Bone Marrow Edema and Tyrosine Kinase Inhibitors Treatment in Chronic Myeloid Leukemia
Abstract
1. Introduction
2. General Considerations for Bone Marrow Edema
2.1. Definition of Bone Marrow Edema
2.2. Etiopathogenesis of Bone Marrow Edema
2.3. Diagnosis of Bone Marrow Edema
2.4. Bone Marrow Edema Therapy
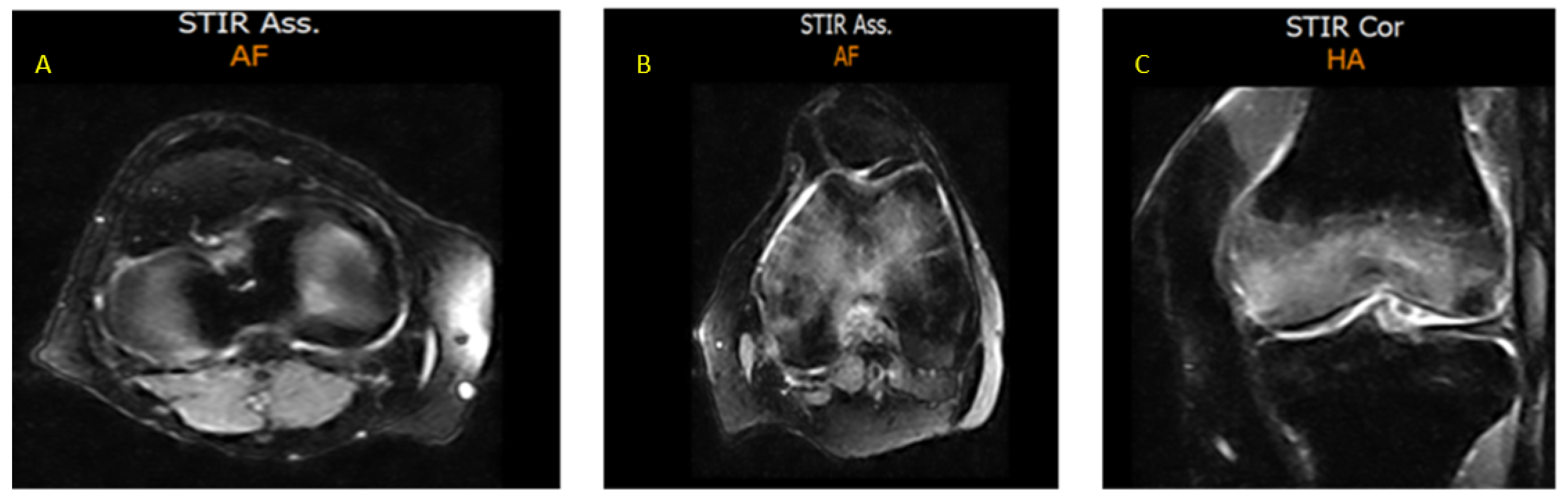
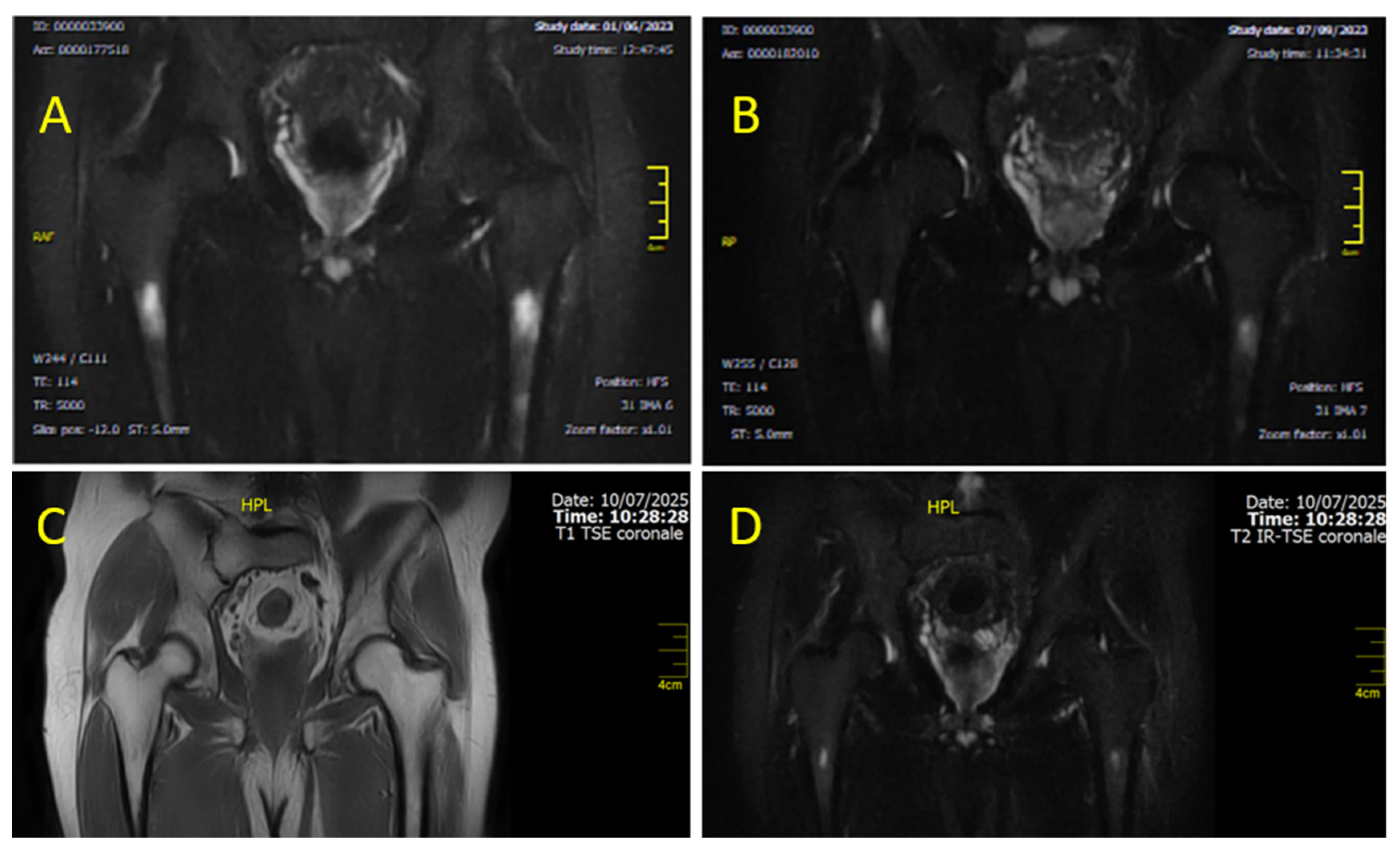
3. Case Presentation
- CASE 1: A CML patient under dasatinib and imatinib treatment
- CASE 2: 60-year-old patient with imatinib therapy
- CASE 3: 66-year-old patient with imatinib therapy
4. Discussion
4.1. From Clinical Observation to Mechanistic Hypothesis
4.2. The Role of PDGFR-β in Imatinib-Induced Edema Formation
4.3. PDGFR- and c-KIT-Mediated Modulation of the Osteochondral Unit Under TKI Therapy
4.4. From Muscle Edema to Bone Marrow Edema: Vascular Effects of TKIs
4.5. Therapeutic Switching to Bosutinib: Clinical and Mechanistic Justification
5. Suggested Diagnostic/Therapeutic Algorithm and Conclusions
- (1)
- Diagnostic Confirmation: Perform targeted MRI to confirm or exclude the presence of BME;
- (2)
- Initial Therapeutic Adjustment: Temporary discontinuation of the ongoing TKI if BME is confirmed.
- (3)
- Supportive Bone-Directed Therapy: Bisphosphonates in combination with vitamin D and calcium supplementation. This timely approach allows us to enhance skeletal stability, stabilize the skeletal microenvironment, and counteract secondary bone metabolic alterations.
- (4)
- Symptom Control: Associate symptomatic management with NSAIDs when clinically indicated to improve QoL.
