Prognostic Value of the PILE Score in Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Chemoradiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Treatment Protocol
2.3. Data Collection
2.4. Inflammatory Indexes and PILE Score
2.5. Outcome Definitions
2.6. Statistical Analysis
2.7. Language Editing Statement
3. Results
3.1. Patient Characteristics
3.2. ROC Analysis for Discriminatory Performance
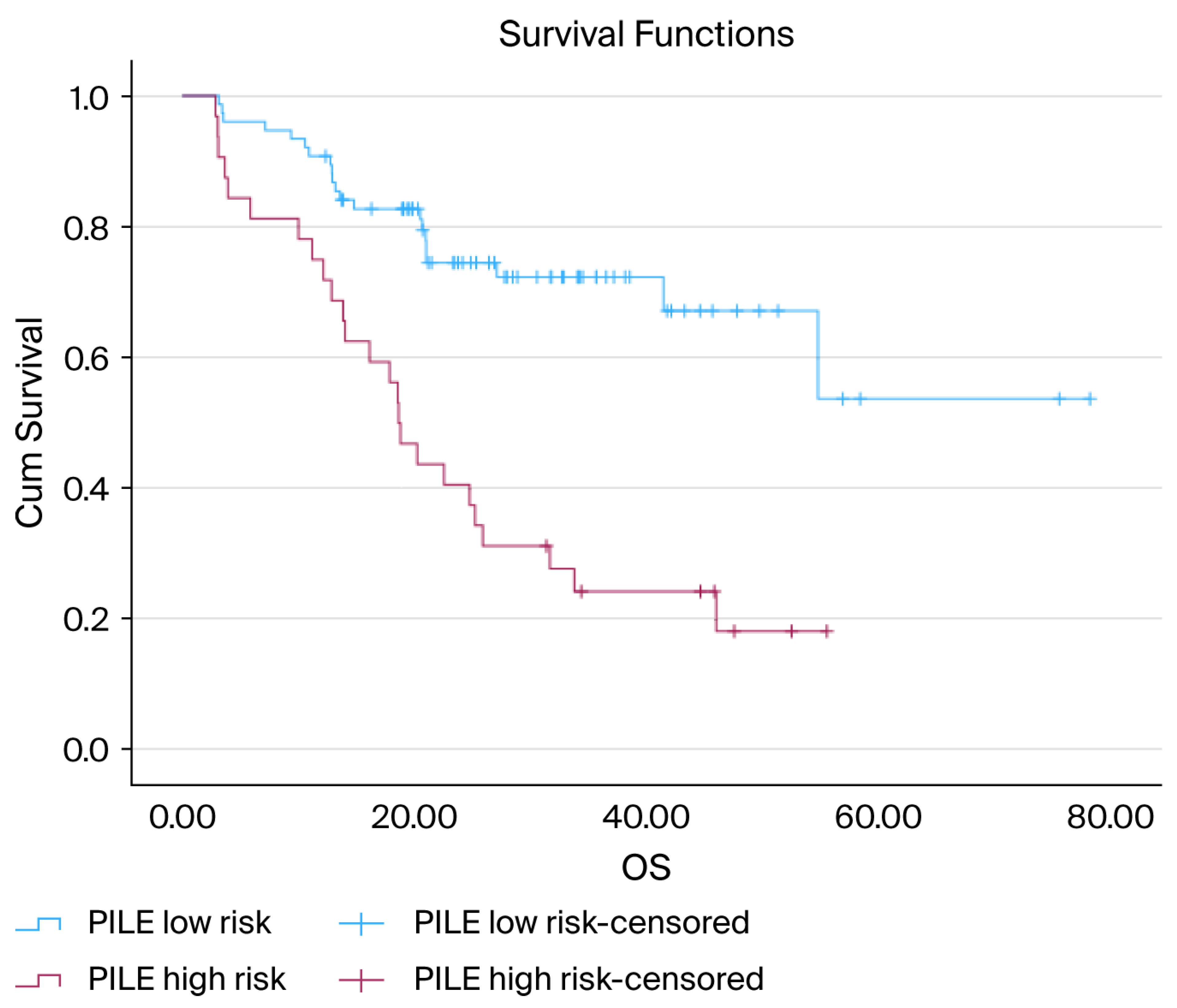
3.3. Overall Survival Analysis
3.4. Cox Regression Analysis for OS
Subgroup and Interaction Analysis for OS
3.5. Progression-Free Survival Analysis
3.6. Cox Regression Analysis for PFS
Subgroup and Interaction Analysis for PFS
3.7. Association Between PILE Score and pCR
3.8. Model Performance
4. Discussion
Clinical Implications and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| CAD | Coronary Artery Disease |
| C-index | Concordance Index |
| CALLY Index | CRP–Albumin–Lymphocyte Index |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| dCRT | Definitive Chemoradiotherapy |
| DM | Diabetes Mellitus |
| ECOG | Eastern Cooperative Oncology Group |
| ESCC | Esophageal Squamous Cell Carcinoma |
| GRIm Score | Gustave Roussy Immune Score |
| Gy | Gray |
| HR | Hazard Ratio |
| HT | Hypertension |
| LDH | Lactate Dehydrogenase |
| nCRT | Neoadjuvant Chemoradiotherapy |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| OS | Overall Survival |
| pCR | Pathological complete response |
| PFS | Progression-Free Survival |
| PILE | Composite inflammation–laboratory evaluation score derived from PIV, LDH, and ECOG |
| PIV | Pan-Immune-Inflammation Value |
| PLR | Platelet-to-Lymphocyte Ratio |
| R0 | Microscopically margin-negative resection |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| ULN | Upper Limit of Normal |
References
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from Globocan 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Mignozzi, S.; Malvezzi, M.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. Global Trends in Esophageal Cancer Mortality with Predictions to 2025, and in Incidence by Histotype. Cancer Epidemiol. 2023, 87, 102486. [Google Scholar] [CrossRef] [PubMed]
- Klingelhofer, D.; Zhu, Y.; Braun, M.; Bruggmann, D.; Schoffel, N.; Groneberg, D.A. A World Map of Esophagus Cancer Research: A Critical Accounting. J. Transl. Med. 2019, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xia, C.; Cao, M.; Yang, F.; Yan, X.; He, S.; Cao, M.; Zhang, S.; Li, Q.; Tan, N.; et al. Esophageal Cancer Global Burden Profiles, Trends, and Contributors. Cancer Biol. Med. 2024, 21, 656–666. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Zheng, Y.; Gao, Y.; He, S.; Li, H.; Zou, K.; Li, N.; Tian, J.; Chen, W.; et al. Esophageal Cancer: Epidemiology, Risk Factors and Screening. Chin. J. Cancer Res. 2021, 33, 535–547. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Xiu, Y.; Zhang, H.; Zhang, Y.; Ying, W. Burden of Esophageal Cancer in Global, Regional and National Regions from 1990 to 2021 and Its Projection until 2050: Results from the Gbd Study 2021. Front. Oncol. 2025, 14, 1518567. [Google Scholar] [CrossRef]
