Line-Field Confocal Optical Coherence Tomography of Basal Cell Carcinoma: Systematic Correlation with Histopathology
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient and Tumour Characteristics
3.2. Objective Evaluation
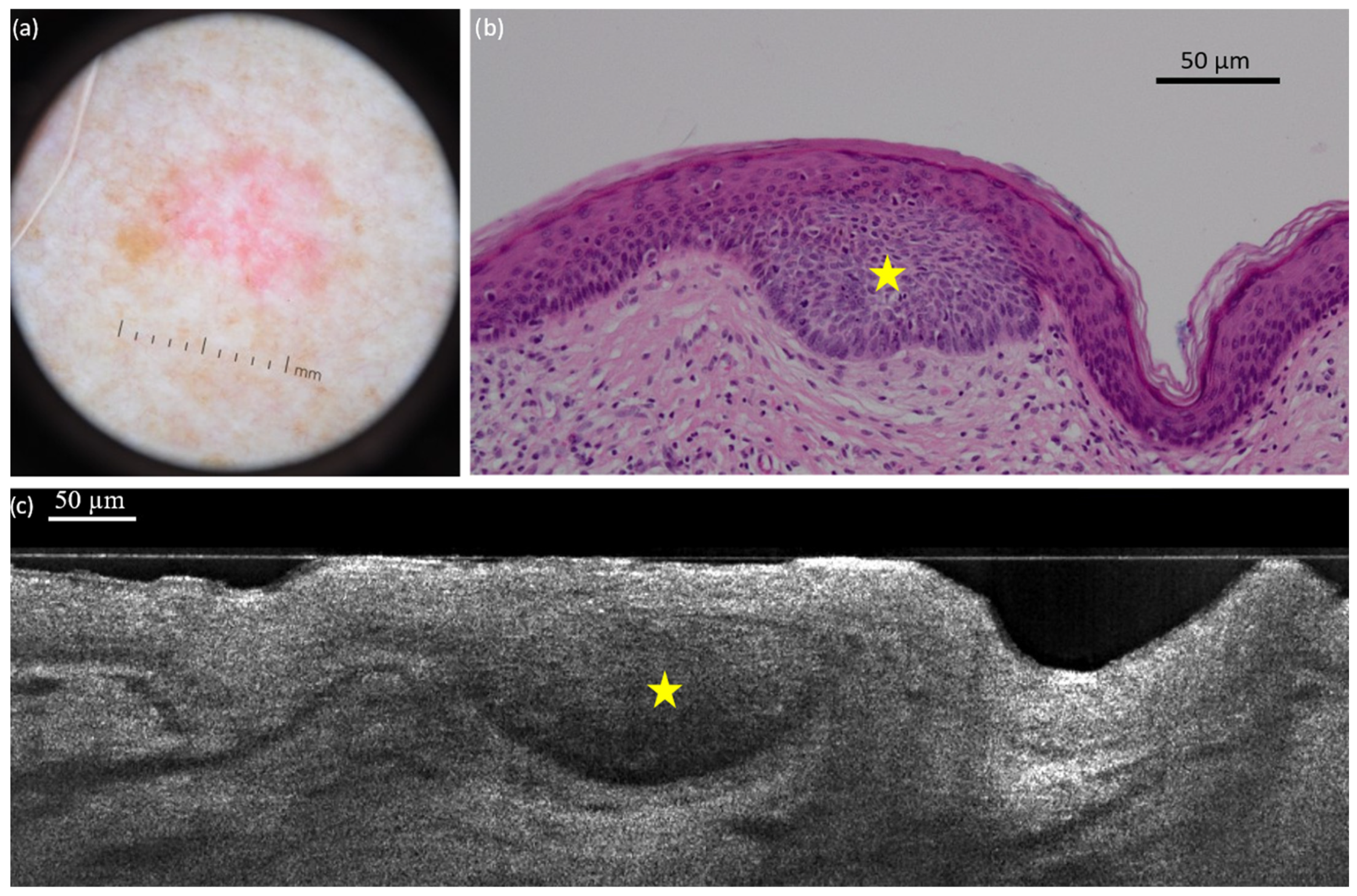
- The highest concordance rates (80–100%) between LC-OCT and histopathology were found for the following criteria: lobule (100%), blood vessels (100%), bright cells within epidermis (99.2%), lobule core (99.2%), lobule location (92.9%), bright cells within lobules (88.2%), disorganized epidermis (86.6%), round/ovoid morphology (81.9%), and disruption of the dermal-epidermal junction (DEJ) (81.1%).
