The study found significant differences in nuclear size, shape, and chromatin distribution between the study and control groups. The SVM model had a Pearson correlation coefficient of 0.6472, while the CNN model had a coefficient of 0.7790, indicating that the SVM model was more accurate. The availability of multidimensional datasets and advancements in AI have led to increased use in oncology research, with potential improvements in early detection and patient outcomes. |
| 25 | Early Diagnosis and Detection | Color Intensity-Based Textural Features | Sharma PN [46] | The research indicated that some textural characteristics, including entropy and contrast, were elevated in oral squamous cell cancer (OSCC) relative to the control group. Analysis of the receiver operating characteristic curve indicated that the accuracy, sensitivity, and specificity of utilizing these features for diagnosis were 88%, 91%, and 81%, respectively. This indicates that computer-assisted textural analysis may be beneficial for the early diagnosis of oral cancer. |
| 26 | Early Diagnosis and Detection | Clinical-histopathological Images | Zayed SO [47] | The study found that the Diagnosis Oral Diseases Software (DODS) had a correct diagnosis rate comparable to oral pathologists using microscopic examination. The software had high reliability and accuracy, making it a useful diagnostic tool. However, it had lower accuracy, sensitivity, and specificity compared to oral pathologists with a master’s degree. |
| 27 | Early Diagnosis and Detection | Oral biopsy Histopathological images | Soni A [48] | The EfficientNetB0 model attained remarkable performance metrics, exceeding leading methodologies in the domain. It exhibited elevated accuracy, sensitivity, specificity, precision, F1 score, Matthew’s correlation coefficient (MCC), and kappa statistics. The model’s incorporation of deep learning methodologies demonstrates potential for the automated early diagnosis of oral cancer, enhancing treatment approaches. |
| 28 | Early Diagnosis and Detection | Radiographs (Contrast-enhanced CT) | Struckmeier A-K [49] | The research indicated that CT scans had a sensitivity of 76.85% and a specificity of 82.20% in identifying bone invasion in patients. The percentages of false positives and false negatives were 11.27% and 5.99%, respectively. Artefacts influenced the assessment in several individuals, excluding those with bone invasion. Factors including tumor size, depth of invasion, tumor localization, lymphatic invasion, and perineural invasion were linked to the erroneous identification of bone invasion. The research indicates that integrating several techniques and employing artificial intelligence or monitoring electrolyte imbalances may enhance the precision of bone invasion identification prior to histopathological examination. |
| 29 | Early Diagnosis and Detection | Genetic Analysis (SEMA3C) | Dou H [50] | SEMA3C, a gene associated with cell adhesion and regulation of various cellular activities, was found to be highly expressed in TSCC (tongue squamous cell carcinoma). High expression of SEMA3C was linked to poor prognosis in TSCC patients. Further analysis confirmed its expression in tumor cells and showed that its knockdown inhibited tumor cell proliferation, migration, and invasion. This study suggests that SEMA3C could serve as a biomarker for TSCC diagnosis, prevention, prognosis, and personalized medicine. |