- Christodoulidis, G.; Agko, S.E.; Koumarelas, K.E.; Kouliou, M.N.; Zacharoulis, D. Advancements and Challenges in the Treatment of Esophageal Cancer: A Comprehensive Review. World J. Clin. Oncol. 2024, 15, 1463–1467. [Google Scholar] [CrossRef]
- Hou, S.; Pan, Z.; Hao, X.; Hang, Q.; Ding, Y. Recent Progress in the Neoadjuvant Treatment Strategy for Locally Advanced Esophageal Cancer. Cancers 2021, 13, 5162. [Google Scholar] [CrossRef]
- Lorenz, E.; Weitz, A.; Reinstaller, T.; Hass, P.; Croner, R.S.; Benedix, F. Neoadjuvant Radiochemotherapy with Cisplatin/5-Flourouracil or Carboplatin/Paclitaxel in Patients with Resectable Cancer of the Esophagus and the Gastroesophageal Junction—Comparison of Postoperative Mortality and Complications, Toxicity, and Pathological Tumor Response. Langenbecks Arch. Surg. 2023, 408, 429. [Google Scholar]
- Logarajah, S.; Jeyarajah, P.; Darwish, M.; Moslim, M.; Jureller, M.; Osman, H.; Jeyarajah, D.R. Does Chemotherapy Regimen Matter in the Neoadjuvant Treatment of Esophageal Cancer? J. Gastrointest. Oncol. 2022, 13, 2713–2720. [Google Scholar] [CrossRef]
- Munch, S.; Pigorsch, S.U.; Feith, M.; Slotta-Huspenina, J.; Weichert, W.; Friess, H.; Combs, S.E.; Habermehl, D. Comparison of Neoadjuvant Chemoradiation with Carboplatin/Paclitaxel or Cisplatin/5-Fluoruracil in Patients with Squamous Cell Carcinoma of the Esophagus. Radiat. Oncol. 2017, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- van Meerten, E.; Muller, K.; Tilanus, H.W.; Siersema, P.D.; Eijkenboom, W.M.; van Dekken, H.; Tran, T.C.; van der Gaast, A. Neoadjuvant Concurrent Chemoradiation with Weekly Paclitaxel and Carboplatin for Patients with Oesophageal Cancer: A Phase Ii Study. Br. J. Cancer 2006, 94, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Lee, C.T.; Tsai, Y.N.; Tseng, C.M.; Chen, T.H.; Hsu, M.H.; Wang, C.C.; Wang, W.L. Prognostic Significance of Systemic Inflammatory Response Markers in Patients with Superficial Esophageal Squamous Cell Carcinomas. Sci. Rep. 2022, 12, 18241. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, T.; Dai, Y.; Li, J. Preoperative Systemic Inflammation Score (Sis) Is Superior to Neutrophil to Lymphocyte Ratio (Nlr) as a Predicting Indicator in Patients with Esophageal Squamous Cell Carcinoma. BMC Cancer 2019, 19, 721. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, W.; Cai, S.; Zhang, F.; Shao, F.; Zhang, G.; Liu, T.; Tan, F.; Li, N.; Xue, Q.; et al. Systemic Immune-Inflammation Index (Sii) Is Useful to Predict Survival Outcomes in Patients with Surgically Resected Esophageal Squamous Cell Carcinoma. J. Cancer 2019, 10, 3188–3196. [Google Scholar] [CrossRef]