- Intermediate concordance rates (50–80%) between LC-OCT and histopathology were found for: hemispheric morphology (73.2%), outer bright rim (72.4%), erosion/ulceration (71.4%), stromal stretching (65.4%), crust (64.6%), branched morphology (63.8%), parakeratosis (58.7%), stromal brightness (56.7%) and polymorphic morphology (55.9%).
- Low concordance rates (<50%) between LC-OCT and histopathology were found for: inner dark rim (48.0%) and palisading (7.9%). This last criterion was detected in 125/127 (98.4%) histopathological slides but in only 8/127 (6.3%) LC-OCT images/videos/3D reconstructions.
3.3. Subjective Evaluation
- Table 4 shows good concordance among the three observers in the subjective correlation assessment. Most cases were rated as ‘strong’ by all observers (86.6–98.4%), whereas the ‘weak’ category was rarely assigned. The overall LC-OCT/histopathology concordance rate was 81.1%. Interobserver agreement was slight but statistically significant (κ = 0.10, p = 0.02), reflecting variability in individual ratings.
- Table 5 summarizes the pairwise interobserver agreement of the subjective evaluations. A moderate agreement was observed between observer 1 and observer 3 (concordance rate 86.0%; κ = 0.41; p < 0.001). In contrast, only slight agreement was noted between observer 1 and observer 2 (concordance rate 80.1%; κ = 0.09; p = 0.09) and between observer 2 and observer 3 (concordance rate 85.4%; κ = 0.09; p = 0.13), despite relatively high concordance rates. This discrepancy between high concordance rates and low κ values likely reflects the well-recognized prevalence effect and differences in observer experience, both of which can substantially lower κ despite good absolute agreement.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCC | Basal cell carcinoma |
| sBCC | Superficial basal cell carcinoma |
| OCT | Optical coherence tomography |
| RCM | Reflectance confocal microscopy |
| HD-OCT | High-definition optical coherence tomography |
| LC-OCT | Line-field confocal optical coherence tomography |
| nBCC | Nodular basal cell carcinoma |
| iBCC | Infiltrative/morpheaform basal cell carcinoma |
| 3D | Three-dimensional |
| DEJ | Dermal-epidermal junction |
Appendix A
| Feature | Histopathology | LC-OCT |
|---|---|---|
| Lobule | Aggregates of basaloid cells growing into the dermis (BCC tumour islands) | Structure with variable shape, size and location within the dermis, characterized by a grey core usually surrounded by a darker rim |
| Core | Dense cellularity within the tumour island, composed of basaloid cells, immune cells, apoptotic bodies and mitotic figures | Grey, laminated structure at the core of the BCC lobule, orientated along the horizontal plane (millefeuille pattern) |
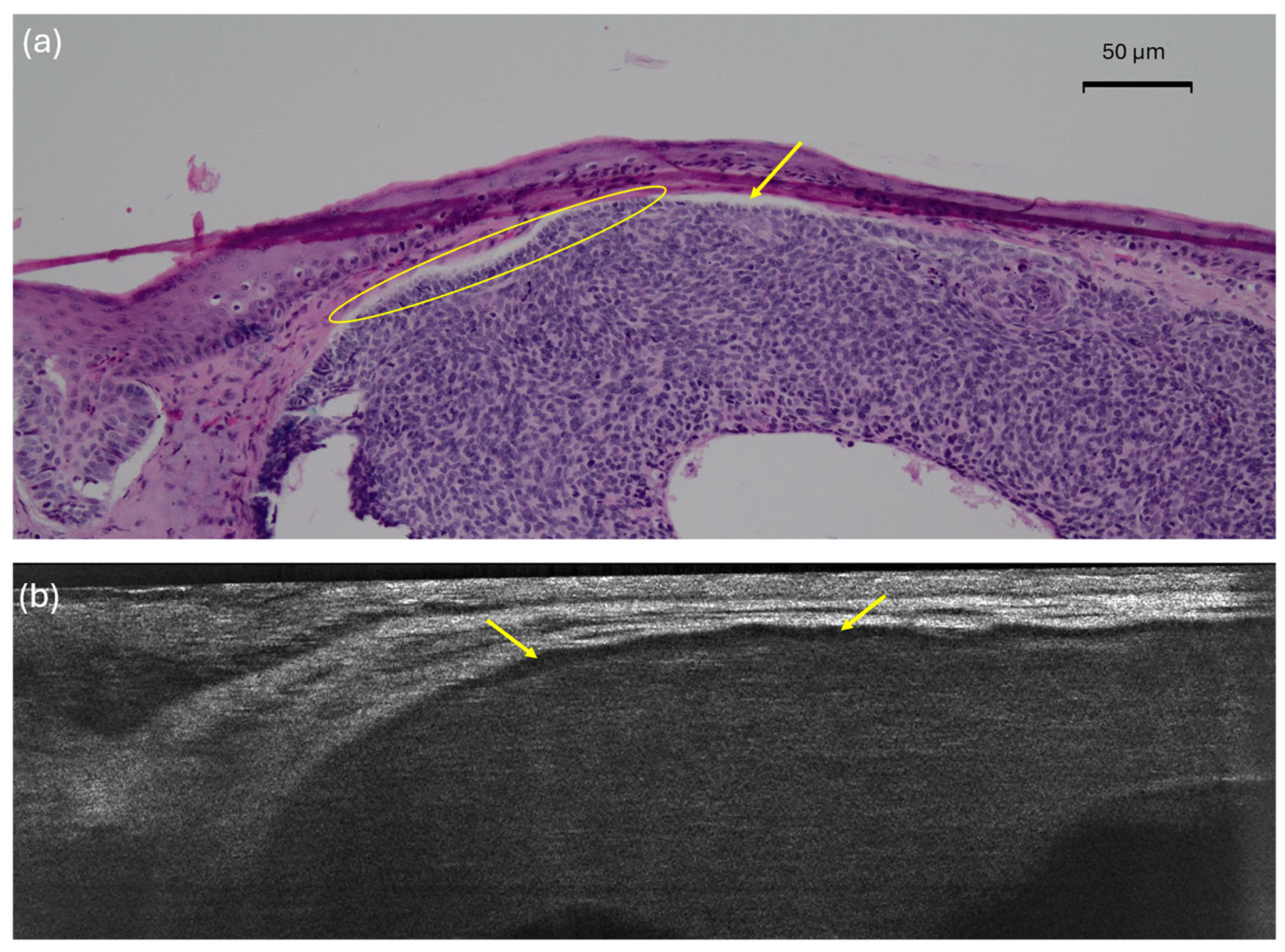
| Palisading | Single layer of basaloïd cells at the periphery of the lobule, arranged parallel to each other and forming a palisade | Single layer of long cells at the periphery of the lobule, arranged parallel to each other |
| Inner rim | Peritumoural mucin deposition | Dark inner rim surrounding the core of the lobule |