| 30 | Early Diagnosis and Detection | Clinical Intraoral Images | Y D, Ramalingam [51] | The research employed a training dataset including 300 clinical photographs to instruct doctors and a machine learning system. A test dataset of 60 novel clinical photographs was subsequently assessed by both the physicians and the algorithm. The findings indicated a moderate concordance between the two, with a Mean Average Precision of 25.4%, precision of 29.8%, and recall of 32.9%. The machine learning model exhibited a specificity of 75% and a sensitivity of 88.9%. The study indicates that machine learning may serve as an effective instrument for the early identification of suspected lesions in dental patients. |
| 31 | Early Diagnosis and Detection | Radiographs (Contrast-enhanced CT) | Struckmeier A-K [52] | The study revealed that sensitivity was greater in the cohort with enlarged lymph nodes than in the cohort with atrophied lymph nodes. Nevertheless, specificity was greater in the cohort with liquefied lymph nodes. The false negative rate for emphasized lymph nodes was 13%, but the false positive rates varied from 8.82% to 51.80%. The diagnostic accuracy for identifying lymph node metastases was superior at levels IIa and IIb compared to level III. Patients exhibiting pronounced lymphadenopathy were more predisposed to possess a tiny, well-differentiated neoplasm. CT scans were deemed adequate for forecasting lymph node metastasis in individuals with oral squamous cell carcinoma, and the prospective incorporation of artificial intelligence and deep learning may enhance the accuracy of CT in identifying lymph node metastasis. Additional inquiry is required. |
| 32 | Early Diagnosis and Detection | Histopathological Images | Ahmad M [53] | The suggested system demonstrated encouraging outcomes for the rapid diagnosis of oral squamous cell cancer (OSCC) utilizing histology images. The support vector machine (SVM) algorithm, integrating DenseNet201 with GLCM, HOG, and LBP features, attained elevated accuracy, precision, sensitivity, specificity, and F-1 score. |
| 33 | Early Diagnosis and Detection | Radiographs (Optical Coherence) | Yang Z [54] | Three varieties of (CNNs) were developed and assessed utilizing four measures. The CNN-based methodologies were juxtaposed with machine learning techniques utilizing the identical dataset. The results indicated that CNNs achieved a superior classification accuracy of 96.76% in contrast to the machine-learning-based technique, which attained 92.52%. The model’s capacity to differentiate various oral tissues was assessed by visualizing lesions in OCT images. The research determined that deep learning algorithms can offer decision support for the efficient detection and diagnosis of oral cancer. |
| 34 | Early Diagnosis and Detection | Histopathological Images | Oya K [55] | The proposed method achieved a high accuracy of 99.65% with a specific input size. Grad-CAM analysis revealed that the AI focused on both cellular and structural atypia of SCC, particularly in the region surrounding the basal layer. Training AI with different magnification images simultaneously could be effective for diagnosing oral SCC. |
| 35 | Early Diagnosis and Detection | Radiographs (Contrast-enhanced CT) | Xu X [56] | A DNN model was constructed and achieved high accuracy in localizing lymph nodes and discriminating metastasis. The model performed better than radiologists, surgeons, and students in accurately identifying positive lymph nodes. The model’s clinical accuracy was also higher than that of the Radiology Department, suggesting its potential for accurate diagnosis and personalized treatment planning. |
