Post-Surgical Reassessment of Breast Cancer IHC: Concordance, Δ-Metrics, and Treatment-Relevant Reclassification
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Clinical and Pathological Data
2.3. Biomarker Assessment
2.4. Treatment Data
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Comorbidities
3.2. IHC Marker Changes Pre-Surgery vs. Post-Surgery (ER, PR, HER2)
3.2.1. Changes in Hormone Receptor Expression (ER and PR)
3.2.2. Correlation Between Hormonal Markers (ER and PR)
3.2.3. Correlation Between Pre-Surgery and Post-Surgery HER2
3.2.4. Correlation Between Pre-Surgery and Post-Surgery Ki-67
3.2.5. Clinically Actionable Reclassification (Pre-Specified)
3.3. IHC Analysis with Immunophenotyping
3.3.1. Correlations Between Immunophenotyping and IHC Markers
3.3.2. Significant Differences Between Immunophenotyping and IHC Markers
3.4. Analysis of Relationships and Statistical Differences Between Oncologic Treatments and IHC Markers
3.4.1. Correlations Between Treatments and IHC Markers
3.4.2. Statistically Significant Differences Between Treatments and IHC Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HER2 | Human Epidermal growth factor Receptor 2 |
| CEP17 | Chromosome Enumeration Probe 17 |
| ISH | In Situ Hybridization |
| FISH | Fluorescence In Situ Hybridization |
| ASCO/CAP | American Society of Clinical Oncology/College of American Pathologists |
| PR | Progesterone Receptor |
| ER | Estrogen Receptor |
| IHC | Immunohistochemistry |
| TNBC | Triple-Negative Breast Cancer |
| NACT | Neoadjuvant Chemotherapy |
| OR | Odds Ratio |
| CI | Confidence Interval |
| SD | Standard Deviation |
