Integrating Donor Derived Cell-Free DNA Fraction and Absolute Quantification for Enhanced Rejection Diagnosis in Kidney Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Histological Diagnosis
2.3. Blood Sample Collection and cfDNA Extraction
2.4. Quantification of cfDNA
2.5. SNP Selection, Targeted Sequencing, and Data Analysis
2.6. dd-cfDNA Quantification
2.7. Statistical Analysis
3. Results
3.1. Patient Cohort and Characteristics
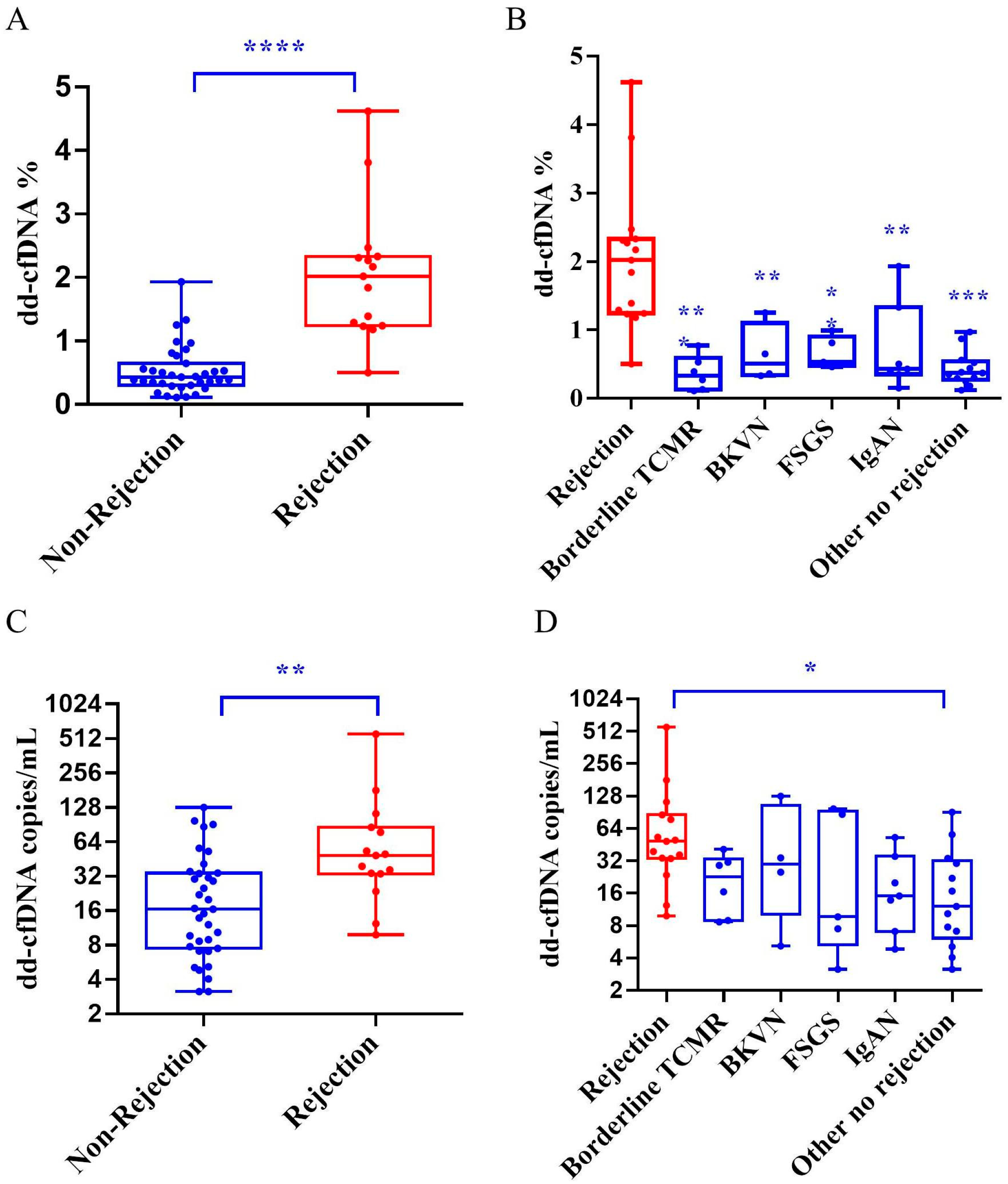
3.2. dd-cfDNA Fraction and Absolute Quantification Among Groups
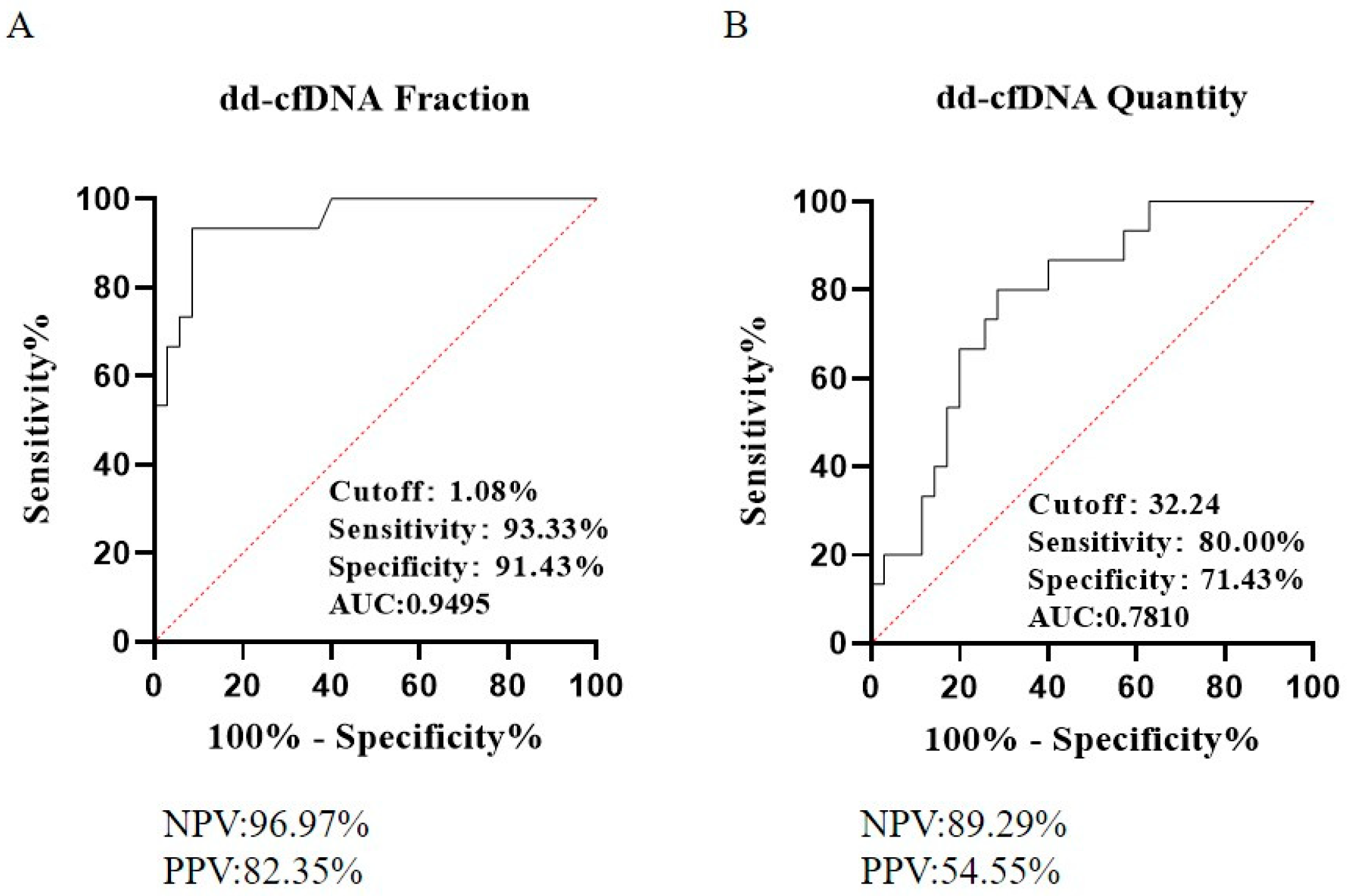
3.3. Diagnostic Performance for Kidney Allograft Rejection
3.4. Integrating dd-cfDNA Fraction and Absolute Quantification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Wilk, A.R.; Castro, S.; Robinson, A.; Wainright, J.L.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am. J. Transplant. 2019, 19 (Suppl. S2), 19–123. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, M.; Shipkova, M.; Asendorf, T.; Walson, P.D.; Schauerte, V.; Mettenmeyer, N.; Kabakchiev, M.; Hasche, G.; Gröne, H.-J.; Friede, T.; et al. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Am. J. Transplant. 2019, 19, 3087–3099. [Google Scholar] [CrossRef]
- Oellerich, M.; Sherwood, K.; Keown, P.; Schütz, E.; Beck, J.; Stegbauer, J.; Rump, L.C.; Walson, P.D. Liquid biopsies: Donor-derived cell-free DNA for the detection of kidney allograft injury. Nat. Rev. Nephrol. 2021, 17, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Whitlam, J.B.; Ling, L.; Skene, A.; Kanellis, J.; Ierino, F.L.; Slater, H.R.; Bruno, D.L.; Power, D.A. Diagnostic application of kidney allograft-derived absolute cell-free DNA levels during transplant dysfunction. Am. J. Transplant. 2019, 19, 1037–1049. [Google Scholar] [CrossRef]
- De Vlaminck, I.; Valantine, H.A.; Snyder, T.M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; Bernstein, D.; Weisshaar, D.; et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci. Transl. Med. 2014, 6, 241ra277. [Google Scholar] [CrossRef]