- (5)
- Long-Term Disease Management: Therapeutic switch to BOS following clinical resolution, ideally guided by mutation analysis and ongoing molecular monitoring to ensure both safety and clinical hematologic efficacy, according to actual guidelines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TKIs | Tyrosine Kinase Inhibitors |
| Ph+/- | Philadelphia Chromosome-Positive/Negative |
| CML | Chronic Myeloid Leukemia |
| QoL | Quality of Life |
| ELN | European Leukemia Network |
| BME | Bone Marrow Edema |
| MRI | Magnetic Resonance Imaging |
| STIR | Short Tau Inversion Recovery |
| DAS | Dasatinib |
| IM | Imatinib |
| BCR::ABL1 | Breakpoint Cluster Region::Abelson Murine Leukemia Viral Oncogene Homolog 1 |
| DECT | Dual-Energy Computed Tomography |
| T1(2)WI | T1/T2-Weighted Imaging |
| FSE | Fast Spin Echo |
| RQ-PCR | Real-Time Quantitative Polymerase Chain Reaction |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| BML | Bone Marrow Lesion |
| ASA | Acetylsalicylic Acid |
| COX-1 | Cyclooxygenase-1 |
| ARCO | Association Research Circulation Osseous |
| ESWT | Extracorporeal Shock Wave Therapy |
| BMP | Bone Morphogenetic Protein |
| MR3 | Major Molecular Response (3-log reduction) |
| MR4 | Deep Molecular Response (4-log reduction) |
| CTCAE | Common Terminology Criteria for Adverse Events |
| BOS | Bosutinib |
| IU | International Units |
| Sokal | Sokal Prognostic Score |
| ELTS | EUTOS Long-Term Survival Score |
| GIST | Gastrointestinal Stromal Tumor |
| PDGFR-α/β | Platelet-Derived Growth Factor Receptor |
| PI3K | Phosphoinositide 3-Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| M-CSF | Macrophage Colony-Stimulating Factor |
| RANKL | Receptor Activator of Nuclear Factor Kappa-B Ligand |
| VEGF | Vascular Endothelial Growth Factor |
| KIT | Stem Cell Factor Receptor (also known as CD117) |
References
- Apperley, J.F.; Milojkovic, D.; Cross, N.C.P.; Hjorth-Hansen, H.; Hochhaus, A.; Kantarjian, H.; Lipton, J.H.; Malhotra, H.; Niederwieser, D.; Radich, J.; et al. European LeukemiaNet Recommendations for the Management of Chronic Myeloid Leukemia. Leukemia 2025, 39, 1797–1813. [Google Scholar] [CrossRef]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.-W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet Recommendations for the Management and Avoidance of Adverse Events of Treatment in Chronic Myeloid Leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. A Phase 2 Trial of Ponatinib in Philadelphia Chromosome–Positive Leukemias. N. Engl. J. Med. 2013, 369, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Brunello, M.; Villari, E.; Cataldi, P.; D’Agostino, C.; Faldini, C. Bone Marrow Edema of the Hip: A Narrative Review. Arch. Orthop. Trauma. Surg. 2023, 143, 6901–6917. [Google Scholar] [CrossRef]
- Villari, E.; Digennaro, V.; Panciera, A.; Ferri, R.; Benvenuti, L.; Cesare, F. Bone Marrow Edema of the Knee: A Narrative Review. Arch. Orthop. Trauma. Surg. 2024, 144, 2305–2316. [Google Scholar] [CrossRef]
- Hayes, C.W.; Conway, W.F.; Daniel, W.W. MR Imaging of Bone Marrow Edema Pattern: Transient Osteoporosis, Transient Bone Marrow Edema Syndrome, or Osteonecrosis. RadioGraphics 1993, 13, 1001–1011. [Google Scholar] [CrossRef]
- Zanetti, M.; Bruder, E.; Romero, J.; Hodler, J. Bone Marrow Edema Pattern in Osteoarthritic Knees: Correlation between MR Imaging and Histologic Findings. Radiology 2000, 215, 835–840. [Google Scholar] [CrossRef]
- Starr, A.M.; Wessely, M.A.; Albastaki, U.; Pierre-Jerome, C.; Kettner, N.W. Bone Marrow Edema: Pathophysiology, Differential Diagnosis, and Imaging. Acta Radiol. 2008, 49, 771–786. [Google Scholar] [CrossRef]