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Mihai, A.-M.; Ianculescu, L.; Cretoiu, D.; Suciu, N. Breast Cancer Screening in Romania: Challenges and Opportunities for Early Detection. Acta Endocrinol. 2024, 20, 45–50. [Google Scholar] [CrossRef]
- Jaime Dos Santos, B.; Balabram, D.; Mara Reis Gomes, V.; Costa Café de Castro, C.; Henrique Costa Diniz, P.; Araújo Buzelin, M.; Buzelin Nunes, C. Changes in Invasive Breast Carcinomas after Neoadjuvant Chemotherapy Can Influence Adjuvant Therapeutic Decisions. Cancer Res. Treat. 2024, 56, 178–190. [Google Scholar] [CrossRef]
- Tu, T.A.; Tin, N.V.; Rhodes, A.; Anh, D.B.Q.; Dao, L.T.H.; Linh, N.T.T.; Nhu, D.T.K.; Nhung, N.T.H.; Cam, L.T.; Hanh, N.T.M.; et al. Reproducibility of Immunohistochemical Testing of Estrogen Receptors, Progesterone Receptors, Human Epidermal Growth Factor Receptor-2 (HER2) and Ki-67 in Vietnam. Br. J. Biomed. Sci. 2025, 82, 15455. [Google Scholar] [CrossRef]
- Louis, D.M.; Nair, L.M.; Vallonthaiel, A.G.; Narmadha, M.P.; Vijaykumar, D.K. Ki 67: A Promising Prognostic Marker in Early Breast Cancer-a Review Article. Indian J. Surg. Oncol. 2023, 14, 122–127. [Google Scholar] [CrossRef]
- Yüksel, C.; Aksel, B.; Doğan, L. Luminal A Breast Cancer: How Feasible is Omitting Axillary Dissection Without Neoadjuvant Therapy. Breast J. 2022, 2022, 8284814. [Google Scholar] [CrossRef] [PubMed]
- Moura, T.; Caramelo, O.; Silva, I.; Silva, S.; Gonçalo, M.; Portilha, M.A.; Moreira, J.N.; Gil, A.M.; Laranjeira, P.; Paiva, A. Early-Stage Luminal B-like Breast Cancer Exhibits a More Immunosuppressive Tumor Microenvironment than Luminal A-like Breast Cancer. Biomolecules 2025, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, A.; Riedel, F.; Gondos, A.; Sinn, P.; Schirmacher, P.; Marmé, F.; Jäger, D.; Kauczor, H.U.; Stieber, A.; Lindel, K.; et al. Prognosis of breast cancer molecular subtypes in routine clinical care: A large prospective cohort study. BMC Cancer 2016, 16, 734. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Coiro, S.; Gasparini, E.; Falco, G.; Santandrea, G.; Foroni, M.; Besutti, G.; Iotti, V.; Di Cicilia, R.; Foroni, M.; Mele, S.; et al. Biomarkers Changes after Neoadjuvant Chemotherapy in Breast Cancer: A Seven-Year Single Institution Experience. Diagnostics 2021, 11, 2249. [Google Scholar] [CrossRef]
- Pruss, M.; Cieslik, J.-P.; Török, J.; Dobrowolski, J.; Neubacher, M.; Helbig, M.; Friebe, V.; Häberle, L.; Krawczyk, N.; Borgmeier, F.; et al. Hormone and HER2-receptor status in breast cancer: Determination using sonographically guided core needle biopsy and correlation with excision specimen—A German single institution diagnostic accuracy study. Arch. Gynecol. Obstet. 2025, 311, 881–891. [Google Scholar] [CrossRef]
- Pang, J.-M.B.; Gorringe, K.L.; Fox, S.B. Ductal carcinoma in situ-update on risk assessment and management. Histopathology 2016, 68, 96–109. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; Chen, H.; Zhou, Y.; Hong, L.; Ma, Y.; Chen, N.; Zhao, W.; Tong, Z. Clinical significance and prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients. Front. Surg. 2023, 9, 1037215. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.S.; Rakha, E.A.; Hodi, Z.; Abbas, A.; Ellis, I.O.; Chan, S. Retesting of oestrogen receptor, progesterone receptor and HER2 status of invasive carcinoma of the breast after neoadjuvant chemotherapy. Histopathology 2025, 87, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Shanmugalingam, A.; Hitos, K.; Hegde, S.; Al-Mashat, A.; Pathmanathan, N.; Edirimmane, S.; Hughes, T.M.; Ngui, N.K. Concordance between core needle biopsy and surgical excision for breast cancer tumor grade and biomarkers. Breast Cancer Res. Treat. 2022, 193, 151–159. [Google Scholar] [CrossRef]