- Aubert, O.; Ursule-Dufait, C.; Brousse, R.; Gueguen, J.; Racapé, M.; Raynaud, M.; Van Loon, E.; Pagliazzi, A.; Huang, E.; Jordan, S.C.; et al. Cell-free DNA for the detection of kidney allograft rejection. Nat. Med. 2024, 30, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Hidestrand, M.; Tomita-Mitchell, A.; Hidestrand, P.M.; Oliphant, A.; Goetsch, M.; Stamm, K.; Liang, H.-L.; Castleberry, C.; Benson, D.W.; Stendahl, G.; et al. Highly sensitive noninvasive cardiac transplant rejection monitoring using targeted quantification of donor-specific cell-free deoxyribonucleic acid. J. Am. Coll. Cardiol. 2014, 63, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- De Vlaminck, I.; Martin, L.; Kertesz, M.; Patel, K.; Kowarsky, M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc. Natl. Acad. Sci. USA 2015, 112, 13336–13341. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Wort, S.J.; Thomas, H.; Collinson, P.; Bennett, E.D. Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit. Care 2006, 10, R60. [Google Scholar] [CrossRef]
- TTug, S.; Helmig, S.; Deichmann, E.R.; Schmeier-Jürchott, A.; Wagner, E.; Zimmermann, T.; Radsak, M.; Giacca, M.; Simon, P. Exercise-induced increases in cell free DNA in human plasma originate predominantly from cells of the haematopoietic lineage. Exerc. Immunol. Rev. 2015, 21, 164–173. [Google Scholar]
- Han, D.S.; Ni, M.; Chan, R.W.; Chan, V.W.; Lui, K.O.; Chiu, R.W.; Lo, Y.D. The Biology of Cell-free DNA Fragmentation and the Roles of DNASE1, DNASE1L3, and DFFB. Am. J. Hum. Genet. 2020, 106, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 2020, 20, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Grskovic, M.; Hiller, D.J.; Eubank, L.A.; Sninsky, J.J.; Christopherson, C.; Collins, J.P.; Thompson, K.; Song, M.; Wang, Y.S.; Ross, D.; et al. Validation of a Clinical-Grade Assay to Measure Donor-Derived Cell-Free DNA in Solid Organ Transplant Recipients. J. Mol. Diagn. JMD 2016, 18, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Osmanodja, B.; Akifova, A.; Budde, K.; Choi, M.; Oellerich, M.; Schütz, E.; Beck, J. Absolute or Relative Quantification of Donor-derived Cell-free DNA in Kidney Transplant Recipients: Case Series. Transplant. Direct 2021, 7, e778. [Google Scholar] [CrossRef]
