Through-the-Needle Biopsy Revisited: How Patient Selection and Standardization Reduce Adverse Events in Pancreatic Cyst Evaluation
Abstract
1. Introduction
2. Materials and Methods
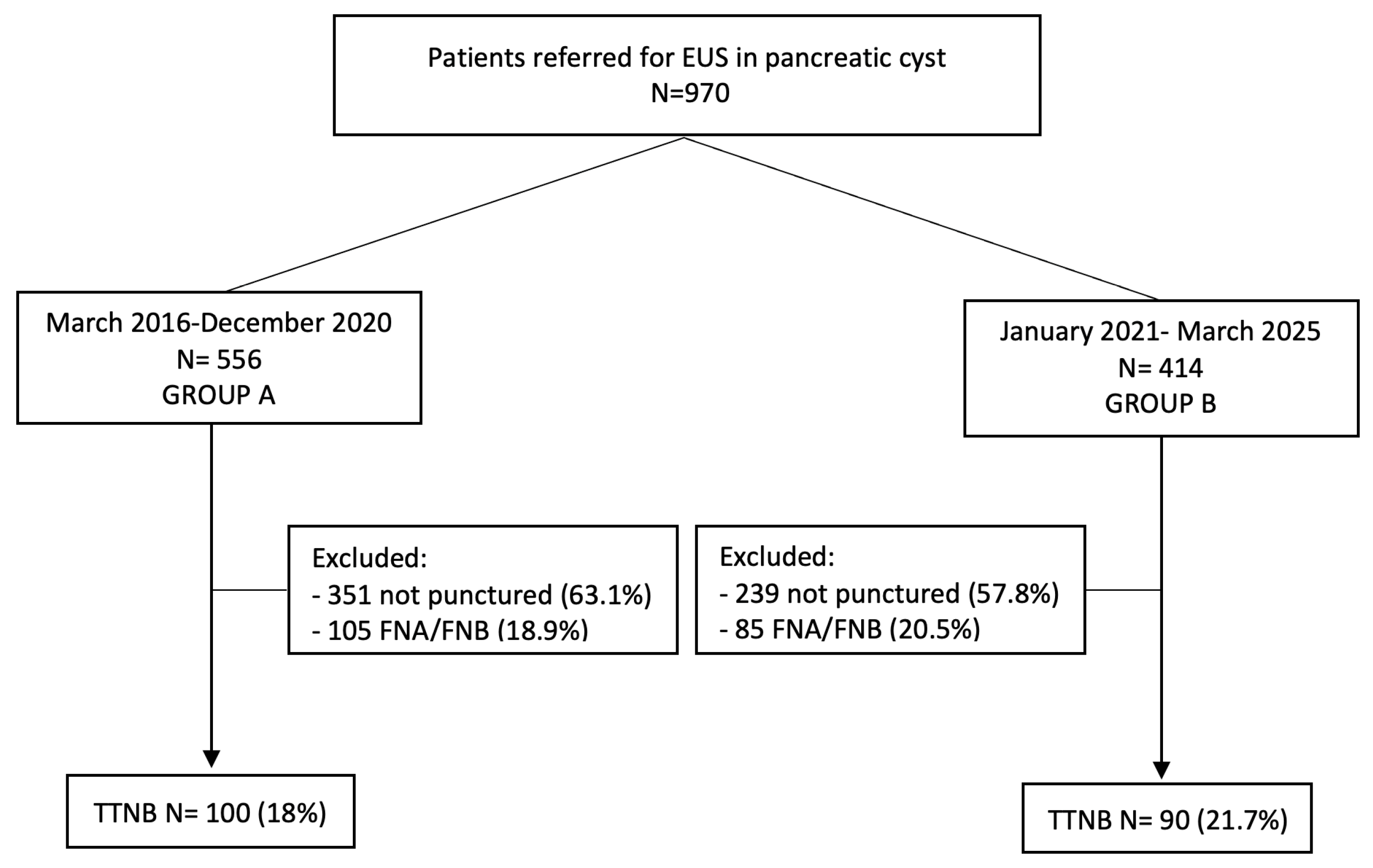
2.1. Patient Selection
2.2. Aims
- Calculated across the entire TTNB population:
- -
- Cytohistological adequacy: Defined as the proportion of TTNB samples sufficient for histological interpretation, per American Gastroenterology Association white paper [13].
- -
- Diagnostic accuracy: Defined as the concordance between TTNB diagnosis and final diagnosis (based on surgical pathology in patients who underwent surgery or composite clinical/imaging/histological criteria).