- Hasvik, E.; Haugen, A.J.; Grøvle, L. Clinical Characteristics of Patients with Bone Marrow Edema Syndrome, Transient Osteoporosis or Migratory Osteoporosis: A Scoping Review. Bone 2025, 198, 117535. [Google Scholar] [CrossRef]
- Singh, V.; Oliashirazi, A.; Tan, T.; Fayyad, A.; Shahi, A. Clinical and Pathophysiologic Significance of MRI Identified Bone Marrow Lesions Associated with Knee Osteoarthritis. Arch. Bone Jt. Surg. 2019, 7, 211–219. [Google Scholar]
- Vilanova, C.; Martín-Noguerol, T.; García-Figueiras, R.; Baleato-González, S.; Vilanova, J.C. Bone Marrow Magnetic Resonance Imaging (MRI): Morphological and Functional Features from Reconversion to Infiltration. Quant. Imaging Med. Surg. 2024, 14, 7969–7982. [Google Scholar] [CrossRef]
- Moradi, K.; Mohammadi, S.; Roemer, F.W.; Momtazmanesh, S.; Hathaway, Q.; Ibad, H.A.; Hunter, D.J.; Guermazi, A.; Demehri, S. Progression of Bone Marrow Lesions and the Development of Knee Osteoarthritis: Osteoarthritis Initiative Data. Radiology 2024, 312, e240470. [Google Scholar] [CrossRef] [PubMed]
- Molfetta, L.; Florian, A.; Saviola, G.; Frediani, B. Bone Marrow Edema: Pathogenetic Features. Clin. Ter. 2022, 173, 434–439. [Google Scholar] [CrossRef]
- Littman, J.; Gil, H.; Aaron, R. Spontaneous Bone Marrow Edema: Perfusion Abnormalities and Treatment with Surgical Decompression. Int. J. Mol. Sci. 2023, 24, 6761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, W.; Chen, Z. Dual Roles of Prostaglandin E2 (PGE2) in Bone Remodeling and Pain Management: Bridging the Gap in Osteoarthritis Research. Mediat. Inflamm. 2025, 2025, 8882429. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, X.; Zhu, R.; Cai, G. Bone Marrow Lesions in Osteoarthritis: Biomarker or Treatment Target? A Narrative Review. Skelet. Radiol. 2025, 54, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Pearl, M.C.; Mont, M.A.; Scuderi, G.R. Osteonecrosis of the Knee. Orthop. Clin. N. Am. 2022, 53, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Li, D.; Marappa-Ganeshan, R. Secondary Osteonecrosis of the Knee; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Patel, S. Primary Bone Marrow Oedema Syndromes. Rheumatology 2014, 53, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Accadbled, F.; Vial, J.; Sales de Gauzy, J. Osteochondritis Dissecans of the Knee. Orthop. Traumatol. Surg. Res. 2018, 104, S97–S105. [Google Scholar] [CrossRef]
- Benchouk, S.; Buchard, P.-A.; Luthi, F. Complex Regional Pain Syndrome and Bone Marrow Oedema Syndrome: Family Ties Potentially Closer than Expected. BMJ Case Rep. 2020, 13, e234600. [Google Scholar] [CrossRef]
- Fowkes, L.A.; Toms, A.P. Bone Marrow Oedema of the Knee. Knee 2010, 17, 1–6. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Liu, X. Role and Mechanism of Mechanical Load in the Homeostasis of the Subchondral Bone in Knee Osteoarthritis: A Comprehensive Review. J. Inflamm. Res. 2024, 17, 9359–9378. [Google Scholar] [CrossRef]
- Matcuk, G.R.; Mahanty, S.R.; Skalski, M.R.; Patel, D.B.; White, E.A.; Gottsegen, C.J. Stress Fractures: Pathophysiology, Clinical Presentation, Imaging Features, and Treatment Options. Emerg. Radiol. 2016, 23, 365–375. [Google Scholar] [CrossRef]
- Akhavan, S.; Martinkovich, S.C.; Kasik, C.; DeMeo, P.J. Bone Marrow Edema, Clinical Significance, and Treatment Options: A Review. J. Am. Acad. Orthop. Surg. 2020, 28, e888–e899. [Google Scholar] [CrossRef]
- Wang, X.; Jin, X.; Blizzard, L.; Antony, B.; Han, W.; Zhu, Z.; Cicuttini, F.; Wluka, A.E.; Winzenberg, T.; Jones, G.; et al. Associations Between Knee Effusion-Synovitis and Joint Structural Changes in Patients with Knee Osteoarthritis. J. Rheumatol. 2017, 44, 1644–1651. [Google Scholar] [CrossRef]