- Al-Saleh, K.; Aldiab, A.; Salah, T.; Arafah, M.; Husain, S.; Al-Rikabi, A.; El-Aziz, N.A. Prognostic Significance of HER2 Expression Changes Following Neoadjuvant Chemotherapy in Saudi Patients with Locally Advanced Breast Cancer. Clin. Breast Cancer 2021, 21, e362–e367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, X.; Tang, M.; Xiao, X. HER2 and hormone receptor conversion after neoadjuvant therapy for breast cancer. Front. Oncol. 2025, 15, 1522460. [Google Scholar] [CrossRef]
- Vemuru, S.; Huang, J.; Colborn, K.; Yoon, Y.; Huynh, V.; Leonard, L.; Ahrendt, G.; Christian, N.; Afghahi, A.; McLemore, L.; et al. Clinical implications of receptor conversions in breast cancer patients who have undergone neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2023, 200, 247–256. [Google Scholar] [CrossRef]
- Feng, R.; Pan, L.; Yao, Y.; Gao, J.; Zhang, X. Impact of Hormone Receptor and HER2 Conversions on Survival After Neoadjuvant Chemotherapy in Breast Cancer Patients. Oncol. Transl. Med. 2025, 11, 73–80. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Z.; Zhao, D.; Zhao, J.; Mao, F.; Sun, Q. Discordance in ER, PR, HER2, and Ki-67 Expression Between Primary and Recurrent/Metastatic Lesions in Patients with Primary Early Stage Breast Cancer and the Clinical Significance: Retrospective Analysis of 75 Cases. Pathol. Oncol. Res. 2021, 27, 599894. [Google Scholar] [CrossRef]