- Xu, X.; Jing, J. Inflammation-Related Parameter Serve as Prognostic Biomarker in Esophageal Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 900305. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Yang, X.; Chen, Q.; Cheng, X. Pretreatment Pan-Immune-Inflammation Value (Piv) in Predicting Therapeutic Response and Clinical Outcomes of Neoadjuvant Immunochemotherapy for Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2024, 31, 272–283. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, L.; Che, G. Could Pretreatment Pan-Immune-Inflammation Value Predict Survival in Esophageal Cancer? Ann. Surg. Oncol. 2024, 31, 3868–3869. [Google Scholar] [CrossRef]
- Zeng, R.; Liu, F.; Fang, C.; Yang, J.; Luo, L.; Yue, P.; Gao, B.; Dong, Y.; Xiang, Y. Piv and Pile Score at Baseline Predict Clinical Outcome of Anti-Pd-1/Pd-L1 Inhibitor Combined with Chemotherapy in Extensive-Stage Small Cell Lung Cancer Patients. Front. Immunol. 2021, 12, 724443. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Zhao, E.; Li, B.; Li, S.; Dong, X.; Yuan, L.; Yang, H. Prognostic Value of Lactate Dehydrogenase in Second-Line Immunotherapy for Advanced Esophageal Squamous Cell Carcinoma. Pathol. Oncol. Res. 2022, 28, 1610245. [Google Scholar] [CrossRef]
- Liu, C.; Han, J.; Han, D.; Huang, W.; Li, B. A New Risk Score Model Based on Lactate Dehydrogenase for Predicting Prognosis in Esophageal Squamous Cell Carcinoma Treated with Chemoradiotherapy. J. Thorac. Dis. 2023, 15, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Chen, Z.; Gao, Y.; He, J. Immunohistochemical Prognostic Markers of Esophageal Squamous Cell Carcinoma: A Systematic Review. Chin. J. Cancer 2017, 36, 65. [Google Scholar] [CrossRef] [PubMed]
- Karadag, I.; Karakaya, S.; Yilmaz, M.E.; Oksuzoglu, O.B.C. The Potential Prognostic Novel Markers Piv and Pile Score to Predict Survival Outcomes at Hepatocellular Cancer. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7679–7686. [Google Scholar] [PubMed]
- Duan, L.; Li, W.; Chen, F. The Candidate Novel Markers Piv and Pile Score to Predict Survival Outcomes and Therapeutic Response in Patients with Primary Central Nervous System Lymphoma. J. Clin. Oncol. 2024, 42, 24. [Google Scholar] [CrossRef]
- Tseng, R.H.; Lai, K.M.; Tsai, C.Y.; Yan, S.L. Elevated Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio after First Cycle of Chemotherapy and Better Survival in Esophageal Cancer Patients Receiving Concurrent Chemoradiotherapy. Curr. Oncol. 2022, 29, 8825–8834. [Google Scholar] [CrossRef]
- Da, L.; Qu, Z.; Zhang, C.; Shen, Y.; Huang, W.; Zhang, Y.; Gu, K. Prognostic Value of Inflammatory Markers and Clinical Features for Survival in Advanced or Metastatic Esophageal Squamous Cell Carcinoma Patients Receiving Anti-Programmed Death 1 Treatment. Front. Oncol. 2023, 13, 1144875. [Google Scholar] [CrossRef]