- Baumbach, S.F.; Pfahler, V.; Bechtold-Dalla Pozza, S.; Feist-Pagenstert, I.; Fürmetz, J.; Baur-Melnyk, A.; Stumpf, U.C.; Saller, M.M.; Straube, A.; Schmidmaier, R.; et al. How We Manage Bone Marrow Edema—An Interdisciplinary Approach. J. Clin. Med. 2020, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, T.R.; Valentin, S.; Gülecyüz, M.F.; Roßbach, B.P.; Ficklscherer, A.; Pietschmann, M.F.; Müller, P.E. Bone Marrow Edema in the Knee and Its Influence on Clinical Outcome After Matrix-Based Autologous Chondrocyte Implantation. Am. J. Sports Med. 2015, 43, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.A.; Ong, Y.X.N.; Weber, M.-A. Pitfalls in Bone Marrow Edema Interpretation on Dual-Energy CT: Challenges and Solutions. In RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgebenden Verfahren; Georg Thieme Verlag KG: Stuttgart, Germany, 2025. [Google Scholar] [CrossRef]
- Shabshin, G.; Shabshin, N. Bone Marrow Lesions Related to Bone Marrow Edema Syndromes and Osteonecrosis. Die Orthop. 2025, 54, 324–331. [Google Scholar] [CrossRef]
- Giraudo, C.; Weber, M.; Puchner, A.; Grisar, J.; Kainberger, F.; Schueller-Weidekamm, C. Which MR Sequences Should We Use for the Reliable Detection and Localization of Bone Marrow Edema in Spondyloarthritis? Radiol. Med. 2017, 122, 752–760. [Google Scholar] [CrossRef]
- Corzo Garcia, P.; Garcia-Duitama, I.; Agustí Claramunt, A.; Duran Jordà, X.; Monfort, J.; Salman-Monte, T.C. Musculoskeletal Involvement in Systemic Lupus Erythematosus: A Contrast-Enhanced Magnetic Resonance Imaging Study in 107 Subjects. Rheumatology 2024, 63, 423–429. [Google Scholar] [CrossRef]
- Compagnoni, R.; Lesman, J.; Ferrua, P.; Menon, A.; Minoli, C.; Gallazzi, M.; Domżalski, M.; Randelli, P. Validation of a New Topographic Classification of Bone Marrow Lesions in the Knee: The Six-letter System. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.G.; Medynski, M.A.; Feller, J.F.; Lawhorn, K.W. Bone Contusion Patterns of the Knee at MR Imaging: Footprint of the Mechanism of Injury. RadioGraphics 2000, 20, S135–S151. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Ronga, M.; Filardo, G.; Farr, J.; Madry, H.; Milano, G.; Andriolo, L.; Shabshin, N. Bone Marrow Lesions and Subchondral Bone Pathology of the Knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Cicuttini, F.M.; Wluka, A.E.; Jones, G.; Hill, C.L.; Ding, C.; Wang, Y. Association between Medial Meniscal Extrusion and Knee Structural Progression in Adults with Symptomatic Knee Osteoarthritis—A Prospective Cohort Study. Skelet. Radiol. 2025, 54, 219–228. [Google Scholar] [CrossRef]
- Paraskevopoulos, K.; Keskinis, A.; Vasios, I.S.; Makiev, K.G.; Tilkeridis, K.; Drosos, G.I.; Ververidis, A.N. Comparison of Various Treatment Modalities for the Management of Bone Marrow Edema Syndrome/Transient Osteoporosis in Men and Non-Pregnant Women: A Systematic Review. Osteoporos. Int. 2023, 34, 269–290. [Google Scholar] [CrossRef]
- Cavalli, L.; Falcone, G.; Cavalli, T.; Pasquetti, P. Therapeutic and Functional Approach for the Treatment of Patients with Bone Marrow Edema in Rehabilitation Medicine. Beyond Rheumatol. 2020, 2, 43–50. [Google Scholar] [CrossRef]
- Jackson, C.; Freeman, A.L.J.; Szlamka, Z.; Spiegelhalter, D.J. The Adverse Effects of Bisphosphonates in Breast Cancer: A Systematic Review and Network Meta-Analysis. PLoS ONE 2021, 16, e0246441. [Google Scholar] [CrossRef]
- Aboubacar, B.H.; Jumelle, Z.N.A.; Odero-Marah, V.; Romuald, K.T.; Laetitia, O.D.Y.; Tarcissus, K. Post Biphosphonate Mandible Osteonecrosis: A Case Study and Literature Review. Oral Oncol. Rep. 2023, 7, 100081. [Google Scholar] [CrossRef]