| 36 | Early Diagnosis and Detection | Biomarkers | Jing F [57] | The research identified specific genes linked to the advancement of (OSCC). These genes effectively predicted the fate of OSCC patients, indicating that those in the high-risk category exhibited a poor prognosis. The seven identified significant genes may function as possible biomarkers for the prognosis of OSCC patients. |
| 37 | Early Diagnosis and Detection | Biomarkers | Tseng Y-J [58] | This study with 337 individuals showed that a predictive model incorporating age and autoantibody levels enhanced the predictive accuracy for high-risk instances of OSCC. The approach can be utilized professionally via an online calculator to deliver individualized information for OSCC diagnosis, potentially diminishing morbidity and fatality rates. |
| 38 | Early Diagnosis and Detection | Biomarkers | Adeoye J [59] | A study discovered 1745 differentially methylated regions (DMRs) and 105 differentially methylated cytosines (DMCs) for the detection of OSCC. The preponderance of DMRs exhibited hypermethylation, although the ratio of hypomethylated to hypermethylated DMCs was comparable. DMRs were predominantly annotated to promoter regions, whereas DMCs were primarily annotated to intergenic regions. A linear SVM model utilizing 11 optimum DMRs showed flawless efficacy in OSCC identification. Saliva samples can facilitate biomarker identification, and machine learning platforms may assist in the screening of OSCC. |
| 39 | Early Diagnosis and Detection | Histopathological Images | Deif MA [60] | The study examined the impact of Reinhard stain normalization on performance. The best features were extracted and selected, and XGBoost was used for classification. The highest accuracy of 96.3% was achieved with Inception V3 and BPSO. This approach improves diagnostic efficiency and reduces costs for OCSCC patients using histopathological images. |
| 40 | Early Diagnosis and Detection | Point of Care Device | Birur N P [61] | Onsite specialists assessed a small percentage of subjects and performed biopsies on some. The study found that telediagnosis had high accuracy compared to onsite specialists, while community health workers had lower sensitivity. The mobile phone and cloud technology accurately identified lesions, making it a useful tool for oral cancer screening in low-resource settings. |
| 41 | Early Diagnosis and Detection | Histopathological Images | Fati SM [62] | A technique employing hybrid features derived from CNN models and diverse algorithms attained exceptional outcomes in diagnosing the OSCC dataset. The dimensionality of the features was decreased by PCA before being input into an ANN algorithm, yielding encouraging accuracy. The proposed approach attained an accuracy of 99.1%, along with elevated specificity, sensitivity, precision, and AUC. |
| 42 | Early Diagnosis and Detection | Radiographs (Contrast-enhanced CT) | Tomita H [63] | The research evaluated the diagnostic efficacy of a deep learning (DL) model against radiologists’ evaluations in categorizing cervical lymph nodes (LNs) in individuals with OSCC. The deep learning model demonstrated markedly superior accuracy in differentiating between benign and metastatic lymph nodes relative to the evaluations of the radiologists. This indicates that deep learning analysis of pretreatment contrast-enhanced CT scans may serve as an effective instrument for diagnosing cervical lymph nodes in patients with oral squamous cell carcinoma. |