- Feng, J.F.; Wang, L.; Yang, X.; Chen, S. Gustave Roussy Immune Score (Grim-Score) Is a Prognostic Marker in Patients with Resectable Esophageal Squamous Cell Carcinoma. J. Cancer 2020, 11, 1334–1340. [Google Scholar] [CrossRef]
- Wu, B.; Liu, J.; Shao, C.; Yu, D.; Liao, J. Integrating Inflammation, Nutrition, and Immunity: The Cally Index as a Prognostic Tool in Digestive System Cancers—A Systematic Review and Meta-Analysis. BMC Cancer 2025, 25, 672. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef]
- Di Ceglie, I.; Carnevale, S.; Rigatelli, A.; Grieco, G.; Molisso, P.; Jaillon, S. Immune Cell Networking in Solid Tumors: Focus on Macrophages and Neutrophils. Front. Immunol. 2024, 15, 1341390. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Bao, Y.; Pang, L.; Yang, C. Neutrophils in Cancer: From Immune Defense to Tumor Promotion. Cancer Biol. Med. 2025, 22, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in Cancer Carcinogenesis and Metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, A.; Zeller, J.; Peter, K.; McFadyen, J.D. Decoding the Role of Platelets in Tumour Metastasis: Enigmatic Accomplices and Intricate Targets for Anticancer Treatments. Front. Immunol. 2023, 14, 1256129. [Google Scholar] [CrossRef]
- Garcia-Leon, M.J.; Liboni, C.; Mittelheisser, V.; Bochler, L.; Follain, G.; Mouriaux, C.; Busnelli, I.; Larnicol, A.; Colin, F.; Peralta, M.; et al. Platelets Favor the Outgrowth of Established Metastases. Nat. Commun. 2024, 15, 3297. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, J.; Bao, S.; Zhang, B.; Li, X.; Wang, H.; Cheng, Y.; Zhang, H.; Zu, L.; Xu, X.; et al. Cancer Progression and Tumor Hypercoagulability: A Platelet Perspective. J. Thromb. Thrombolysis 2024, 57, 959–972. [Google Scholar] [CrossRef]
- Obermann, W.M.J.; Brockhaus, K.; Eble, J.A. Platelets, Constant and Cooperative Companions of Sessile and Disseminating Tumor Cells, Crucially Contribute to the Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 674553. [Google Scholar] [CrossRef]
- Schmied, L.; Hoglund, P.; Meinke, S. Platelet-Mediated Protection of Cancer Cells from Immune Surveillance—Possible Implications for Cancer Immunotherapy. Front. Immunol. 2021, 12, 640578. [Google Scholar] [CrossRef]
- Dovizio, M.; Ballerini, P.; Fullone, R.; Tacconelli, S.; Contursi, A.; Patrignani, P. Multifaceted Functions of Platelets in Cancer: From Tumorigenesis to Liquid Biopsy Tool and Drug Delivery System. Int. J. Mol. Sci. 2020, 21, 9585. [Google Scholar] [CrossRef]
- Cai, C.; Liu, Y.; Lu, R.; Fan, X.; Zeng, S.; Gan, P. Platelets in Cancer and Immunotherapy: Functional Dynamics and Therapeutic Opportunities. Exp. Hematol. Oncol. 2025, 14, 83. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Layeghi, S.M.; Falakian, Z.; Golestani, S.; Kobravi, S.; Talebi, S.; Yousefi, M. An Update to Experimental and Clinical Aspects of Tumor-Associated Macrophages in Cancer Development: Hopes and Pitfalls. Clin. Exp. Med. 2024, 24, 156. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, A.; Yarizadeh, M.; Masjedi, M.N.-K.; Fatehnejad, M.; Jahandideh, R.; Soheili, R.; Eslami, Y.; Zokaei, M.; Ahmadvand, A.; Ghalamkarpour, N.; et al. Macrophage’s Role in Solid Tumors: Two Edges of a Sword. Cancer Cell Int. 2023, 23, 150. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Xia, H.; Chen, Y.H. Monocytes in Tumorigenesis and Tumor Immunotherapy. Cells 2023, 12, 1673. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Nasrollahzadeh, E.; Razi, S.; Keshavarz-Fathi, M.; Mazzone, M.; Rezaei, N. Pro-Tumorigenic Functions of Macrophages at the Primary, Invasive and Metastatic Tumor Site. Cancer Immunol. Immunother. 2020, 69, 1673–1697. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Serafini, P.; Bharadwaj, S.N.; Kortylewski, M. Editorial: Roles of Tumor-Recruited Myeloid Cells in Immune Evasion in Cancer. Front. Immunol. 2021, 12, 749605. [Google Scholar] [CrossRef]
- Jain, A.; Bobdey, S.; Sathwara, J.; Ganesh, B.; Saoba, S.; Khan, A. Role of Monocyte and Lymphocyte Counts in Prognosis of Cervical Cancer. Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 2243–2249. [Google Scholar] [CrossRef]
- Soyfer, V.; Lugovoy, E.; Nikolaevski-Berlin, A.; Korzets, Y.; Schlocker, A.; Gutfeld, O.; Ospovat, I.; Amit, U.; Rabin, T.; Natan-Oz, Y.F.; et al. The Effect of Long-Standing Lymphopenia after Radiation Therapy on Survival in Rectal Cancer. Surg. Oncol. 2024, 56, 102119. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.R.; O’Sullivan, H.; Collins, D.C.; Bambury, R.M.; Power, D.; Grossman, S.; O’Reilly, S. Exploring the Prognostic Impact of Absolute Lymphocyte Count in Patients Treated with Immune-Checkpoint Inhibitors. BJC Rep. 2024, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Palleschi, M.; Schepisi, G.; Casadei, C.; Bleve, S.; Merloni, F.; Sirico, M.; Sarti, S.; Cecconetto, L.; Di Menna, G.; et al. Circulating Inflammatory Cells in Patients with Metastatic Breast Cancer: Implications for Treatment. Front. Oncol. 2022, 12, 882896. [Google Scholar] [CrossRef]
- Claps, G.; Faouzi, S.; Quidville, V.; Chehade, F.; Shen, S.; Vagner, S.; Robert, C. The Multiple Roles of Ldh in Cancer. Nat. Rev. Clin. Oncol. 2022, 19, 749–762. [Google Scholar] [CrossRef]
- Shang, S.; Wang, M.Z.; Xing, Z.; He, N.; Li, S. Lactate Regulators Contribute to Tumor Microenvironment and Predict Prognosis in Lung Adenocarcinoma. Front. Immunol. 2022, 13, 1024925. [Google Scholar] [CrossRef]
- Jang, R.W.; Caraiscos, V.B.; Swami, N.; Banerjee, S.; Mak, E.; Kaya, E.; Rodin, G.; Bryson, J.; Ridley, J.Z.; Le, L.W.; et al. Simple Prognostic Model for Patients with Advanced Cancer Based on Performance Status. J. Oncol. Pr. 2014, 10, e335–e341. [Google Scholar] [CrossRef]