- Zippelius, T.; Strube, P.; Rohe, S.; Schlattmann, P.; Dobrindt, O.; Caffard, T.; Awan Malik, H.; Lindemann, C.; Matziolis, G.; Böhle, S. The Use of Iloprost in the Treatment of Bone Marrow Edema Syndrome of the Proximal Femur: A Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1757. [Google Scholar] [CrossRef]
- Zhao, W.; Gao, Y.; Zhang, S.; Liu, Z.; He, L.; Zhang, D.; Li, W.; Meng, Q. Extracorporeal Shock Wave Therapy for Bone Marrow Edema Syndrome in Patients with Osteonecrosis of the Femoral Head: A Retrospective Cohort Study. J. Orthop. Surg. Res. 2021, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Ivković, A.; Glavčić, M.; Vuletić, F.; Janković, S. Core Decompression Combined with Intraosseous Autologous Conditioned Plasma Injections Decreases Pain and Improves Function in Patients with Symptomatic Knee Bone Marrow Lesions. Biomedicines 2023, 11, 1799. [Google Scholar] [CrossRef]
- Jayavel, S.; Subramanian, M.; Kesavan, P.K.; Jayavel, S. Current and Future of Targeted Therapies against BCR::ABL Kinases. J. Egypt. Natl. Cancer Inst. 2025, 37, 12. [Google Scholar] [CrossRef]
- Cross, N.C.P.; Ernst, T.; Branford, S.; Cayuela, J.-M.; Deininger, M.; Fabarius, A.; Kim, D.D.H.; Machova Polakova, K.; Radich, J.P.; Hehlmann, R.; et al. European LeukemiaNet Laboratory Recommendations for the Diagnosis and Management of Chronic Myeloid Leukemia. Leukemia 2023, 37, 2150–2167. [Google Scholar] [CrossRef] [PubMed]
- Hehlmann, R. Chronic Myeloid Leukemia in 2020. Hemasphere 2020, 4, e468. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 Recommendations for Treating Chronic Myeloid Leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Agostani, E.; Tassistro, E.; Antolini, L.; Gambacorti-Passerini, C. Inflammatory/Immune Adverse Events in Chronic Myeloid Leukemia Patients During Treatment With Bosutinib. Cancer Med. 2025, 14, e70580. [Google Scholar] [CrossRef]
- dos Santos, L.V.; Lima, J.P.; Abdalla, K.C.; Bragagnoli, A.C.; Santos, F.A.; dos Anjos Jácome, A.; Porto, F.E. Imatinib-Induced Bone Edema: Case Report and Review of Literature. J. Natl. Compr. Cancer Netw. 2013, 11, 1187–1191. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, L.; Xu, P.; Hu, W. An Overview of Agents and Treatments for PDGFRA-Mutated Gastrointestinal Stromal Tumors. Front. Oncol. 2022, 12, 927587. [Google Scholar] [CrossRef]
- Marom, H.; Khan, M.; Darvish, N.; Tornetta III, P.; Khoury, A.; Weil, Y.; Skelley, N.; Allison, D.; Meiron, S.; Ehrmann Barr, T. β-Caryophyllene and Statins in Bone Fracture Healing—A Narrative Review. Orthop. Res. Rev. 2025, 17, 31–42. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Adawi, D.H.; Al-Barghouthi, A.; Fredj, N.B.; Lubada, M.; Njjar, D.O.; Ruzaygat, M.; Hallak, H.; Aouam, K. Genetic and Non-Genetic Correlates of Imatinib Pharmacokinetics and Side Effects of Imatinib in Palestinian Patients with Chronic Myeloid Leukemia. Biochem. Genet. 2025, 1–19. [Google Scholar] [CrossRef]
- Mehtap, O. A Rare but Fatal Complication Probably Due to Imatinib: Cerebral Edema. J. Clin. Case Rep. 2012, 2, 11. [Google Scholar] [CrossRef]
- Fazio, A.; Neri, I.; Koufi, F.-D.; Marvi, M.V.; Galvani, A.; Evangelisti, C.; McCubrey, J.A.; Cocco, L.; Manzoli, L.; Ratti, S. Signaling Role of Pericytes in Vascular Health and Tissue Homeostasis. Int. J. Mol. Sci. 2024, 25, 6592. [Google Scholar] [CrossRef] [PubMed]
- Heuchel, R.; Berg, A.; Tallquist, M.; Åhlén, K.; Reed, R.K.; Rubin, K.; Claesson-Welsh, L.; Heldin, C.-H.; Soriano, P. Platelet-Derived Growth Factor β Receptor Regulates Interstitial Fluid Homeostasis through Phosphatidylinositol-3′ Kinase Signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 11410–11415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Warner, K.A.; Mantesso, A.; Nör, J.E. PDGF-BB Signaling via PDGFR-β Regulates the Maturation of Blood Vessels Generated upon Vasculogenic Differentiation of Dental Pulp Stem Cells. Front. Cell Dev. Biol. 2022, 10, 977725. [Google Scholar] [CrossRef]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-β in Recruitment of Vascular Smooth Muscle Cells and Pericytes during Embryonic Blood Vessel Formation in the Mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef]