- Bunnapradist, S.; Homkrailas, P.; Ahmed, E.; Fehringer, G.; Billings, P.R.; Tabriziani, H. Using Both the Fraction and Quantity of Donor-Derived Cell-Free DNA to Detect Kidney Allograft Rejection. J. Am. Soc. Nephrol. 2021, 32, 2439–2441. [Google Scholar] [CrossRef] [PubMed]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.K.; Archila, F.A.; Constantin, T.; Prins, S.A.; Liberto, J.; Damm, I.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; Demko, Z.P.; et al. Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR. J. Clin. Med. 2018, 8, 19. [Google Scholar] [CrossRef]
- Halloran, P.F.; Reeve, J.; Madill-Thomsen, K.S.; Demko, Z.; Prewett, A.; Billings, P.; Trifecta the Trifecta Investigators. The Trifecta Study: Comparing Plasma Levels of Donor-derived Cell-Free DNA with the Molecular Phenotype of Kidney Transplant Biopsies. J. Am. Soc. Nephrol. JASN 2022, 33, 387–400. [Google Scholar] [CrossRef]
- Agbor-Enoh, S.; Shah, P.; Tunc, I.; Hsu, S.; Russell, S.; Feller, E.; Shah, K.; Rodrigo, M.E.; Najjar, S.S.; Kong, H.; et al. Cell-Free DNA to Detect Heart Allograft Acute Rejection. Circulation 2021, 143, 1184–1197. [Google Scholar] [CrossRef]
- Khush, K.K.; Patel, J.; Pinney, S.; Kao, A.; Alharethi, R.; DePasquale, E.; Ewald, G.; Berman, P.; Kanwar, M.; Hiller, D.; et al. Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: A prospective multicenter study. Am. J. Transplant. 2019, 19, 2889–2899. [Google Scholar] [CrossRef] [PubMed]