| 43 | Early Diagnosis and Detection | Point of Care Tele—Cytology | Sunny S [64] | The research assessed the precision of a tele-cytology platform named Cellscope in identifying oral lesions. The platform had an overall accuracy of 84-86% and exhibited comparable performance to traditional cytology. Nonetheless, it exhibited limited sensitivity in identifying specific lesions. The integration of image processing with an artificial neural network-based risk stratification model considerably enhanced sensitivity and overall accuracy. The research finds that tele-cytology, in conjunction with the risk stratification model, serves as an effective instrument for the early diagnosis and screening of oral cancer. |
| 44 | Early Diagnosis and Detection | Point of Care Device | Uthoff RD [65] | A remote specialist and a convolutional neural network (CNN) accurately identified 170 image pairs as ‘suspect’ or ‘not suspicious’, utilizing the on-site professional’s diagnosis as the reference standard. Sensitivities, specificities, and positive predictive values were reported. |
| 45 | Early Diagnosis and Detection | Clinical Images | Zhang H [66] | This research established a deep learning methodology to autonomously determine the extent of malignancy in oral photos. The approach utilized various forms of CNN and applied morphological edge detection for precise feature extraction. The experimental findings demonstrated that the approach was efficacious in diagnosing oral cancer. |
| 46 | Early Diagnosis and Detection | Microbial Analysis | Zhou X [67] | This study found that the average diagnostic accuracy rates for diagnosing oral squamous cell carcinoma (OSCC) using five different sites and saliva were 98.17% and 95.70%, respectively. Cross-validations showed estimated external prediction accuracies of 96.67% and 93.58%, respectively. The false-negative rate was 0%. The study also identified certain bacterial species that were strongly correlated with OSCC. Overall, the study suggests that using oral microbiota data and machine learning methods can provide a noninvasive and inexpensive method of diagnosis. |
| 47 | Early Diagnosis and Detection | Biomarkers | Song X [68] | Machine learning has been used to distinguish OSCC and premalignant lesions from normal conditions with an accuracy of 86.7%. This suggests that combining CPSI-MS and machine learning can be a reliable and automated tool for diagnosing OSCC in clinical settings. |
| 48 | Early Diagnosis and Detection | Point of Care Device | Uthoff RD [69] | A compact smartphone-based intraoral probe has been developed that allows for autofluorescence imaging and polarized white light imaging. It is small and flexible, allowing for imaging of high-risk areas for oral cancer. Remote diagnosis is possible through cloud-based technology and a neural network. Field-testing data are provided. |
| 49 | Early Diagnosis AND Prediction | Biomarkers | Yang Z [70] | The study found that AUNIP, a gene, could be a useful biomarker for diagnosing and predicting the prognosis of oral squamous cell carcinoma (OSCC). AUNIP’s expression increased with the severity of OSCC, and its suppression inhibited cancer cell growth. This suggests that targeting AUNIP could be a potential strategy for preventing and treating OSCC. |
| 50 | Prediction Modelling and Risk Evaluation | Histological Images | Nikkuni Y [71] | The models achieved areas under the curve ranging from 0.71 to 0.84, indicating the usefulness of PET radiomics analysis for preoperative prediction of the grade. |
| 51 | Prediction Modelling and Risk Evaluation | CT-MRI Radiographic Images | Deng C [72] | The AI models had an area under the curve (AUC) of 0.92 and high sensitivity and specificity. The performance of AI was better than that of experienced radiologists. This suggests that AI could be a useful tool in clinical practice for diagnosing LN metastases in OSCC patients. |