| Outer rim | Compression/alteration of the collagen fibres of the stroma by the tumour island (mass effect and tumour–stroma interaction) | Bright outer rim surrounding the lobule characterized by a brighter colour than the stroma |
| Lobule location | Location of the lobule related to the epidermis (0, connected; 1, both; 2, separated) | Location of the lobule related to the epidermis (0, connected; 1, both; 2, separated) |
| Round/ovoid | Lobule with clear contour definition | Lobule with clear contour definition |
| Hemispheric | Lobule leaning towards the epidermis | Lobule leaning towards the epidermis |
| Branched | Lobule divided into one or more subdivisions with progressive loss of contour definition | Lobule divided into one or more subdivisions with progressive loss of contour definition |
| Polymorphic | Lobule presenting more than one type of morphology | Lobule presenting more than one type of morphology |
| Blood vessels | Dermal blood vessels, particularly prominent when next to tumour islands (neo-angiogenesis); blood cell can be visualized within them | Well-defined, hypo-reflective structures of various shape/size localized within the dermis and especially next to lobules. In the LC–OCT in vivo acquisition modality and videos, hyper--reflective elements can be seen flowing within them |
| Stretching | Global distortion of the collagen/elastic fibres of the stroma due to the presence of tumour islands (mass effect and tumour–stroma interaction) | The dermis surrounding the lobules appears stretched, polarized in one direction |
| Brightness | Increased reflectivity of the stroma due to the presence of tumour islands (mass effect and tumour–stroma interaction) | The dermis surrounding the lobules appears whiter (brighter) than the overlying epidermis |
| Parakeratosis | Nucleated keratinocytes in the stratum corneum | Dark little roundish structures in the upper layer of the epidermis |
| Disorganized epidermis | Pleomorphism in the epidermis | Variability of size and shape of the keratinocytes’ nuclei within a particular layer of the epidermis |
| Disrupted dermal-epidermal junction | Loss of a clear separation between the epidermis and the dermis. It may be related to the presence of hemispheric lobules leaning towards the epidermis, ulceration, or crusts | Loss of a clear separation between the epidermis and the dermis. It may be related to the presence of hemispheric lobules leaning towards the epidermis, ulceration, or crusts |
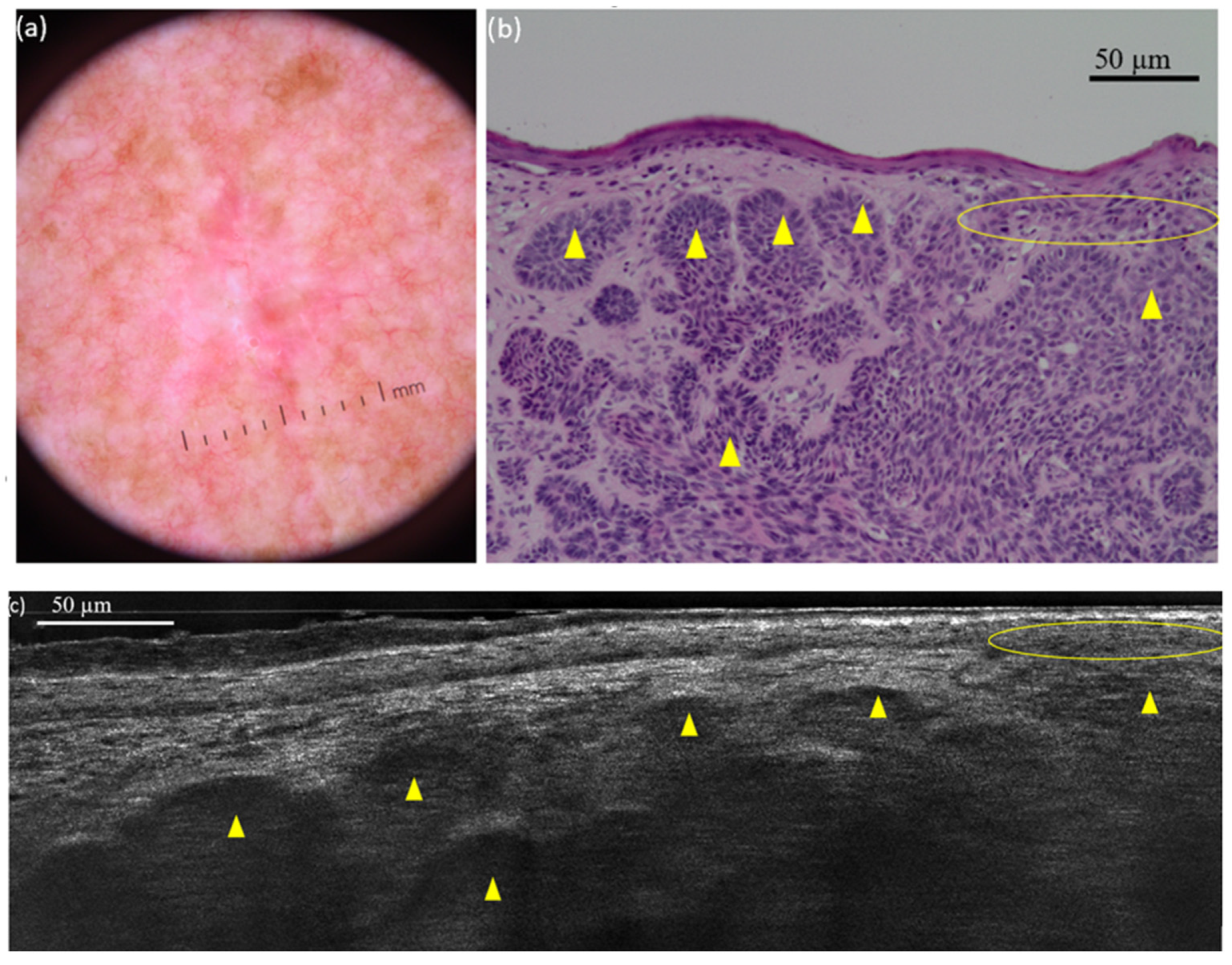
| Bright cells | Immunologically competent skin cells (Langerhans cells and granulocytes) and activated melanocytes | Hyper-reflective structures within the epidermis |
| Erosion/ulceration | Partial/complete loss of the epidermis (without/with involvement of the basal membrane) | Partial/complete loss of continuity of the epidermis (without/with involvement of the dermal-epidermal junction) |
| Crust | Dried material (sebum, pus, blood, serum) usually mixed with epithelial debris, at the surface of the lesion | Accumulation of hyper- and hypo-reflective structures overlying the epidermis |
References
