A Particular Case of Myocardial Injury
Abstract
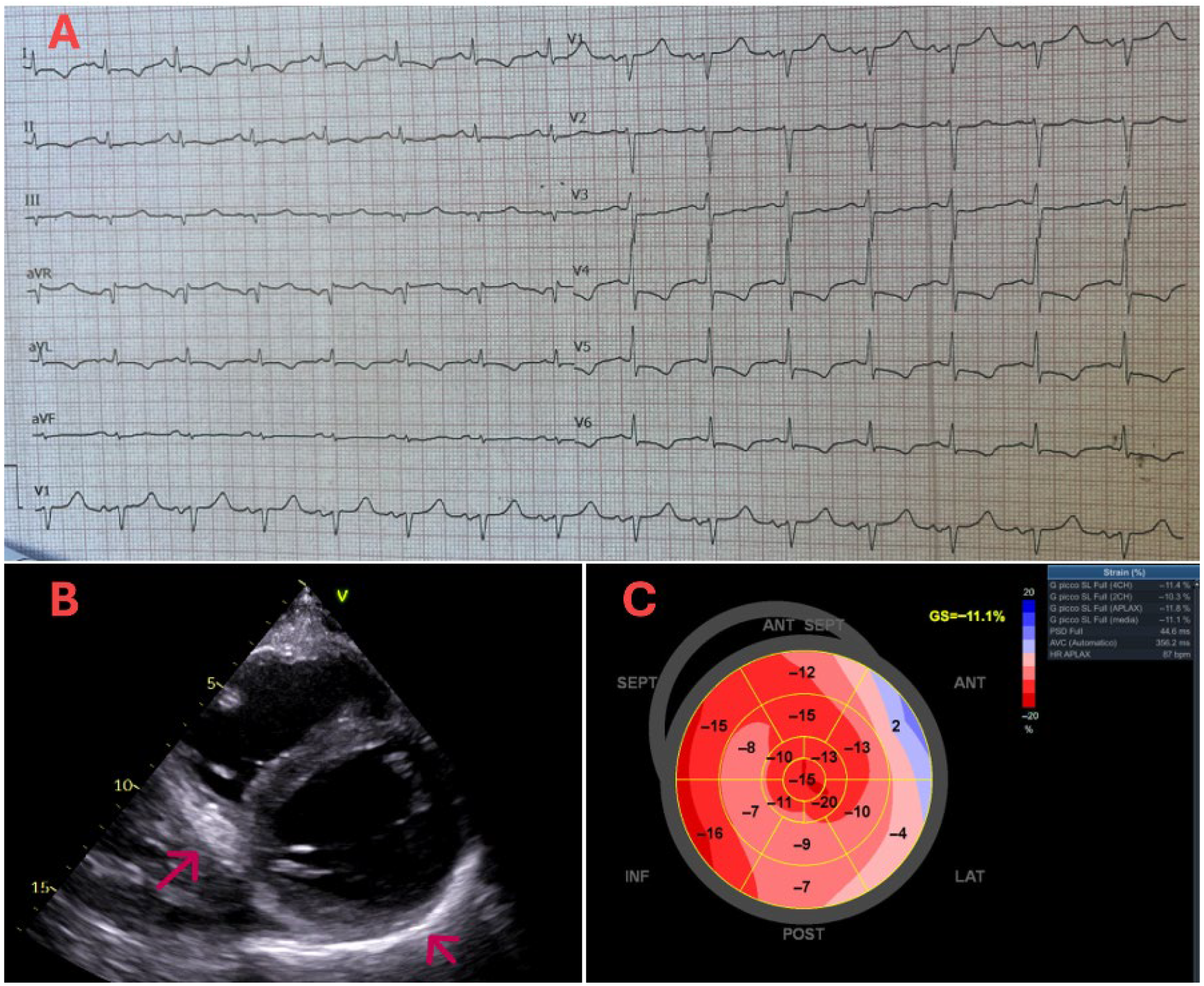
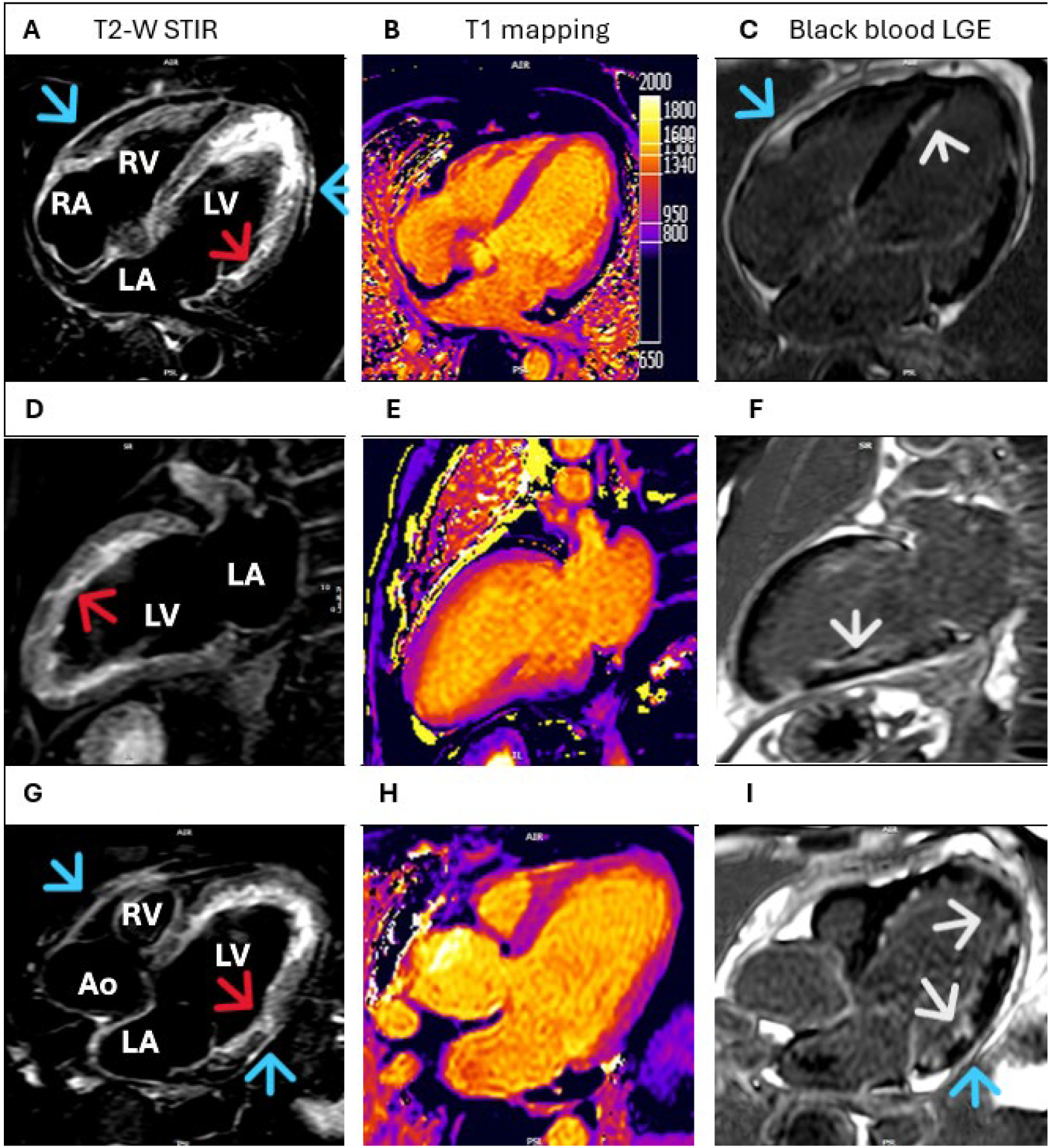
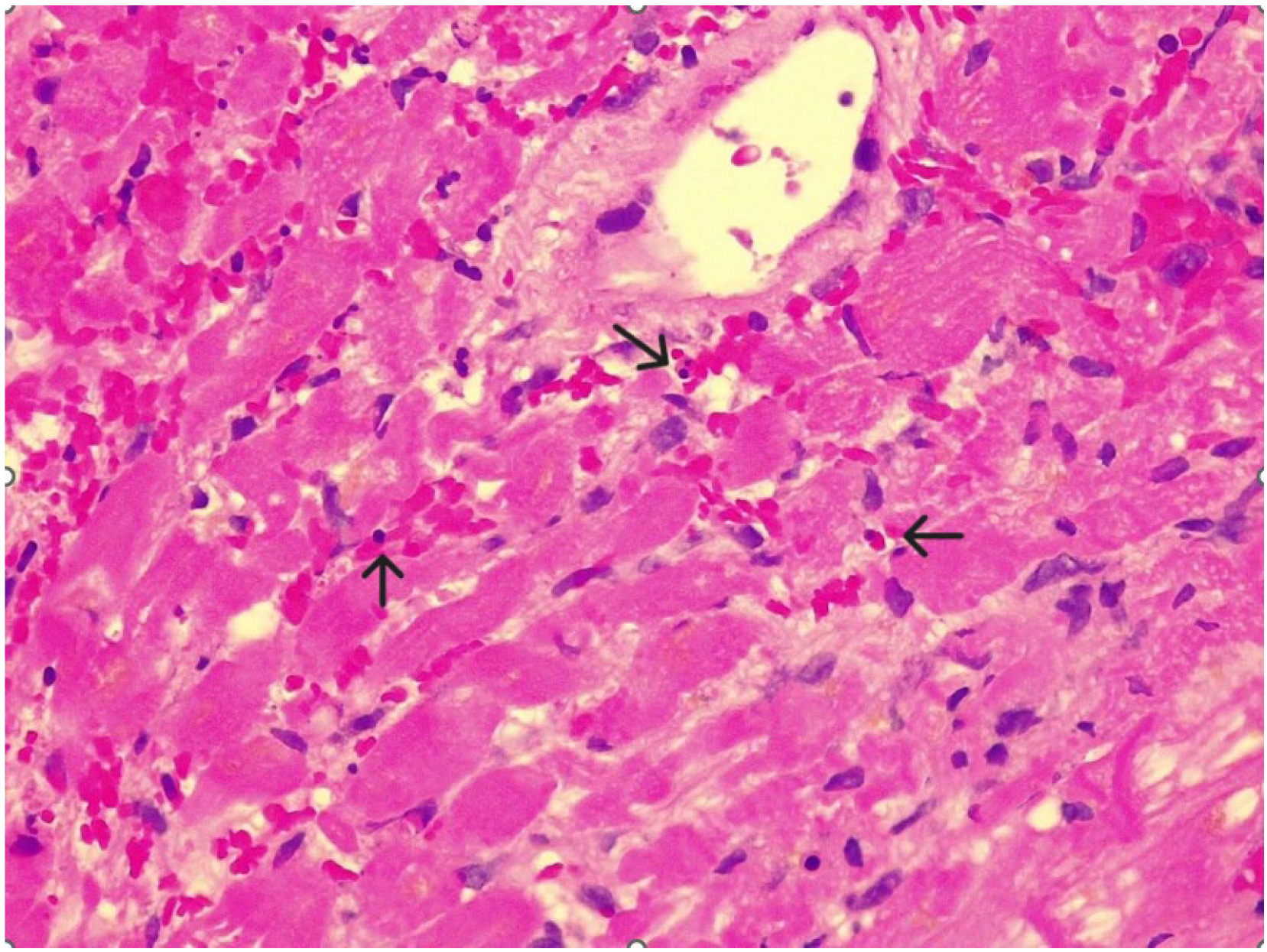
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caminati, M.; Olivieri, B.; Dama, A.; Micheletto, C.; Paggiaro, P.; Pinter, P.; Senna, G.; Schiappoli, M. Dupilumab-induced hypereosinophilia: Review of the literature and algorithm proposal for clinical management. Expert. Rev. Respir. Med. 2022, 16, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Collini, V.; Gröschel, J.; Adler, Y.; Brucato, A.; Christian, V.; Ferreira, V.M.; Gandjbakhch, E.; Heidecker, B.; Kerneis, M.; et al. ESC Scientific Document Group. 2025 ESC Guidelines for the management of myocarditis and pericarditis. Eur. Heart J. 2025, 29, ehaf192. [Google Scholar]