- Yousef, E.M.; Alswilem, A.M.; Alfaraj, Z.S.; Alhamood, D.J.; Ghashi, G.K.; Alruwaily, H.S.; Al Yahya, S.S.; Alsaeed, E. Incidence and Prognostic Significance of Hormonal Receptors and HER2 Status Conversion in Recurrent Breast Cancer: A Retrospective Study in a Single Institute. Medicina 2025, 61, 563. [Google Scholar] [CrossRef] [PubMed]
- You, K.; Park, S.; Ryu, J.M.; Kim, I.; Lee, S.K.; Yu, J.; Kim, S.W.; Nam, S.J.; Lee, J.E. Comparison of Core Needle Biopsy and Surgical Specimens in Determining Intrinsic Biological Subtypes of Breast Cancer with Immunohistochemistry. J. Breast Cancer 2017, 20, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Hacking, S.M.; Wang, Y. Practical Issues of Ki-67 Evaluation in Breast Cancer Clinical Practice. J. Clin. Transl. Pathol. 2022, 2, 53–56. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Zengel, B.; Kocatepe Çavdar, D.; Yılmaz, C.; Durusoy, R. Prognostic Value of Receptor Change After Neoadjuvant Chemotherapy in Breast Cancer Patients. Eur. J. Breast Health 2022, 18, 167–171. [Google Scholar] [CrossRef]
- Srivastava, P.; Wang, T.; Clark, B.Z.; Yu, J.; Fine, J.L.; Villatoro, T.M.; Carter, G.J.; Brufsky, A.M.; Gorantla, V.C.; Huggins-Puhalla, S.L.; et al. Clinical-pathologic characteristics and response to neoadjuvant chemotherapy in triple-negative low Ki-67 proliferation (TNLP) breast cancers. npj Breast Cancer 2022, 8, 51. [Google Scholar] [CrossRef]
- Pan, X.-B.; Chen, R.-J.; Huang, S.-T.; Jiang, Y.-M.; Zhu, X.-D. Systematic review and meta-analysis of the efficacy of breast conservation therapy followed by radiotherapy in four breast cancer subtypes. Oncotarget 2017, 8, 57414–57420. [Google Scholar] [CrossRef]
- Pistilli, B.; Lohrisch, C.; Sheade, J.; Fleming, G.F. Personalizing Adjuvant Endocrine Therapy for Early-Stage Hormone Receptor-Positive Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 60–72. [Google Scholar] [CrossRef]