- Comparison between Group A and Group B:
- -
- Diagnostic yield: Defined as the proportion of EUS procedures that provided a clinically actionable diagnosis (e.g., mucinous vs. non-mucinous cyst), per American Gastroenterology Association white paper [13].
- -
- Impact on clinical management: Assessed as the proportion of patients in whom TTNB findings influenced decision-making (e.g., surgical indication, cessation of surveillance, definitive diagnosis in unilocular cysts).
- Exploratory analysis:
- -
- Utility of antibiotic prophylaxis: Evaluated by comparing AE rates in patients who received antibiotics versus those who did not [14].
2.3. Procedures
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Procedural Details
3.3. Fluid Analysis and Cytology
3.4. Primary Outcome
3.5. Secondary Outcomes
3.6. Outcomes and Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| CI | Confidence interval |
| EUS | Endoscopic ultrasound |
| FNA | Fine-needle aspiration |
| FNB | Fine-needle biopsy |
| IPMN | Intraductal papillary mucinous neoplasm |
| PCL | pancreatic cystic lesions |
| OR | Odds ratio |
| TTNB | Through-the-needle biopsy |
Appendix A
| Patient | Fluid Analysis | Cytology | TTNB Result | Surgical Result |
|---|---|---|---|---|
| Female, 21-year-old, Group A | Not performed | Cells suggestive of solid pseudopapillary tumor | Adequate but not clear for a specific histotype | SPN |
| Male 49-year-old, Group A | Not performed | Poor cellularity: amorphous debris and rare macrophages | Adequate but not clear for a specific histotype | IPMN |
| Female 57-year-old, Group A | ↑ CEA, ↑ Amylase | Acellular fibrin clot | MCN | SCOP |
| Female 73-year-old, Group A | ↑ CEA | Proteinaceous material with macrophages and rare mucin-secreting cells | Adequate but not clear for a specific histotype | IPMN |
| Female 81-year-old, Group A | ↓ CEA, ↑ Amylase | Acellular material | Inadequate | MCN |
| Female 75-year-old, Group A | Not performed | Muco-proteinaceous material with macrophages | Adequate but not clear for a specific histotype | IPMN |
| Female, 45-year-old, Group A | ↑ CEA | Numerous macrophages with very rare epithelial cells showing cytologic atypia | Adequate, not clear for a specific histotype, but mucinous | MCN |
| Male, 54-year-old, Group A | Not performed | Not available | Adequate but not clear for a specific histotype | Dysontogenetic cyst |
| Male, 67-year-old, Group A | ↑ CEA | Bloody/amorphous material with siderophages and rare epithelial elements | Adequate, some atypical changes but not conclusive | Adenocarcinoma associated with SMC |
| Female, 59-year-old, Group B | ↑ CEA | Mucus with amorphous background and foamy macrophages | Adequate but not clear for a specific histotype, but mucinous | MCN |
| Female, 46-year-old, Group B | Not performed | Not performed | Inadequate | IPMN |
| Male, 66-year-old, Group B | ↑ CEA, ↑ Amylase | Bloody material | Inadequate | SMC |
| Female, 28-year-old, Group B | Not evaluable | Bloody material | Adequate but not clear for a specific histotype | SPN |
| Male, 42-year-old, Group B | ↑ Amylase | Pancreatic acini | Inadequate | Pseudocyst |
References
- Salvia, R.; Marchegiani, G.; Pennacchio, S.; Paiella, S.; Paini, M.; Pea, A.; Butturini, G.; Pederzoli, P.; Bassi, C. Pancreatic resections for cystic neoplasms: From the surgeon’s presumption to the pathologist’s reality. Surgery 2012, 152, S135–S142. [Google Scholar] [CrossRef] [PubMed]