- Gielis, E.M.; Beirnaert, C.; Dendooven, A.; Meysman, P.; Laukens, K.; De Schrijver, J.; Van Laecke, S.; Van Biesen, W.; Emonds, M.-P.; De Winter, B.Y.; et al. Plasma donor-derived cell-free DNA kinetics after kidney transplantation using a single tube multiplex PCR assay. PLoS ONE 2018, 13, e0208207. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Coll, V.; Band, J.E.K.E.; Llorente, S.; González-López, R.; Fernández-González, M.; Martínez-Banaclocha, H.; Galián, J.A.; Botella, C.; Moya-Quiles, M.R.; Minguela, A.; et al. All That Glitters in cfDNA Analysis Is Not Gold or Its Utility Is Completely Established Due to Graft Damage: A Critical Review in the Field of Transplantation. Diagnostics 2023, 13, 1982. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Coll, V.; Llorente, S.; Boix, F.; Alfaro, R.; Galián, J.A.; Martinez-Banaclocha, H.; Botella, C.; Moya-Quiles, M.R.; Muro-Pérez, M.; Minguela, A.; et al. Monitoring of Serological, Cellular and Genomic Biomarkers in Transplantation, Computational Prediction Models and Role of Cell-Free DNA in Transplant Outcome. Int. J. Mol. Sci. 2023, 24, 3908. [Google Scholar] [CrossRef]
- Schütz, E.; Asendorf, T.; Beck, J.; Schauerte, V.; Mettenmeyer, N.; Shipkova, M.; Wieland, E.; Kabakchiev, M.; Walson, P.D.; Schwenger, V.; et al. Time-Dependent Apparent Increase in dd-cfDNA Percentage in Clinically Stable Patients Between One and Five Years Following Kidney Transplantation. Clin. Chem. 2020, 66, 1290–1299. [Google Scholar] [CrossRef]
- Oellerich, M.; Budde, K.; Osmanodja, B.; Bornemann-Kolatzki, K.; Beck, J.; Schütz, E.; Walson, P.D. Donor-derived cell-free DNA as a diagnostic tool in transplantation. Front. Genet. 2022, 13, 1031894. [Google Scholar] [CrossRef]
- Halloran, P.F.; Reeve, J.; Madill-Thomsen, K.S.; Kaur, N.; Ahmed, E.; Cantos, C.; Baddar, N.A.H.; Demko, Z.; Liang, N.; Swenerton, R.K.; et al. Combining Donor-derived Cell-free DNA Fraction and Quantity to Detect Kidney Transplant Rejection Using Molecular Diagnoses and Histology as Confirmation. Transplantation 2022, 106, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Sethi, S.; Peng, A.; Najjar, R.; Mirocha, J.; Haas, M.; Vo, A.; Jordan, S.C. Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am. J. Transplant. 2019, 19, 1663–1670. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Non-Rejection (n = 35) | Rejection (n = 15) | p | ||