- Pandya, J.; Joshi, S.A.; Barot, H.J. Effect of Neoadjuvant Chemotherapy on Immunohistochemistry in Breast Cancer. Cureus 2025, 17, e8757. [Google Scholar] [CrossRef] [PubMed]
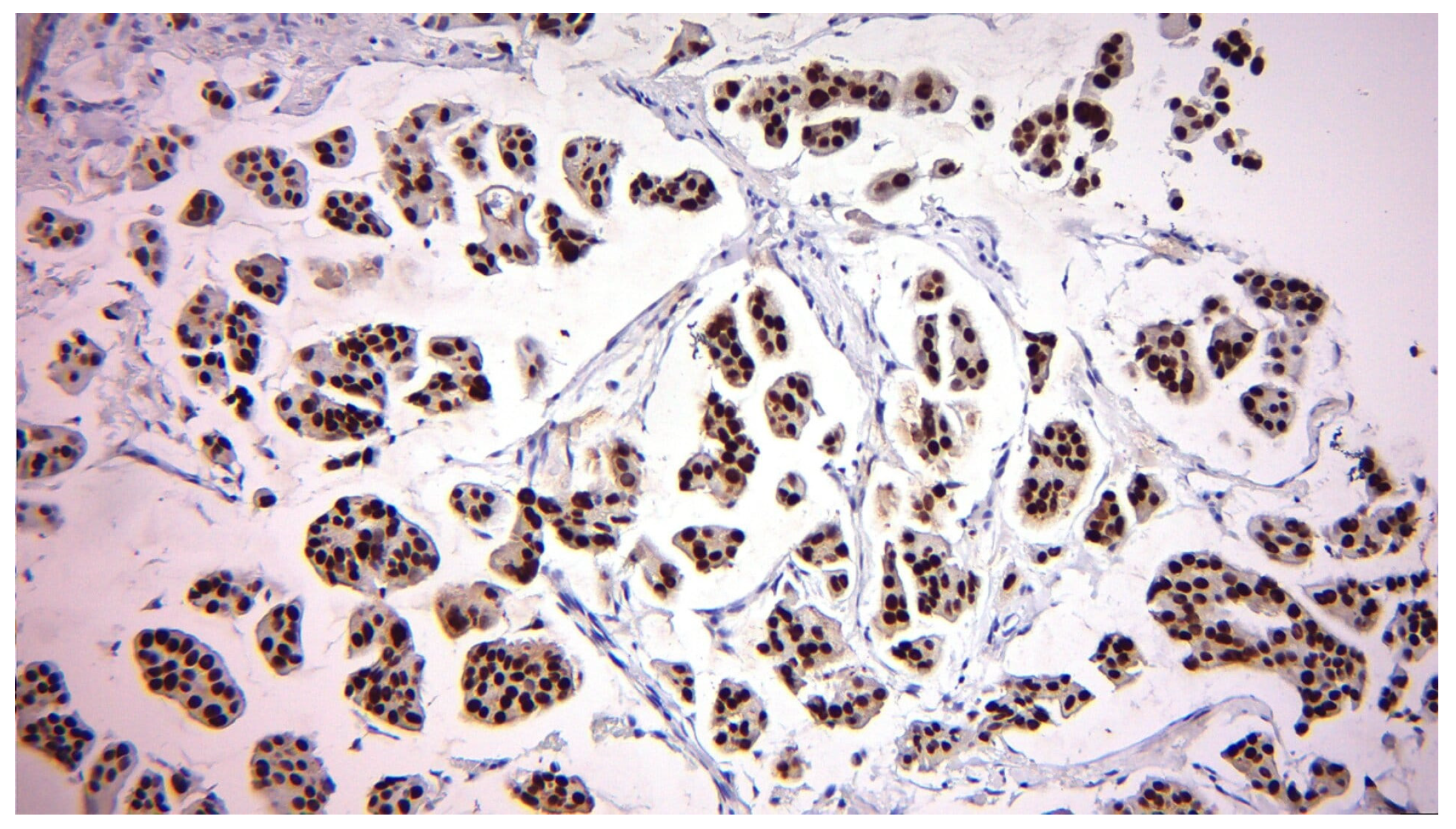
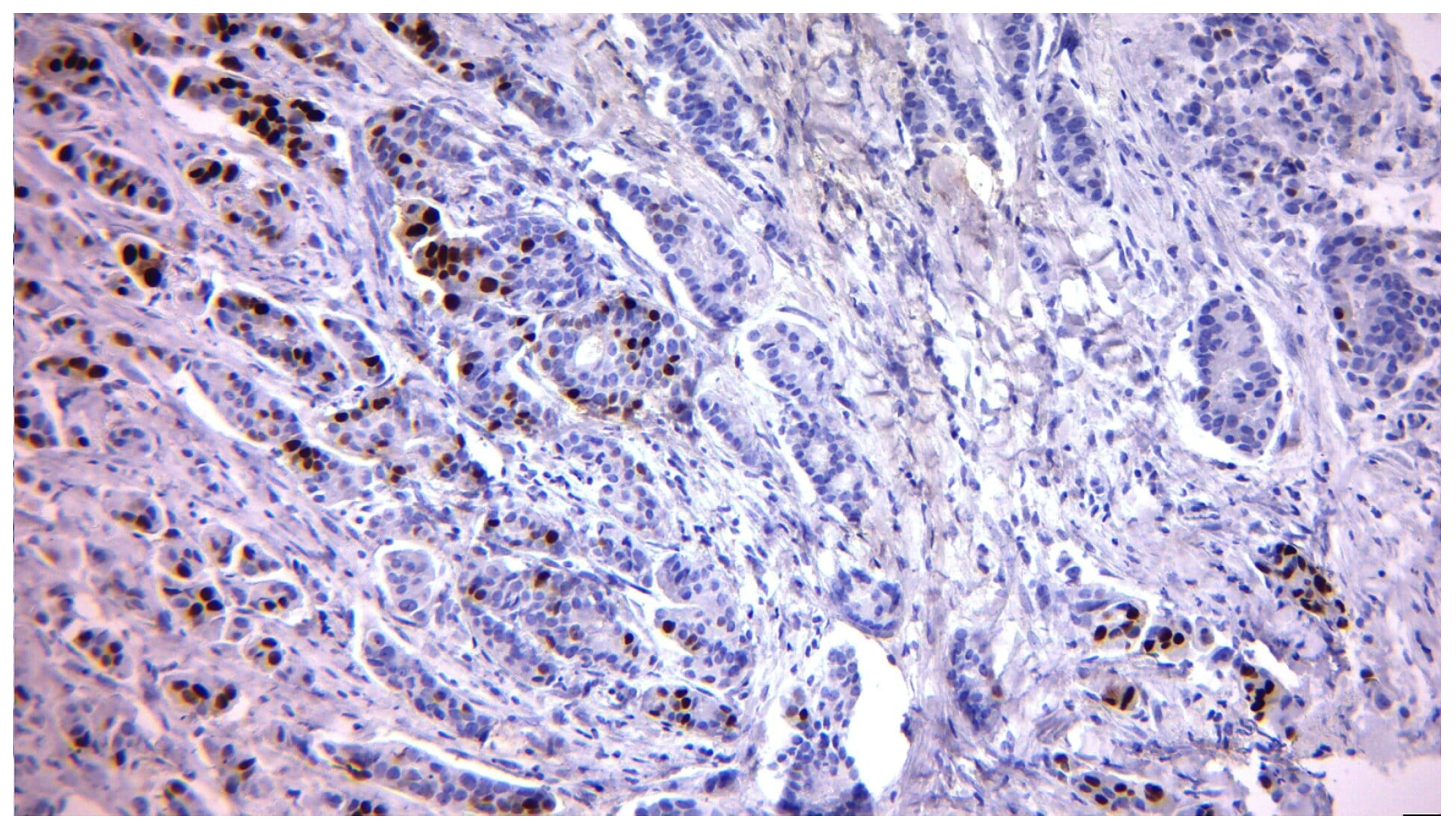
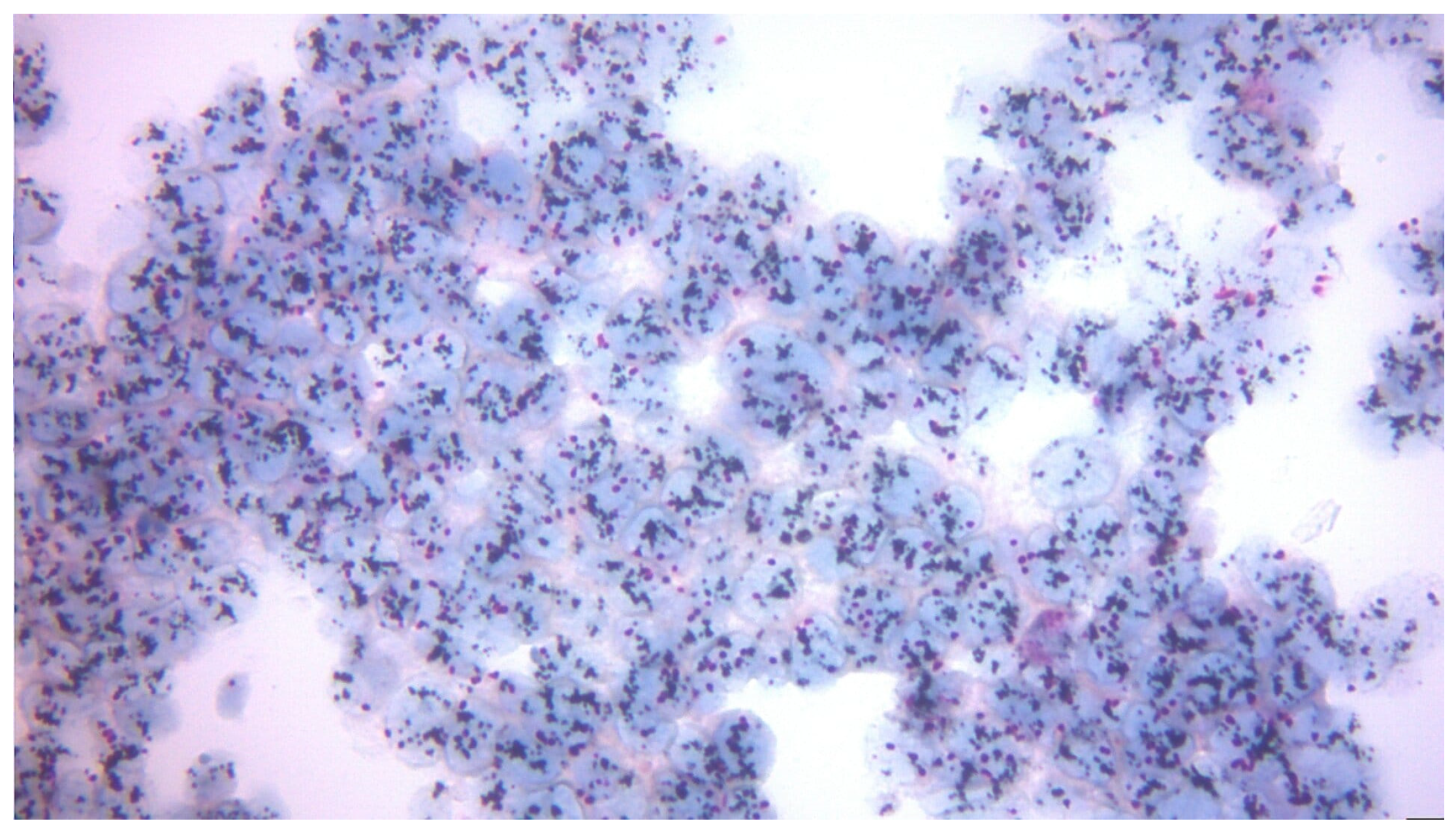
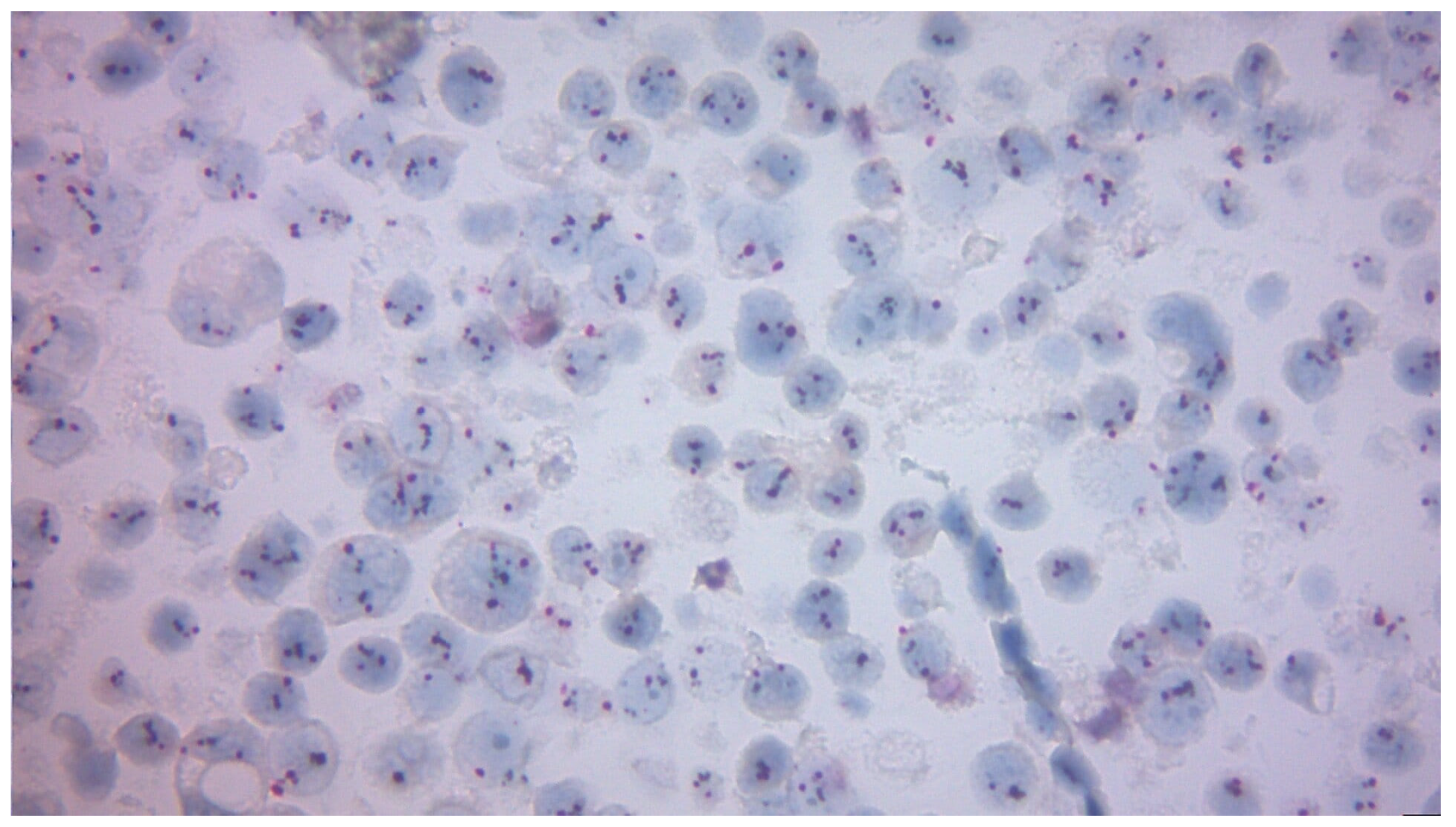
| Variable | n (%) or Mean ± SD |
|---|---|
| Total patients | 79 |
| Age (years) | 57.1 ± 11.5 (range: 30–83) |
| Gender | Female: 78 (98.7%), Male: 1 (1.3%) |
| Hypertension (HTA) | 18 (22.8%) |
| Type II Diabetes Mellitus | 6 (7.6%) |
| Dyslipidemia | 9 (11.4%) |
| Heart failure | 9 (11.4%) |
| Marker | Category | Pre-Surgery, n (%) | Post-Surgery, n (%) |
|---|---|---|---|
| ER | Negative | 8 (10.13) | 11 (13.92) |
| Positive | 71 (89.87) | 68 (86.08) | |
| PR | Negative | 12 (15.19) | 22 (27.85) |
| Positive | 67 (84.81) | 57 (72.15) | |
| HER2 | 0 | 44 (55.7) | 44 (55.7) |
| 1+ | 10 (12.66) | 11 (13.92) | |
| 2+ | 14 (17.72) | 19 (24.05) | |
| 3+ | 9 (11.39) | 5 (6.33) | |
| Ki-67 | <20% | 30 (37.97) | 45 (56.96) |
| 20–30% | 49 (62.03) | 34 (43.04) | |
| >30% | - | - |
| Marker | Category | Pre-Surgery, NACT (n = 54) n (%) | Post-Surgery, NACT (n = 54) n (%) | Pre-Surgery, Upfront Surgery (n = 25) n (%) | Post-Surgery, Upfront Surgery (n = 25) n (%) |
|---|---|---|---|---|---|
| ER | Negative | 7 (12.96) | 8 (14.81) | 1 (4.00) | 3 (12.00) |
| Positive | 47 (87.04) | 46 (85.19) | 24 (96.00) | 22 (88.00) | |
| PR | Negative | 12 (22.22) | 16 (29.63) | 0 (0.00) | 6 (24.00) |
| Positive | 42 (77.78) | 38 (70.37) | 25 (100.00) | 19 (76.00) | |