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef]
- Suppa, M.; Micantonio, T.; Di Stefani, A.; Soyer, H.P.; Chimenti, S.; Fargnoli, M.C.; Peris, K. Dermoscopic variability of basal cell carcinoma according to clinical type and anatomic location. J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 1732–1741. [Google Scholar] [CrossRef]
- Roozeboom, M.H.; Kreukels, H.; Nelemans, P.J.; Mosterd, K.; Winnepenninckx, V.J.L.; Abdul Hamid, M.A.; de Haas, E.R.; Kelleners-Smeets, N.W. Subtyping basal cell carcinoma by clinical diagnosis versus punch biopsy. Acta Derm Venereol. 2015, 95, 996–998. [Google Scholar] [CrossRef]
- Dorrell, D.N.; Strowd, L.C. Skin Cancer Detection Technology. Dermatol Clin. 2019, 37, 527–536. [Google Scholar] [CrossRef]
- Dasgeb, B.; Kainerstorfer, J.; Mehregan, D.; Van Vreede, A.; Gandjbakhche, A. An introduction to primary skin imaging. Int. J. Dermatol. 2013, 52, 1319–1330. [Google Scholar] [CrossRef]
- Hussain, A.A.; Themstrup, L.; Jemec, G.B.E. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch. Dermatol. Res. 2015, 307, 1–10. [Google Scholar] [CrossRef]
- Boone, M.A.L.M.; Suppa, M.; Pellacani, G.; Marneffe, A.; Miyamoto, M.; Alarcon, I.; Ruini, C.; Hofmann-Wellenhof, R.; Malvehy, J.; Jemec, G.B.; et al. High-definition optical coherence tomography algorithm for discrimination of basal cell carcinoma from clinical BCC imitators and differentiation between common subtypes. J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 1771–1780. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. In vivo reflectance confocal microscopy image interpretation for the dermatopathologist. J. Cutan. Pathol. 2018, 45, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; Del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J. Biomed. Opt. 2018, 23, 106007. [Google Scholar] [CrossRef] [PubMed]
- Latriglia, F.; Ogien, J.; Tavernier, C.; Fischman, S.; Suppa, M.; Perrot, J.L.; Dubois, A. Line-Field Confocal Optical Coherence Tomography (LC-OCT) for Skin Imaging in Dermatology. Life 2023, 13, 2268. [Google Scholar] [CrossRef] [PubMed]
- Niculet, E.; Craescu, M.; Rebegea, L.; Bobeica, C.; Nastase, F.; Lupasteanu, G.; Stan, D.J.; Chioncel, V.; Anghel, L.; Lungu, M.; et al. Basal cell carcinoma: Comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (Review). Exp. Ther. Med. 2022, 23, 60. [Google Scholar] [CrossRef]
- Reddy, N.; Nguyen, B.T. The utility of optical coherence tomography for diagnosis of basal cell carcinoma: A quantitative review. Br. J. Dermatol. 2019, 180, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.L.M.; Norrenberg, S.; Jemec, G.B.E.; Del Marmol, V. Imaging of basal cell carcinoma by high-definition optical coherence tomography: Histomorphological correlation. A pilot study. Br. J. Dermatol. 2012, 167, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Lallas, A.; Kyrgidis, A.; Rabinovitz, H.; Moscarella, E.; Ciardo, S.; Zalaudek, I.; Oliviero, M.; Losi, A.; Gonzalez, S.; et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J. Am. Acad. Dermatol. 2014, 71, 716–724.e1. [Google Scholar] [CrossRef]