| 52 | Prediction Modelling and Risk Evaluation | Biomarkers | Einhaus J [73] | A machine learning process was employed to precisely diagnose the histological grade of OSCC. Three model parameters were identified as correlating with clinical outcomes: granulocyte MAPKAPK2 signaling, stromal CD4+ memory T cell size, and the distance of fibroblasts from the tumor margin. This study offers a framework for the analysis of intricate imaging data and the identification of prognostic biomarkers for recurrence risk assessment and the development of immunomodulatory therapies. |
| 53 | Prediction Modelling and Risk Evaluation | International Cancer Genome Consortium (ICGC) data portal. | Siddalingappa R, Kanagaraj S [74] | The experimental results indicate that the k-fold approach surpasses the hold-out method, achieving a mean absolute error score of 0.015. The model effectively categorizes patients into various stages of cancer, demonstrating good accuracy, recall, precision, and F-measure. The research suggests that elderly patients with a greater number of mutations have an elevated risk of reduced survival and advanced cancer stages. |
| 54 | Prediction Modelling and Risk Evaluation | Histopathological Images | Zhang X [75] | The study found that high-risk oral leukoplakia (OL) patients were almost 4 times more likely to develop oral cancer (OC) compared to low-risk patients. The time-to-progression also differed significantly between the two groups. The 5-year probability of OC development was higher in high-risk patients. The OMRS model remained predictive even after adjusting for age, OL site, and dysplasia grading. This model can help identify high-risk OL patients and improve early diagnosis and prevention of OC. |
| 55 | Prediction Modelling and Risk Evaluation | Genetics | Singh T [76] | The study aimed to increase the number of instances in oral cancer gene datasets and identify key genes for gingivobuccal cancer (GBC) prognosis. Supervised and unsupervised machine learning methods were used to annotate and analyze the datasets, emphasizing the significance of automated gene identification for GBC and other oral cancer types. |
| 56 | Prediction Modelling and Risk Evaluation | Genetics | Zhang L [77] | A study developed an IRS model using machine learning to predict the survival and disease-free survival of OSCC patients. The model included several genes and was found to be more accurate than traditional indicators. The model was also an independent risk factor and could be used in combination with other factors for clinical application. The IRS was associated with tumor microenvironment and could predict response to immunotherapy and chemotherapy. The study suggests that the model could help identify biomarkers and targets for immunotherapy. |
| 57 | Prediction Modelling and Risk Evaluation | Biomarkers | Kim Y [78] | A classifier was used to predict patient survival patterns using an ORCA dataset. Seven genes were identified as differentially expressed between risk groups. This study highlights the significance of TILs in the tumor microenvironment and suggests the potential of a deep learning approach for cancer prognosis. |
| 58 | Prediction Modelling and Risk Evaluation | Clinical Images | Camalan S [79] | A method for analyzing clinical photographic images was tested on datasets from the UK and Brazil. The system achieved accuracies of 73.6% and 90.9% using different validation methods. The study also found that using patches instead of the whole image improved performance and identified predictive regions using class activation map analysis. |