- Brugge, W.R.; Lewandrowski, K.; Lee-Lewandrowski, E.; Centeno, B.A.; Szydlo, T.; Regan, S.; del Castillo, C.F.; Warshaw, A.L. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology 2004, 126, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, M.J.; Jamouss, K.T.; Afghani, E.; Lim, S.J.; Rodriguez Franco, S.; Mayo, H.; Spann, M.; Wang, H.; Singhi, A.; Lennon, A.M.; et al. Predictive ability of pancreatic cyst fluid biomarkers: A systematic review and meta-analysis. Pancreatology 2023, 23, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Thosani, N.; Thosani, S.; Qiao, W.; Fleming, J.B.; Bhutani, M.S.; Guha, S. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: A systematic review and meta-analysis. Dig. Dis. Sci. 2010, 55, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, Z.J.; Pan, C.Y.; Wu, C.; Li, Z.S.; Jin, Z.D.; Wang, K.X. Comparative Performance of Endoscopic Ultrasound-Based Techniques in Patients with Pancreatic Cystic Lesions: A Network Meta-Analysis. Am. J. Gastroenterol. 2023, 118, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Westerveld, D.R.; Ponniah, S.A.; Draganov, P.V.; Yang, D. Diagnostic yield of EUS-guided through-the-needle microforceps biopsy versus EUS-FNA of pancreatic cystic lesions: A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E656–E667. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Del Prete, V.; Antonino, M.; Buccino, V.R.; Wani, S. Diagnostic yield of EUS-guided through-the-needle biopsy in pancreatic cysts: A meta-analysis. Gastrointest. Endosc. 2020, 92, 1–8.e3. [Google Scholar] [CrossRef] [PubMed]
- Gopakumar, H.; Puli, S.R. Value of Endoscopic ultrasound-guided through-the-needle biopsy in pancreatic cystic lesions. a systematic review and meta-analysis. J. Gastrointest. Cancer 2024, 55, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Trindade, A.J.; Yachimski, P.; Benias, P.; Nieto, J.; Manvar, A.; Ho, S.; Esnakula, A.; Gamboa, A.; Sethi, A.; et al. Histologic analysis of endoscopic ultrasound-guided through the needle microforceps biopsies accurately identifies mucinous pancreas cysts. Clin. Gastroenterol. Hepatol. 2019, 17, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Rift, C.V.; Scheie, D.; Toxvaerd, A.; Kovacevic, B.; Klausen, P.; Vilmann, P.; Hansen, C.P.; Lund, E.L.; Hasselby, J.P. Diagnostic accuracy of EUS-guided through-the-needle-biopsies and simultaneously obtained fine needle aspiration for cytology from pancreatic cysts: A systematic review and meta-analysis. Pathol. Res. Pract. 2021, 220, 153368. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Kovacevic, B.; Yang, D.; Vilas-Boas, F.; Martínez-Moreno, B.; Stigliano, S.; Rizzatti, G.; Sacco, M.; Arevalo-Mora, M.; Villarreal-Sanchez, L.; et al. Predictors of adverse events after endoscopic ultrasound-guided through-the-needle biopsy of pancreatic cysts: A recursive partitioning analysis. Endoscopy 2022, 54, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Nass, K.J.; Zwager, L.W.; Van Der Vlugt, M.; Dekker, E.; Bossuyt, P.M.M.; Ravindran, S.; Thomas-Gibson, S.; Fockens, P. Novel classification for adverse events in GI endoscopy: The AGREE classification. Gastrointest. Endosc. 2022, 95, 1078–1085.e8. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Muthusamy, V.R.; McGrath, C.M.; Sepulveda, A.R.; Das, A.; Messersmith, W.; Kochman, M.L.; Shah, J. AGA White Paper: Optimizing Endoscopic Ultrasound-Guided Tissue Acquisition and Future Directions. Clin. Gastroenterol. Hepatol. 2018, 16, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Arvanitakis, M.; Crinò, S.F.; Fabbri, C.; Fornelli, A.; Leeds, J.; Archibugi, L.; Carrara, S.; Dhar, J.; Gkolfakis, P.; et al. Endoscopic ultrasound-guided tissue sampling: European Society of Gastrointestinal Endoscopy (ESGE) Technical and Technology Review. Endoscopy 2025, 57, 390–418. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A.; Fabbri, C.; Crinò, S.F.; Correale, L.; Chiarello, G.; Barresi, L.; Van Velthuysen, M.L.; Poley, J.W.; Rahal, D.; Carrara, S.; et al. Interobserver agreement among expert pathologists on through-the-needle microforceps biopsy samples for evaluation of pancreatic cystic lesions. Gastrointest. Endosc. 2019, 90, 784–792.e4. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, B.; Antonelli, G.; Klausen, P.; Hassan, C.; Larghi, A.; Vilmann, P.; Karstensen, J.G. EUS-guided biopsy versus confocal laser endomicroscopy in patients with pancreatic cystic lesions: A systematic review and meta-analysis. Endosc. Ultrasound 2021, 10, 270–279. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.; Rustagi, T. Endoscopic ultrasound-guided through-the-needle microforceps biopsy improves diagnostic yield for pancreatic cystic lesions: A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E1280–E1290. [Google Scholar] [PubMed]
- Facciorusso, A.; Arevalo-Mora, M.; Conti Bellocchi, M.C.; Bernardoni, L.; Ramai, D.; Gkolfakis, P.; Loizzi, D.; Muscatiello, N.; Ambrosi, A.; Tartaglia, N.; et al. Impact of Antibiotic Prophylaxis on Infection Rate after Endoscopic Ultrasound Through-the-Needle Biopsy of Pancreatic Cysts: A Propensity Score-Matched Study. Diagnostics 2022, 12, 211. [Google Scholar] [PubMed]
- Ohtsuka, T.; Fernandez-del Castillo, C.; Furukawa, T.; Hijioka, S.; Jang, J.Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2024, 24, 255–270. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall n = 190 | Group A n = 100 | Group B n = 90 | p Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 52.1 ± 14.7 | 51.2 ± 14.9 | 53.5 ± 14.8 | 0.288 |
| Sex | 0.208 | |||
| Male | 49 (26%) | 22 (22%) | 27 (30%) | |
| Female | 141 (74%) | 78 (78%) | 63 (70%) | |
| BMI (kg/m2) | 25.3 ± 4.9 | 25.5 ± 5 | 25.1 ± 4.9 | 0.790 |
| CCI | 1 | 1 | 1 | NS |
| Imaging performed | NS | |||
| US | 88 (47.1%) | 59 (60.9%) | 29 (32.2%) | |
| CT | 102 (54.5%) | 43 (44.3%) | 59 (65.6%) | |
| MRI | 162 (86.6%) | 88 (90.7%) | 74 (82.2%) | |
| Previous EUS | 28 (15%) | 11 (11.3%) | 17 (18.9%) | |
| Size on cross-sectional (mm) | ||||
| Mean ± SD | 48.2 ± 3.5 | 45.7 ± 17.6 | 51.2 ± 28 | 0.114 |
| Suggested diagnosis on cross-sectional imaging * | ||||
| 0.004 | ||||
| Serous cystic neoplasm | 48 (25.2%) | 31 (31%) | 17 (18.8%) | |
| Mucinous cyst | 93 (48.9%) | 59 (59%) | 34 (37.7%) | |
| Not specified | 68 (35.2%) | 28 (28%) | 40 (44.4%) | |
| Other diagnoses | 6 (3.1%) | 1 (1%) | 5 (2.6%) |
| Variables | Overall n = 190 | Group A n = 100 | Group B n = 90 | p Value |
|---|---|---|---|---|
| Size of lesion (mm) on EUS | ||||
| Mean ± SD | 46.7 ± 19.5 | 44 ± 16.6 | 49.8 ± 22 | 0.044 |
| Site of lesion | 0.349 | |||
| Head/uncinate process | 56 (29.5%) | 32 (32%) | 24 (26.7%) | |
| Body/tail | 127 (66.8%) | 66 (66%) | 61 (67.7%) | |
| Extra pancreatic | 7 (3.7%) | 2 (2%) | 5 (5.6%) | |
| Morphology | 0.061 | |||
| Unilocular | 109 (57.4%) | 51 (51%) | 58 (64.4%) | |
| Oligocystic | 81 (42.6%) | 49 (49%) | 32 (35.6%) | |
| Cyst walls | 0.263 | |||
| Thin | 130 (68.4%) | 72 (72%) | 58 (64.4%) | |
| Thickened | 60 (31.6%) | 28 (28%) | 32 (35.6%) | |
| Cyst content | 0.118 | |||
| Anechoic | 100 (52.6%) | 58 (58%) | 42 (46.7%) | |
| Inhomogeneous | 90 (47.4%) | 42 (42%) | 48 (53.3%) | |
| Intracystic lesion | ||||
| No | 167 (87.9%) | 83 (83%) | 84 (93.3%) | |
| Yes | 23 (12.1%) | 17 (17%) | 6 (6.7%) | |
| Thickened septum/nodule | 6/17 | 6/11 | 0/6 | 0.029 |
| TTNB passes | ||||
| ≤2 passes | 74 (38.9%) | 25 (25%) | 49 (54.4%) | <0.001 |
| ≥3 passes | 116 (61.1%) | 75 (75%) | 41 (45.6%) | |
| Visible specimens (median) | 2 | |||
| Complete cyst aspiration | ||||
| Yes | 132 (69.5%) | 69 (69%) | 63 (70%) | |