- Simcock, R.; Wright, J. Beyond Performance Status. Clin. Oncol. R. Coll. Radiol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Hess, L.M.; Smith, D.; Cui, Z.L.; Montejano, L.; Liepa, A.M.; Schelman, W.; Bowman, L. The Relationship between Eastern Cooperative Oncology Group Performance Status and Healthcare Resource Utilization among Patients with Advanced or Metastatic Colorectal, Lung or Gastric Cancer. J. Drug Assess. 2020, 10, 10–17. [Google Scholar] [CrossRef]
- Kumar, D.; Neeman, E.; Zhu, S.; Sun, H.; Kotak, D.; Liu, R. Revisiting the Association of Ecog Performance Status with Clinical Outcomes in Diverse Patients with Cancer. J. Natl. Compr. Cancer Netw. 2024, 22, e237111. [Google Scholar] [CrossRef]
- Sorensen, J.B.; Klee, M.; Palshof, T.; Hansen, H.H. Performance Status Assessment in Cancer Patients. An Inter-Observer Variability Study. Br. J. Cancer 1993, 67, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yue, J.; Wang, S.; Zhao, F.; Zhang, W.; Qie, S.; Jiang, H. Prognostic Role of the Pathological Status Following Neoadjuvant Chemoradiotherapy and Surgery in Esophageal Squamous Cell Carcinoma. BMC Cancer 2025, 25, 61. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Tan, L.; Shen, Y.; Wang, H.; Lin, M.; Feng, M.; Xu, S.; Guo, W.; Qian, C.; Liu, T.; et al. Cmisg1701: A Multicenter Prospective Randomized Phase Iii Clinical Trial Comparing Neoadjuvant Chemoradiotherapy to Neoadjuvant Chemotherapy Followed by Minimally Invasive Esophagectomy in Patients with Locally Advanced Resectable Esophageal Squamous Cell Carcinoma (Ct3–4aN0–1M0) (Nct03001596). BMC Cancer 2017, 17, 450. [Google Scholar]
- Chen, C.Y.; Li, C.C.; Chien, C.R. Neoadjuvant Vs Definitive Concurrent Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma Patients. World J. Surg. Oncol. 2018, 16, 141. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Long-Term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The Neocrtec5010 Randomized Clinical Trial. JAMA Surg. 2021, 156, 721–729. [Google Scholar]
- Eyck, B.M.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van der Wilk, B.J.; Shapiro, J.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled Cross Trial. J. Clin. Oncol. 2021, 39, 1995–2004. [Google Scholar] [CrossRef]
- Toyokawa, T.; Tamura, T.; Sakurai, K.; Kubo, N.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Ohira, M. Postoperative Inflammation Is an Independent Prognostic Factor in Patients with Thoracic Esophageal Squamous Cell Carcinoma. Anticancer. Res. 2019, 39, 2777–2784. [Google Scholar] [CrossRef]
- Booka, E.; Kikuchi, H.; Hiramatsu, Y.; Takeuchi, H. The Impact of Infectious Complications after Esophagectomy for Esophageal Cancer on Cancer Prognosis and Treatment Strategy. J. Clin. Med. 2021, 10, 4614. [Google Scholar] [CrossRef]