|---|---|---|---|---|---|
| Recipients Characteristics | |||||
| Age (years, IQR) | 35 (28–45) | 43 (27–57) | 0.268 | ||
| Male sex (n, %) | 27 (77.1%) | 12 (80.0%) | 1.000 | ||
| Median time posttransplant (years, IQR) | 1.16 (0.43–2.43) | 6.17 (0.28–8.55) | 0.035 | ||
| Baseline characteristics at biopsy | |||||
| Renal function | |||||
| Creatinine (umol/L) | 156 (112–247) | 144 (110–256) | 0.719 | ||
| eGFR (mL/min/1.73 m2, IQR) | 54.81 (29.77–80.20) | 60.04 (31.18–80.30) | 0.857 | ||
| Urine protein | - | ||||
| 0 (n, %) | 21(60.0%) | 7 (46.7%) | |||
| 1+ (n, %) | 8 (22.9%) | 7 (46.7%) | |||
| 2+ (n, %) | 4 (11.4%) | 1 (6.7%) | |||
| 3+ (n, %) | 2 (5.7%) | 0 (0.0%) | |||
| White blood cell (×109/L, IQR) | 6.51 (5.61–8.52) | 6.64 (4.99–7.86) | 0.735 | ||
| Neutrophils (×109/L, IQR) | 4.96 (3.25–7.41) | 4.20 (3.16–5.68) | 0.816 | ||
| Cause for biopsy | - | ||||
| Raised creatinine (n, %) | 17 (48.6%) | 8 (53.3%) | |||
| Proteinuria (n, %) | 8 (22.9%) | 3 (20.0%) | |||
| Hematuria (n, %) | 5 (14.3%) | 0 (0.0%) | |||
| PRA Positive (n, %) | 0 (0.0%) | 3 (20.0%) | |||
| Others (n, %) | 5 (14.3%) | 1 (6.7%) | |||
| CNI | 1.000 | ||||
| Tacrolimus (n, %) | 32 (91.4%) | 14 (93.3%) | |||
| Cyclosporin (n, %) | 3 (8.6%) | 1 (6.7%) | |||
| CNI trough concentration | |||||
| Tacrolimus (ng/mL, IQR) | 7.10 (6.05–8.35) | 5.80 (4.18–7.88) | 0.471 | ||
| Cyclosporin (ng/mL, IQR) | 85.80 (68.00–) | 49.30– | - | ||
| DSA positive (n, %) | 2 (5.7%) | 13 (86.7%) | 0.000 | ||
| Donor Characteristics | |||||
| Donor type | 1.000 | ||||
| Deceased-donor (n, %) | 28 (80.0%) | 12 (80.0%) | |||
| Living-donor (n, %) | 7 (20.0%) | 3 (20.0%) | |||
| All Recipients (n = 50) | |||
|---|---|---|---|
| Banff Histology | Frequency (n, %) | dd-cfDNA Fraction (%, Q1–Q3) | dd-cfDNA Absolute Quantification (copies/mL, Q1–Q3) |
| ABMR * | 14 (28.0%) | 2.10 (1.28–2.36) | 43.73 (31.02–79.40) |
| TCMR | 1 (2.0%) | 0.50– | 557.55– |