- Suppa, M.; Fontaine, M.; Dejonckheere, G.; Cinotti, E.; Yélamos, O.; Diet, G.; Tognetti, L.; Miyamoto, M.; Orte Cano, C.; Perez-Anker, J.; et al. Line-field confocal optical coherence tomography of basal cell carcinoma: A descriptive study. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, 1099–1110. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E. Line-field optical coherence tomography: In vivo diagnosis of basal cell carcinoma subtypes compared with histopathology. Clin. Exp. Dermatol. 2021, 46, 1471–1481. [Google Scholar] [CrossRef]
- Mtimet, L.; Boussingault, L.; Aktas, D.; Fontaine, M.; Orte Cano, C.; Diet, G.; Lenoir, C.; Miyamoto, M.; Cinotti, E.; Tognetti, L.; et al. Line-field confocal optical coherence tomography of basal cell carcinoma: A retrospective study on diagnostic performance. J. Eur. Acad. Dermatol. Venereol. JEADV 2024, 39, 1468–1480. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Agero, A.L.C.; Busam, K.J.; Benvenuto-Andrade, C.; Scope, A.; Gill, M.; Marghoob, A.A.; González, S.; Halpern, A.C. Reflectance confocal microscopy of pigmented basal cell carcinoma. J. Am. Acad. Dermatol. 2006, 54, 638–643. [Google Scholar] [CrossRef]
- Coleman, A.J.; Richardson, T.J.; Orchard, G.; Uddin, A.; Choi, M.J.; Lacy, K.E. Histological correlates of optical coherence tomography in non-melanoma skin cancer. Skin Res. Technol. 2013, 19, 10–19. [Google Scholar] [CrossRef]
- Pelosini, L.; Smith, H.B.; Schofield, J.B.; Meeckings, A.; Dithal, A.; Khandwala, M. A novel imaging approach to periocular basal cell carcinoma: In vivo optical coherence tomography and histological correlates. Eye 2015, 29, 1092–1098. [Google Scholar] [CrossRef]
- Lupu, M.; Popa, I.M.; Voiculescu, V.M.; Boda, D.; Caruntu, C.; Zurac, S.; Giurcaneanu, C. A Retrospective Study of the Diagnostic Accuracy of In Vivo Reflectance Confocal Microscopy for Basal Cell Carcinoma Diagnosis and Subtyping. J. Clin. Med. 2019, 8, 449. [Google Scholar] [CrossRef]
- Peppelman, M.; Wolberink, E.A.W.; Blokx, W.A.M.; van de Kerkhof, P.C.M.; van Erp, P.E.J.; Gerritsen, M.J.P. In vivo diagnosis of basal cell carcinoma subtype by reflectance confocal microscopy. Dermatology 2013, 227, 255–262. [Google Scholar] [CrossRef] [PubMed]
- McCormack, C.J.; Kelly, J.W.; Dorevitch, A.P. Differences in age and body site distribution of the histological subtypes of basal cell carcinoma. A possible indicator of differing causes. Arch. Dermatol. 1997, 133, 593–596. [Google Scholar] [CrossRef]
- Scrivener, Y.; Grosshans, E.; Cribier, B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br. J. Dermatol. 2002, 147, 41–47. [Google Scholar] [CrossRef]
- Lenoir, C.; Diet, G.; Cinotti, E.; Tognetti, L.; Orte Cano, C.; Rocq, L.; Trépant, A.L.; Monnier, J.; Perez-Anker, J.; Rubegni, P.; et al. Line-field confocal optical coherence tomography of sebaceous hyperplasia: A case series. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, pe509. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, C.; Perez-Anker, J.; Diet, G.; Tognetti, L.; Cinotti, E.; Trépant, A.l.; Rubegni, P.; Puig, S.; Perrot, J.L.; Malvehy, J.; et al. Line-field confocal optical coherence tomography of benign dermal melanocytic proliferations: A case series. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, e399–e401. [Google Scholar] [CrossRef]