- Aoyama, T.; Ju, M.; Komori, K.; Tamagawa, H.; Tamagawa, A.; Maezawa, Y.; Hashimoto, I.; Kano, K.; Hara, K.; Cho, H.; et al. The Systemic Inflammation Score Is an Independent Prognostic Factor for Esophageal Cancer Patients Who Receive Curative Treatment. Anticancer. Res. 2022, 42, 2711–2717. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Lei, M.; He, H.; Zhang, D.; Lin, J.; Lin, H.; Wei, W.; Chen, P.; Zhuang, F.; et al. Comparison of Neoadjuvant Chemoimmunotherapy and Neoadjuvant Chemotherapy for Resectable Esophageal Squamous Cell Carcinoma: A Retrospective Study with 3-Year Survival Analysis. J. Cancer Res. Clin. Oncol. 2024, 150, 477. [Google Scholar] [CrossRef]
- Li, K.; Hao, W.; Liu, X.; Li, Y.; Sun, H.; Liu, S.; Xing, W.; Zheng, Y. The Role of Adjuvant Chemotherapy in the Treatment of Esophageal Squamous Cell Carcinoma after Neoadjuvant Chemotherapy. J. Cancer 2023, 14, 3130–3138. [Google Scholar] [CrossRef]
- Fan, L.; Yang, Z.; Chang, M.; Chen, Z.; Wen, Q. Ct-Based Delta-Radiomics Nomogram to Predict Pathological Complete Response after Neoadjuvant Chemoradiotherapy in Esophageal Squamous Cell Carcinoma Patients. J. Transl. Med. 2024, 22, 579. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Zhou, J.; Li, X.; Lian, S.; Yang, Y. Comprehensive Risk Score of the E-Pass Scoring System Serves a Prognostic Indicator for Patients after Neoadjuvant Therapy and Curative Esophageal Cancer Surgery: A Multicenter Retrospective Study. Front. Oncol. 2025, 15, 1617683. [Google Scholar] [CrossRef]
- Li, C.; Lin, J.W.; Yeh, H.L.; Chuang, C.Y.; Chen, C.C. Good Prediction of Treatment Responses to Neoadjuvant Chemoradiotherapy for Esophageal Cancer Based on Preoperative Inflammatory Status and Tumor Glucose Metabolism. Sci. Rep. 2021, 11, 11626. [Google Scholar] [CrossRef]


| Variable | n (%)/Mean ± SD |
|---|---|
| Age (years), mean ± SD | 62.30 ± 10.84 |
| Sex | |
| Male | 39 (36.1%) |
| Female | 69 (63.9%) |
| Smoking status | |
| Non-smoker | 72 (66.7%) |
| Active smoker | 32 (29.6%) |
| Former smoker | 4 (3.7%) |
| ECOG performance status | |
| 0 | 28 (25.9%) |
| 1 | 58 (53.7%) |
| 2 | 22 (20.4%) |
| Comorbidity | |
| Absent | 57 (52.8%) |
| Present | 51 (47.2%) |
| Comorbidity types | |
| None | 57 (52.8%) |
| DM | 10 (9.3%) |
| HT | 22 (20.4%) |
| CAD | 11 (10.2%) |
| COPD | 3 (2.8%) |
| Other | 5 (4.6%) |
| Pathological type | |
| Squamous cell carcinoma | 108 (100%) |
| Tumor location | |
| Middle third | 27 (25.0%) |
| Lower third | 81 (75.0%) |
| Clinical T stage | |
| T2 | 40 (37.0%) |
| T3 | 68 (63.0%) |
| Clinical N stage | |
| N0 | 26 (24.1%) |
| N1 | 35 (32.4%) |
| N2 | 35 (32.4%) |
| N3 | 12 (11.1%) |
| Clinical stage | |
| Stage II | 40 (37.0%) |
| Stage III | 56 (51.9%) |
| Stage IVA | 12 (11.1%) |
| Surgery performed | 34 (31.5%) |
| pCR | 25 (23%) |
| Progression | 43 (39.8%) |
| Distant metastasis | 14 (13.0%) |
| Lung | 7 (50.0%) |
| Liver | 3 (21.4%) |
| Other | 4 (28.6%) |
| Status | |
| Alive | 62 (57.4%) |
| Deceased | 46 (42.6%) |
| Laboratory parameters (mean ± SD) | |
| LDH (U/L) | 216.83 ± 51.55 |
| Lymphocyte count (×109/L) | 2.176 ± 0.680 |
| Neutrophil count (×109/L) | 5.348 ± 2.013 |
| Monocyte count (×109/L) | 0.612 ± 0.195 |