| HER2 | 0 | 29 (53.70) | 25 (46.30) | 15 (60.00) | 19 (76.00) |
| 1+ | 6 (11.11) | 9 (16.67) | 4 (16.00) | 2 (8.00) | |
| 2+ | 9 (16.67) | 15 (27.78) | 5 (20.00) | 4 (16.00) | |
| 3+ | 8 (14.81) | 5 (9.26) | 1 (4.00) | 0 (0.00) | |
| Ki-67 | <20% | 14 (25.93) | 29 (53.70) | 16 (64.00) | 16 (64.00) |
| ≥20% | 40 (74.07) | 25 (46.30) | 9 (36.00) | 9 (36.00) |
| Biomarker | Clinically Actionable Change (Pre → Post) | Patients n (%) | Typical Therapeutic Implication |
|---|---|---|---|
| PR | PR-positive → PR-negative | 12 (15.2) | Less favorable endocrine profile; may support chemotherapy escalation |
| PR-negative → PR-positive | 2 (2.5) | Endocrine therapy becomes more clearly indicated | |
| HER2 | HER2-positive → HER2-negative | 7 (8.9) | Potential de-escalation or discontinuation of anti-HER2 targeted therapy |
| HER2-negative → HER2-positive | 2 (2.5) | New eligibility for adding anti-HER2 targeted therapy | |
| KI-67 | High (≥20%) → Low (<20%) | 22 (27.8) | Supports de-escalation of chemotherapy in responding, high-proliferative BC |
| Low (<20%) → High (≥20%) | 7 (8.9) | Favors intensification of systemic therapy | |
| Any marker | ≥1 clinically actionable change in PR, HER2, or Ki-67 (above) | 42 (53.2) | Treatment plan revisited in multidisciplinary discussion |
| Histologic Grade | Luminal A | Luminal B | Triple-Negative | |
|---|---|---|---|---|
| Pre-surgery | G1 | 4 | 7 | 0 |
| G2 | 14 | 9 | 1 | |
| G3 | 5 | 0 | 2 | |
| Post-surgery | G1 | 6 | 0 | 0 |
| G2 | 4 | 3 | 3 | |
| G3 | 1 | 1 | 1 | |
| Therapy | n (%) |
|---|---|
| Mastectomy | 60 (75.9%) |
| Lumpectomy | 19 (24.1%) |
| Lymphadenectomy | 68 (86.1%) |
| Neoadjuvant chemotherapy | 54 (68.4%) |
| Adjuvant chemotherapy | 21 (26.6%) |