- Donelli, C.; Suppa, M.; Tognetti, L.; Perrot, J.L.; Calabrese, L.; Pérez-Anker, J.; Malvehy, J.; Rubegni, P.; Cinotti, E. Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas: Real-Life Data over Three Years. Curr. Oncol. 2023, 30, 8853–8864. [Google Scholar] [CrossRef] [PubMed]
- Gust, C.; Schuh, S.; Welzel, J.; Daxenberger, F.; Hartmann, D.; French, L.E.; Ruini, C.; Sattler, E.C. Line-Field Confocal Optical Coherence Tomography Increases the Diagnostic Accuracy and Confidence for Basal Cell Carcinoma in Equivocal Lesions: A Prospective Study. Cancers 2022, 14, 1082. [Google Scholar] [CrossRef]
- Altamura, D.; Menzies, S.W.; Argenziano, G.; Zalaudek, I.; Soyer, H.P.; Sera, F.; Avramidis, M.; DeAmbrosis, K.; Fargnoli, M.C.; Peris, K. Dermatoscopy of basal cell carcinoma: Morphologic variability of global and local features and accuracy of diagnosis. J. Am. Acad. Dermatol. 2010, 62, 67–75. [Google Scholar] [CrossRef]
- Lallas, A.; Tzellos, T.; Kyrgidis, A.; Apalla, Z.; Zalaudek, I.; Karatolias, A.; Ferrara, G.; Piana, S.; Longo, C.; Moscarella, E.; et al. Accuracy of dermoscopic criteria for discriminating superficial from other subtypes of basal cell carcinoma. J. Am. Acad. Dermatol. 2014, 70, 303–311. [Google Scholar] [CrossRef]
- Cappilli, S.; Suppa, M.; Ricci, C.; Del Marmol, V.; Peris, K.; Di Stefani, A. Line-field confocal optical coherence tomography of cutaneous vascular lesions: Morphological assessment and histopathological correlations. J. Eur. Acad. Dermatol. Venereol. JEADV 2023, 37, 1664–1668. [Google Scholar] [CrossRef]
- Lenoir, C.; Suppa, M.; Puig, S.; Del Marmol, V.; Albero, R.; Also, L.; Orte Cano, C.; Diet, G.; Fontaine, M.; Tognetti, L.; et al. Correlation of Vascular Patterns in Skin Lesions with LC-OCT and Dermoscopy with a Tridimensional Perspective: A Pilot Study. Dermatol. Pract. Concept. 2025, 15, 5297. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Roewert-Huber, J.; González, S.; Rius-Diaz, F.; Stockfleth, E.; Kanitakis, J. Peritumoral clefting in basal cell carcinoma: Correlation of in vivo reflectance confocal microscopy and routine histology. J. Cutan. Pathol. 2011, 38, 190–195. [Google Scholar] [CrossRef]
- Gambichler, T.; Plura, I.; Kampilafkos, P.; Valavanis, K.; Sand, M.; Bechara, F.G.; Stücker, M. Histopathological correlates of basal cell carcinoma in the slice and en face imaging modes of high-definition optical coherence tomography. Br. J. Dermatol. 2014, 170, 1358–1361. [Google Scholar] [CrossRef]
- Blasdale, C.; Charlton, F.G.; Weatherhead, S.C.; Ormond, P.; Lawrence, C.M. Effect of tissue shrinkage on histological tumour-free margin after excision of basal cell carcinoma. Br. J. Dermatol. 2010, 162, 607–610. [Google Scholar] [CrossRef]
- Orte Cano, C.; Suppa, M.; Del Marmol, V. Where Artificial Intelligence Can Take Us in the Management and Understanding of Cancerization Fields. Cancers 2023, 15, 5264. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; D’Onghia, M.; Cartocci, A.; Lo Conte, S.; Barbarossa, L.; Tavernier, C.; Rubegni, G.; Tognetti, L.; Suppa, M.; Rubegni, P. Line-Field Confocal Optical Coherence Tomography: Is One Hour of Training Sufficient for Diagnosing Basal Cell Carcinoma? Cancers 2025, 17, 826. [Google Scholar] [CrossRef]