- Pachika, P.S.; Dande, R.R.; Ngo, P. Imatinib-Induced Recurrent Pleural Effusions. Cureus 2025, 17, e83153. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Naot, D.; Callon, K.; Porteous, F.; Horne, A.; Wattie, D.; Watson, M.; Cornish, J.; Browett, P.; Grey, A. Imatinib Promotes Osteoblast Differentiation by Inhibiting PDGFR Signaling and Inhibits Osteoclastogenesis by Both Direct and Stromal Cell-Dependent Mechanisms. J. Bone Miner. Res. 2007, 22, 1679–1689. [Google Scholar] [CrossRef]
- Sims, N.A. Osteoclast-Derived Coupling Factors: Origins and State-of-Play Louis V Avioli Lecture, ASBMR 2023. J. Bone Miner. Res. 2024, 39, 1377–1385. [Google Scholar] [CrossRef]
- Brun, J.; Andreasen, C.M.; Ejersted, C.; Andersen, T.L.; Caverzasio, J.; Thouverey, C. PDGF Receptor Signaling in Osteoblast Lineage Cells Controls Bone Resorption Through Upregulation of Csf1 Expression. J. Bone Miner. Res. 2020, 35, 2458–2469. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, D.; Cortes, J.E.; Medeiros, L.J.; Jabbour, E.J.; Hidalgo, J.E.; Kanagal-Shamanna, R.; Bueso-Ramos, C.E. Multiparameter Analysis of Off-Target Effects of Dasatinib on Bone Homeostasis in Patients With Newly Diagnosed Chronic Myelogenous Leukemia. Clin. Lymphoma Myeloma Leuk. 2016, 16, S86–S92. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Torino, F.; Barnabei, A.; Iannantuono, G.M.; Corsello, A.; Locantore, P.; Corsello, S.M. Bone Metabolism Effects of Medical Therapy in Advanced Renal Cell Carcinoma. Cancers 2023, 15, 529. [Google Scholar] [CrossRef]
- Garcia-Gomez, A.; Ocio, E.M.; Crusoe, E.; Santamaria, C.; Hernández-Campo, P.; Blanco, J.F.; Sanchez-Guijo, F.M.; Hernández-Iglesias, T.; Briñón, J.G.; Fisac-Herrero, R.M.; et al. Dasatinib as a Bone-Modifying Agent: Anabolic and Anti-Resorptive Effects. PLoS ONE 2012, 7, e34914. [Google Scholar] [CrossRef][Green Version]
- Cheng, F.; Xu, Q.; Li, Q.; Cui, Z.; Li, W.; Zeng, F. Adverse Reactions after Treatment with Dasatinib in Chronic Myeloid Leukemia: Characteristics, Potential Mechanisms, and Clinical Management Strategies. Front. Oncol. 2023, 13, 1113462. [Google Scholar] [CrossRef]
- Gover-Proaktor, A.; Leshem-Lev, D.; Winograd-Katz, S.; Partouche, S.; Samara, A.; Shapira, S.; Nardi-Agmon, I.; Harari, E.; Younis, A.; Najjar, A.; et al. Dasatinib Induces Endothelial Dysfunction Leading to Impaired Recovery from Ischaemia. Br. J. Haematol. 2024, 205, 1011–1016. [Google Scholar] [CrossRef]
- Nekoukar, Z.; Moghimi, M.; Salehifar, E. A Narrative Review on Adverse Effects of Dasatinib with a Focus on Pharmacotherapy of Dasatinib-Induced Pulmonary Toxicities. Blood Res. 2021, 56, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kang, Y.-Y.; Zhu, C.-Y.; Ma, Y.; Yu, P.-H.; Yang, T.; Liu, Y.-Q.; Zhang, Z.-Y.; Suzuki, N.; Ogra, Y.; et al. Heat Stress Targets and Degrades BCR::ABL1 Oncoproteins to Overcome Drug-Resistance in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Leukemia 2025, 39, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Brümmendorf, T.H.; Cortes, J.E.; de Souza, C.A.; Guilhot, F.; Duvillié, L.; Pavlov, D.; Gogat, K.; Countouriotis, A.M.; Gambacorti-Passerini, C. Bosutinib versus Imatinib in Newly Diagnosed Chronic-phase Chronic Myeloid Leukaemia: Results from the 24-month Follow-up of the BELA Trial. Br. J. Haematol. 