| No | 58 (30.5%) | 31 (31%) | 27 (30%) | 0.881 |
| Antibiotic administration | ||||
| Yes | 149 (78.4%) | 87 (87%) | 62 (68.9%) | |
| No | 41 (21.6%) | 13 (13%) | 28 (31.1%) | 0.002 |
| Patient, Sex (Years) | Group A/B | Adverse Event | Severity * | Final Diagnosis |
|---|---|---|---|---|
| Female (57) | A | Fever/infection | II | MCN |
| Female (27) | A | Hypotension | I | MCN |
| Female (40) | A | Mild acute pancreatitis | II | MCN |
| Female (47) | A | Hematoma | I | SCN |
| Male (75) | A | Fever/infection | II | Undefined |
| Female (70) | A | Fever/infection | II | Undefined |
| Female (62) | A | Orticaria | II | SCN |
| Male (75) | A | Severe acute pancreatitis (ICU admission) | IIIb | IPMN |
| Female (63) | A | Necrotic pancreatitis with infected collections | IV | MCN |
| Female (65) | A | Xantogranulomatous reaction/gastric mass | IIIb | MCN |
| Female (45) | A | Mild pancreatitis | II | MCN |
| Female (65) | B | Mild pancreatitis | II | IPMN |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| AE Yes/No | p Value | p Value | OR (95% CI) | |
| Group | 0.004 | 0.023 | 11.5 (1.4–94.1) | |
| A | 11/89 | |||
| B | 1/89 | |||
| Complete aspiration | 0.833 | - | ||
| Yes | 5/127 | |||
| No | 3/55 | |||
| Antibiotic administration | 0.646 | - | ||
| Yes | 6/143 | |||
| No | 2/39 | |||
| Final diagnosis | 0.062 | 0.025 | 6.2 (1.2–32.1) | |
| Mucinous | 8/75 | |||
| Non-mucinous | 2/96 | |||
| Unknown | 2/17 | |||
| Overall | Group A | Group B | p Value | |
|---|---|---|---|---|
| Sensitivity, % (95% CI) | 82.6% (76–88) | 79% (68.9–87.1) | 86.4% (77–93.2) | 0.152 |
| Specificity, % (95% CI) | 83.3% (35.8–99.5) | 66.7% (9.4–99.1) | 100% (15.8–100) | 0.438 |
| Accuracy, % (95% CI) | 82.6% (76.2–87.9) | 78.6% [68.7–86.6] | 86.7% (77.5–3.2) | 0.124 |
| Final Diagnosis | Number (%) | Accuracy of TTNB | Surgery |
|---|---|---|---|
| SCN | 60 (31.6%) | 54/60 (90%) | 1/60 (1.6%) |
| MCN | 58 (30.5%) | 53/58 (91.4%) | 44/58 (75.8%) |
| IPMN | 16 (8.6%) | 5 /16 (31.2%) | 4/16 (25%) |
| SPN | 2 (1%) | 1/2 (50%) | 2/2 (100%) |
| Rare histotype | 31 (16.3%) | 26/31 (83.9%) | 14/28 (50%) |
| Pseudocyst | 6 (3.2%) | 4/6 (66.7%) | 3/6 (50%) |
| Undefined | 17 (8.9%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti Bellocchi, M.C.; Teso, M.V.; Spagnolo, S.; Manfrin, E.; Sina, S.; Pea, A.; de Pretis, N.; Salvia, R.; Frulloni, L.; Crinò, S.F. Through-the-Needle Biopsy Revisited: How Patient Selection and Standardization Reduce Adverse Events in Pancreatic Cyst Evaluation. Diagnostics 2025, 15, 3096. https://doi.org/10.3390/diagnostics15243096
Conti Bellocchi MC, Teso MV, Spagnolo S, Manfrin E, Sina S, Pea A, de Pretis N, Salvia R, Frulloni L, Crinò SF. Through-the-Needle Biopsy Revisited: How Patient Selection and Standardization Reduce Adverse Events in Pancreatic Cyst Evaluation. Diagnostics. 2025; 15(24):3096. https://doi.org/10.3390/diagnostics15243096
Chicago/Turabian StyleConti Bellocchi, Maria Cristina, Maria Vittoria Teso, Sofia Spagnolo, Erminia Manfrin, Sokol Sina, Antonio Pea, Nicolò de Pretis, Roberto Salvia, Luca Frulloni, and Stefano Francesco Crinò. 2025. "Through-the-Needle Biopsy Revisited: How Patient Selection and Standardization Reduce Adverse Events in Pancreatic Cyst Evaluation" Diagnostics 15, no. 24: 3096. https://doi.org/10.3390/diagnostics15243096
APA StyleConti Bellocchi, M. C., Teso, M. V., Spagnolo, S., Manfrin, E., Sina, S., Pea, A., de Pretis, N., Salvia, R., Frulloni, L., & Crinò, S. F. (2025). Through-the-Needle Biopsy Revisited: How Patient Selection and Standardization Reduce Adverse Events in Pancreatic Cyst Evaluation. Diagnostics, 15(24), 3096. https://doi.org/10.3390/diagnostics15243096