- Vatamanesku, I.; Parasca, S.V.; Parasca, O.M.; Vaida, F.A.; Mehedinţi, M.C.; Grosu, F.; Ciurea, M.E. Basal cell carcinoma of the nasal pyramid excision margins: A retrospective study. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2019, 60, 1261–1268. [Google Scholar]
- Boostani, M.; Pellacani, G.; Wortsman, X.; Suppa, M.; Goldust, M.; Cantisani, C.; Pietkiewicz, P.; Lőrincz, K.; Bánvölgyi, A.; Wikonkál, N.M.; et al. FDA and EMA-approved noninvasive imaging techniques for basal cell carcinoma subtyping: A systematic review. JAAD Int. 2025, 21, 73–86. [Google Scholar] [CrossRef]
- Fischman, S.; Viel, T.; Perrot, J.L.; Pérez-Anker, J.; Suppa, M.; Cinotti, E.; Lenoir, C.; Orte Cano, C.; Welzel, J.; Schuh, S.; et al. AI-assisted basal cell carcinoma diagnosis with LC-OCT: A multicentric retrospective study. J. Eur. Acad. Dermatol. Venereol. JEADV 2025. [Google Scholar] [CrossRef] [PubMed]
- Niculet, E.; Bobeica, C.; Craescu, M.; Nicolescu, A.C.; Tocu, G.; Onisor, C.; Arbune, M.; Tatu, A.L. Multimodal Considerations Concerning Basal Cell Carcinoma Clefting—Profile of Structural and Aggressive Traits—Perspectives. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2087–2095. [Google Scholar] [CrossRef]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-Field Confocal Optical Coherence Tomography May Enhance Monitoring of Superficial Basal Cell Carcinoma Treated with Imiquimod 5% Cream: A Pilot Study. Cancers 2021, 13, 4913. [Google Scholar] [CrossRef]
- Adan, F.; Nelemans, P.J.; Essers, B.A.B.; Brinkhuizen, T.; Dodemont, S.R.P.; Kessels, J.P.H.M.; Quaedvlieg, P.J.F.; Dermont, G.J.; Winnepenninckx, V.J.L.; Abdul Hamid, M.; et al. Optical coherence tomography versus punch biopsy for diagnosis of basal cell carcinoma: A multicentre, randomised, non-inferiority trial. Lancet Oncol. 2022, 23, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, O.; Schwartz, M.; Feldman, E.; Bienenfeld, A.; Bieber, A.K.; Ellis, J.; Alapati, U.; Lebwohl, M.; Siegel, D.M. Evaluation of Optical Coherence Tomography as a Means of Identifying Earlier Stage Basal Cell Carcinomas while Reducing the Use of Diagnostic Biopsy. J. Clin. Aesthetic Dermatol. 2015, 8, 14–20. [Google Scholar]
- González-Valdés, S.; Bustos, S.; Donoso, F.; Caussade, M.C.; Antunez-Lay, A.; Droppelmann, K.; Uribe, P.; Pellacani, G.; Abarzúa-Araya, A.; Navarrete-Dechent, C. Reflectance confocal microscopy reduces biopsies in adults and children: A prospective real-life study. J. Am. Acad. Dermatol. 2025. [Google Scholar] [CrossRef]
- Damae Medical. Evaluation of the Efficiency and Economic Impact of LC-OCT (Line-field Confocal Optical Coherence Tomography) for the Diagnosis and Management of Basal Cell Carcinomas (ECOBASO) [Internet]. ClinicalTrials.gov. Report No.: NCT06271603. February 2024. Available online: https://clinicaltrials.gov/study/NCT06271603 (accessed on 11 November 2025).