| Adjuvant radiotherapy | 45 (57.0%) |
| Neoadjuvant hormone therapy | 7 (8.9%) |
| Adjuvant hormone therapy | 67 (84.8%) |
| Targeted molecular therapy | 12 (15.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioroianu, R.A.; Schenker, M.; Berisha, T.C.; Rădulescu, V.-M.; Cioroianu, G.O.; Chirculescu, R.; Petrescu, A.M.; Popescu, M.; Dijmărescu, A.L.; Mogoantă, S.Ș. Post-Surgical Reassessment of Breast Cancer IHC: Concordance, Δ-Metrics, and Treatment-Relevant Reclassification. Diagnostics 2025, 15, 3128. https://doi.org/10.3390/diagnostics15243128
Cioroianu RA, Schenker M, Berisha TC, Rădulescu V-M, Cioroianu GO, Chirculescu R, Petrescu AM, Popescu M, Dijmărescu AL, Mogoantă SȘ. Post-Surgical Reassessment of Breast Cancer IHC: Concordance, Δ-Metrics, and Treatment-Relevant Reclassification. Diagnostics. 2025; 15(24):3128. https://doi.org/10.3390/diagnostics15243128
Chicago/Turabian StyleCioroianu, Ramona Andreea, Michael Schenker, Tradian Ciprian Berisha, Virginia-Maria Rădulescu, George Ovidiu Cioroianu, Raluca Chirculescu, Ana Maria Petrescu, Mihaela Popescu, Anda Lorena Dijmărescu, and Stelian Ștefăniță Mogoantă. 2025. "Post-Surgical Reassessment of Breast Cancer IHC: Concordance, Δ-Metrics, and Treatment-Relevant Reclassification" Diagnostics 15, no. 24: 3128. https://doi.org/10.3390/diagnostics15243128
APA StyleCioroianu, R. A., Schenker, M., Berisha, T. C., Rădulescu, V.-M., Cioroianu, G. O., Chirculescu, R., Petrescu, A. M., Popescu, M., Dijmărescu, A. L., & Mogoantă, S. Ș. (2025). Post-Surgical Reassessment of Breast Cancer IHC: Concordance, Δ-Metrics, and Treatment-Relevant Reclassification. Diagnostics, 15(24), 3128. https://doi.org/10.3390/diagnostics15243128