| Platelet count (×109/L) | 296.42 ± 101.13 |
| PIV | 550.05 ± 577.63 |
| NLR | 2.75 ± 1.68 |
| PLR | 148.4 ± 66.9 |
| OS (months) | 26.34 ± 15.32 |
| PFS (months) | 22.01 ± 15.39 |
| PILE score | |
| 0 | 34 (31.5%) |
| 1 | 42 (38.9%) |
| 2 | 26 (24.1%) |
| 3 | 6 (5.6%) |
| PILE risk classification | |
| Low (0–1) | 76 (70.4%) |
| High (2–3) | 32 (29.6%) |
| Variable | Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value |
|---|---|---|---|---|
| Age (years) | 1.032 (1.005–1.060) | 0.021 | 1.018 (0.984–1.053) | 0.312 |
| Sex | 0.748 (0.413–1.354) | 0.338 | 0.696 (0.366–1.323) | 0.269 |
| Comorbidity | 1.242 (0.695–2.219) | 0.464 | 0.834 (0.406–1.715) | 0.623 |
| Clinical stage | — | 0.111 | — | 0.514 |
| Stage III vs. II | 1.186 (0.608–2.313) | 0.616 | 1.235 (0.608–2.507) | 0.559 |
| Stage IVA vs. II | 2.377 (1.024–5.518) | 0.044 | 1.706 (0.689–4.227) | 0.249 |
| Surgery | 0.181 (0.071–0.460) | <0.001 | 0.249 (0.090–0.683) | 0.007 |
| PILE risk (High vs. Low) | 3.531 (1.974–6.318) | <0.001 | 2.527 (1.380–4.629) | 0.003 |
| Variable | Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value |
|---|---|---|---|---|
| Age (years) | 1.030 (1.002–1.060) | 0.035 | 0.986 (0.952–1.022) | 0.447 |
| Sex | 1.366 (0.701–2.663) | 0.359 | 1.343 (0.649–2.778) | 0.427 |
| Comorbidity | 1.938 (1.049–3.579) | 0.034 | 1.615 (0.780–3.345) | 0.197 |
| Clinical stage | — | 0.312 | — | 0.439 |
| Stage III vs. II | 1.162 (0.594–2.272) | 0.661 | 1.596 (0.780–3.262) | 0.2 |
| Stage IVA vs. II | 1.989 (0.801–4.938) | 0.138 | 1.285 (0.475–3.473) | 0.621 |
| Surgery | 0.116 (0.041–0.328) | <0.001 | 0.131 (0.044–0.394) | <0.001 |
| PILE risk (High vs. Low) | 3.561 (1.936–6.551) | <0.001 | 2.932 (1.525–5.639) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turhan, A.; Büyükbayram, M.E.; Hannarici, Z.; Çağlar, A.A.; Bilici, M.; Tekin, S.B. Prognostic Value of the PILE Score in Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Chemoradiotherapy. Diagnostics 2025, 15, 3158. https://doi.org/10.3390/diagnostics15243158
Turhan A, Büyükbayram ME, Hannarici Z, Çağlar AA, Bilici M, Tekin SB. Prognostic Value of the PILE Score in Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Chemoradiotherapy. Diagnostics. 2025; 15(24):3158. https://doi.org/10.3390/diagnostics15243158
Chicago/Turabian StyleTurhan, Aykut, Mehmet Emin Büyükbayram, Zekeriya Hannarici, Alperen Akansel Çağlar, Mehmet Bilici, and Salim Başol Tekin. 2025. "Prognostic Value of the PILE Score in Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Chemoradiotherapy" Diagnostics 15, no. 24: 3158. https://doi.org/10.3390/diagnostics15243158
APA StyleTurhan, A., Büyükbayram, M. E., Hannarici, Z., Çağlar, A. A., Bilici, M., & Tekin, S. B. (2025). Prognostic Value of the PILE Score in Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Chemoradiotherapy. Diagnostics, 15(24), 3158. https://doi.org/10.3390/diagnostics15243158