| Borderline TCMR | 6 (12.0%) | 0.33 (0.13–0.59) | 22.72 (8.85–33.47) |
| BKVN | 4 (8.0%) | 0.51 (0.34–1.10) | 29.56 (10.14–104.07) |
| IgAN/FSGS | 12 (24.0%) | 0.49 (0.40–0.95) | 14.42 (7.17–48.32) |
| Other no rejection | 13 (26.0%) | 0.37 (0.27–0.54) | 12.08 (6.10–32.00) |
| Cut-Off Value (%, Copy Number) | PPV | NPV | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| dd-cfDNA Fraction | 1.08 | 82.35% | 96.97% | 93.33% | 91.43% |
| dd-cfDNA copy number | 32 | 54.55% | 89.29% | 80.00% | 71.43% |
| Double-negative | 1.08,32 | 55.56% | 100.00% | 100.00% | 65.71% |
| Double-positive | 1.08, 32 | 91.67% | 89.47% | 73.33% | 97.14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, W.; Wang, Y.; Fu, Q.; Wu, C.; Deng, R.; Yu, X.; Ye, C.; Liu, X.; Xu, B.; Sun, P.; et al. Integrating Donor Derived Cell-Free DNA Fraction and Absolute Quantification for Enhanced Rejection Diagnosis in Kidney Transplant Recipients. Diagnostics 2025, 15, 237. https://doi.org/10.3390/diagnostics15030237
Nie W, Wang Y, Fu Q, Wu C, Deng R, Yu X, Ye C, Liu X, Xu B, Sun P, et al. Integrating Donor Derived Cell-Free DNA Fraction and Absolute Quantification for Enhanced Rejection Diagnosis in Kidney Transplant Recipients. Diagnostics. 2025; 15(3):237. https://doi.org/10.3390/diagnostics15030237
Chicago/Turabian StyleNie, Weijian, Yan Wang, Qian Fu, Chenglin Wu, Ronghai Deng, Xiaolin Yu, Caiguo Ye, Xiangjun Liu, Bowen Xu, Pingping Sun, and et al. 2025. "Integrating Donor Derived Cell-Free DNA Fraction and Absolute Quantification for Enhanced Rejection Diagnosis in Kidney Transplant Recipients" Diagnostics 15, no. 3: 237. https://doi.org/10.3390/diagnostics15030237
APA StyleNie, W., Wang, Y., Fu, Q., Wu, C., Deng, R., Yu, X., Ye, C., Liu, X., Xu, B., Sun, P., Liu, L., Li, J., Zhang, H., & Wang, C. (2025). Integrating Donor Derived Cell-Free DNA Fraction and Absolute Quantification for Enhanced Rejection Diagnosis in Kidney Transplant Recipients. Diagnostics, 15(3), 237. https://doi.org/10.3390/diagnostics15030237