| 59 | Prediction Modelling and Risk Evaluation | Biomarkers | Hamana K [80] | A study found that a majority of DNA samples from tumor tissues and serum showed abnormalities in at least one gene. The patterns of abnormalities in serum DNA matched those found in tumor DNA. Abnormalities were often detected before and after surgery. Patients with no abnormalities after surgery had no recurrence and were disease-free, while those with abnormalities had a higher risk of distant metastasis and death. This suggests that analyzing microsatellite status in serum DNA could help predict disease prognosis. |
| 60 | Prediction Modelling and Risk Evaluation | Genetics | Cao R [81] | A 3-mRNA signature consisting of CLEC3B, C6, and CLCN1 was found to be a prognostic biomarker pattern for oral squamous cell carcinoma (OSCC). The risk score was calculated based on the expression levels of these mRNAs. The signature showed good predictive accuracy for 3- and 5-year survival in both the training and validation cohorts. Machine learning analysis also confirmed the significance of this signature in predicting survival. The pathway analysis revealed that neuroactive ligand–receptor interaction was the most enriched pathway associated with OSCC. Overall, the 3-mRNA signature is a promising tool for predicting OSCC patient survival. |
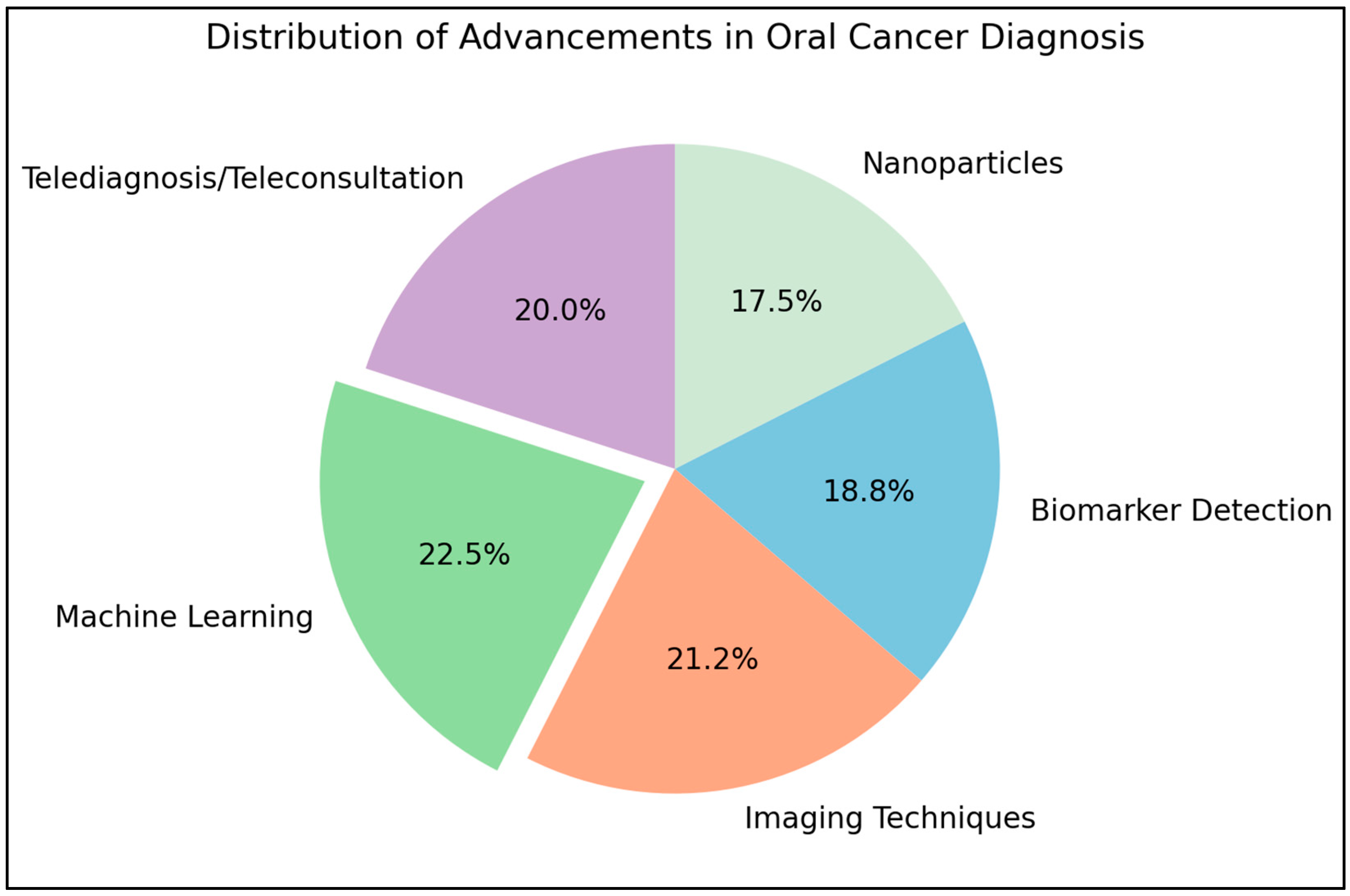
| 61 | Recent Advancement and Future Research | - | Ojha A [82] | This review discusses the advancements in imaging methods like narrow band imaging, fluorescence imaging, and optical coherence tomography for early lesion detection. It also highlights the use of biomarker detection in saliva and targeted nanoparticles for early diagnosis, along with the role of machine learning in improving diagnostic accuracy. However, further clinical and large-scale studies are needed for widespread adoption. |
| 62 | Recent Advancement and Future Research | - | Dos Santos RTN [83] | Digital strategies such as telediagnosis and teleconsultations are being used to diagnose oral cancer using clinical or histopathological images. The success of these strategies is determined by the level of agreement in the better performance of the strategy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinay, V.; Jodalli, P.; Chavan, M.S.; Buddhikot, C.S.; Luke, A.M.; Ingafou, M.S.H.; Reda, R.; Pawar, A.M.; Testarelli, L. Artificial Intelligence in Oral Cancer: A Comprehensive Scoping Review of Diagnostic and Prognostic Applications. Diagnostics 2025, 15, 280. https://doi.org/10.3390/diagnostics15030280
Vinay V, Jodalli P, Chavan MS, Buddhikot CS, Luke AM, Ingafou MSH, Reda R, Pawar AM, Testarelli L. Artificial Intelligence in Oral Cancer: A Comprehensive Scoping Review of Diagnostic and Prognostic Applications. Diagnostics. 2025; 15(3):280. https://doi.org/10.3390/diagnostics15030280
Chicago/Turabian StyleVinay, Vineet, Praveen Jodalli, Mahesh S. Chavan, Chaitanya. S. Buddhikot, Alexander Maniangat Luke, Mohamed Saleh Hamad Ingafou, Rodolfo Reda, Ajinkya M. Pawar, and Luca Testarelli. 2025. "Artificial Intelligence in Oral Cancer: A Comprehensive Scoping Review of Diagnostic and Prognostic Applications" Diagnostics 15, no. 3: 280. https://doi.org/10.3390/diagnostics15030280
APA StyleVinay, V., Jodalli, P., Chavan, M. S., Buddhikot, C. S., Luke, A. M., Ingafou, M. S. H., Reda, R., Pawar, A. M., & Testarelli, L. (2025). Artificial Intelligence in Oral Cancer: A Comprehensive Scoping Review of Diagnostic and Prognostic Applications. Diagnostics, 15(3), 280. https://doi.org/10.3390/diagnostics15030280