2015, 168, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Khoury, H.J.; Kantarjian, H.M.; Lipton, J.H.; Kim, D.; Schafhausen, P.; Matczak, E.; Leip, E.; Noonan, K.; Brümmendorf, T.H.; et al. Long-term Bosutinib for Chronic Phase Chronic Myeloid Leukemia after Failure of Imatinib plus Dasatinib and/or Nilotinib. Am. J. Hematol. 2016, 91, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Iurlo, A.; Bucelli, C.; Intermesoli, T.; Elena, C.; D’Adda, M.; Agostani, E.; Fiamenghi, C.; Maffioli, M.; Orofino, N.; Lunghi, F.; et al. Bosutinib Treatment of Chronic Myeloid Leukemia in Lombardy. Acta Haematol. 2024, 148, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Ault, D.P.S.; Rose, P.J.; Nodzon, P.L.A.; Kaled, R.E.S. Bosutinib Therapy in Patients With Chronic Myeloid Leukemia: Practical Considerations for Management of Side Effects. J. Adv. Pract. Oncol. 2016, 7, 160–175. [Google Scholar] [CrossRef]
- Hoch, M.; Huth, F.; Manley, P.W.; Loisios-Konstantinidis, I.; Combes, F.P.; Li, Y.F.; Fu, Y.; Sy, S.K.B.; Obourn, V.; Chakraborty, A.; et al. Clinical Pharmacology of Asciminib: A Review. Clin. Pharmacokinet. 2024, 63, 1513–1528. [Google Scholar] [CrossRef]
- Pierro, F.; Stella, S.; Fazio, M.; Russo, S.; Massimino, M.; Mirabile, G.; Belletti, D.; Allegra, A.; Stagno, F. Chronic Myeloid Leukemia and the T315I BCR::ABL1 Mutation. Int. J. Mol. Sci. 2025, 26, 11285. [Google Scholar] [CrossRef]
- Hughes, T.P.; Mauro, M.J.; Cortes, J.E.; Minami, H.; Rea, D.; DeAngelo, D.J.; Breccia, M.; Goh, Y.-T.; Talpaz, M.; Hochhaus, A.; et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibitor Failure. N. Engl. J. Med. 2019, 381, 2315–2326. [Google Scholar] [CrossRef]
- Lauseker, M.; Hehlmann, R.; Hochhaus, A.; Saußele, S. Survival with Chronic Myeloid Leukaemia after Failing Milestones. Leukemia 2023, 37, 2231–2236. [Google Scholar] [CrossRef]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Guilhot, F.; Hehlmann, R. Long-Term Outcomes of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. Blood 2025, 145, 910–920. [Google Scholar] [CrossRef] [PubMed]




| BME Pathogenesis | Cause | Characteristics | Ref |
|---|---|---|---|
| ISCHEMIC | Reduced blood perfusion | Knee osteonecrosis:
| [6] |
| MECHANICAL “Bone bruise ” | Mainly traumatic events [Acute/chronic trauma and repeated stresses may lead to a breakdown of the marrow trabeculae, with interstitial fluid leakage and hemorrhage to BM spaces] | Most common form. It includes the following: Bone contusions [23]:
| [6] |
| REACTIVE | Inflammatory response to adjacent pathological processes | Generally associated with the following: | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, S.; Fazio, M.; Mirabile, G.; Sciaccotta, R.; Stagno, F.; Allegra, A. Bone Marrow Edema and Tyrosine Kinase Inhibitors Treatment in Chronic Myeloid Leukemia. Diagnostics 2025, 15, 3112. https://doi.org/10.3390/diagnostics15243112
Russo S, Fazio M, Mirabile G, Sciaccotta R, Stagno F, Allegra A. Bone Marrow Edema and Tyrosine Kinase Inhibitors Treatment in Chronic Myeloid Leukemia. Diagnostics. 2025; 15(24):3112. https://doi.org/10.3390/diagnostics15243112
Chicago/Turabian StyleRusso, Sabina, Manlio Fazio, Giuseppe Mirabile, Raffaele Sciaccotta, Fabio Stagno, and Alessandro Allegra. 2025. "Bone Marrow Edema and Tyrosine Kinase Inhibitors Treatment in Chronic Myeloid Leukemia" Diagnostics 15, no. 24: 3112. https://doi.org/10.3390/diagnostics15243112
APA StyleRusso, S., Fazio, M., Mirabile, G., Sciaccotta, R., Stagno, F., & Allegra, A. (2025). Bone Marrow Edema and Tyrosine Kinase Inhibitors Treatment in Chronic Myeloid Leukemia. Diagnostics, 15(24), 3112. https://doi.org/10.3390/diagnostics15243112