- Pöyhönen, P.; Rågback, J.; Mäyränpää, M.I.; Nordenswan, H.-K.; Lehtonen, J.; Shenoy, C.; Kupari, M. Cardiac magnetic resonance in histologically proven eosinophilic myocarditis. J. Cardiovasc. Magn. Reson. 2023, 25, 79. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catapano, D.; Ascrizzi, A.; Falco, L.; Dellegrottaglie, S.; Scatteia, A.; Loffredo, F.S.; Pezzullo, E.; Gravino, R.; Golino, P.; di Lorenzo, E.; et al. A Particular Case of Myocardial Injury. Diagnostics 2025, 15, 3136. https://doi.org/10.3390/diagnostics15243136
Catapano D, Ascrizzi A, Falco L, Dellegrottaglie S, Scatteia A, Loffredo FS, Pezzullo E, Gravino R, Golino P, di Lorenzo E, et al. A Particular Case of Myocardial Injury. Diagnostics. 2025; 15(24):3136. https://doi.org/10.3390/diagnostics15243136
Chicago/Turabian StyleCatapano, Dario, Antonia Ascrizzi, Luigi Falco, Santo Dellegrottaglie, Alessandra Scatteia, Francesco Saverio Loffredo, Enrica Pezzullo, Rita Gravino, Paolo Golino, Emilio di Lorenzo, and et al. 2025. "A Particular Case of Myocardial Injury" Diagnostics 15, no. 24: 3136. https://doi.org/10.3390/diagnostics15243136
APA StyleCatapano, D., Ascrizzi, A., Falco, L., Dellegrottaglie, S., Scatteia, A., Loffredo, F. S., Pezzullo, E., Gravino, R., Golino, P., di Lorenzo, E., & Masarone, D. (2025). A Particular Case of Myocardial Injury. Diagnostics, 15(24), 3136. https://doi.org/10.3390/diagnostics15243136