- Bruno, G.M.; Di Matteo, S.; Longo, C.; Stanganelli, I.; Farnetani, F.; Borsari, S.; Mazzoni, L.; Ciardo, S.; Raucci, M.; Magi, S.; et al. Cost-Benefit Analysis of in vivo Reflectance Confocal Microscopy for Melanoma Diagnosis in a Real-World Clinical Setting. Risk Manag. Healthc. Policy 2025, 18, 163–172. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 127) | Head/Neck (n = 62) | Trunk (n = 37) | Limbs (n = 28) | |
|---|---|---|---|---|
| Pure BCC subtypes | 91 (71.7) | 38 (61.3) | 28 (75.7) | 25 (89.3) |
| Superficial | 59 (46.5) | 11 (17.7) | 24 (64.9) | 24 (85.7) |
| Nodular | 27 (21.3) | 22 (35.5) | 4 (10.8) | 1 (3.6) |
| Infiltrative | 5 (3.9) | 5 (8.1) | 0 (0) | 0 (0) |
| Mixed BCC subtypes | 36 (28.3) | 24 (38.7) | 9 (24.3) | 3 (10.7) |
| Superficial and nodular | 24 (18.9) | 14 (22.6) | 7 (18.9) | 3 (10.7) |
| Nodular and infiltrative | 7 (5.5) | 5 (8.1) | 2 (5.4) | 0 (0) |
| Superficial and infiltrative | 3 (2.4) | 3 (4.8) | 0 (0) | 0 (0) |
| Superficial, nodular, and infiltrative | 2 (1.6) | 2 (3.2) | 0 (0) | 0 (0) |
| Histopathology | LC-OCT | Concordance (%) | |
|---|---|---|---|
| Lobule | 127 (100) | 127 (100) | 100 |
| Lobule composition | |||
| Core a | 127 (100) | 126 (99.2) | 99.2 |
| Palisading | 125 (98.4) | 8 (6.3) | 7.9 |
| Inner rim b | 94 (74.0) | 62 (48.8) | 48.0 |
| Outer rim c | 123 (96.9) | 90 (70.9) | 72.4 |
| Lobule location | 92.9 | ||
| Separated from epidermis | 16 (12.6) | 18 (14.2) | |
| Connected to epidermis | 45 (35.4) | 41 (32.3) | |
| Both | 66 (52.0) | 68 (53.5) | |
| Lobule morphology | |||
| Round/ovoid | 107 (84.3) | 92 (72.4) | 81.9 |
| Hemispheric | 81 (63.8) | 69 (54.3) | 73.2 |
| Branched | 50 (39.4) | 20 (15.8) | 63.8 |
| Polymorphic | 102 (80.3) | 56 (44.1) | 55.9 |
| Blood vessels | 127 (100) | 127 (100) | 100 |
| Stroma involvement | |||
| Stretching | 53 (41.7) | 67 (52.8) | 65.4 |
| Brightness d | 0 (100) | 55 (43.3) | 56.7 |
| Epidermal changes | |||
| Parakeratosis | 55 (43.3) | 14 (15.2) | 58.7 |
| Disorganized epidermis | 11 (8.7) | 8 (6.3) | 86.6 |
| Disrupted DEJ | 105 (82.7) | 103 (81.1) | 81.1 |
| Bright cells e | |||
| within epidermis | 127 (100) | 126 (99.2) | 99.2 |
| within lobules | 127 (100) | 112 (88.2) | 88.2 |
| Other | |||
| Erosion/ulceration | 40 (31.5) | 8 (6.3) | 71.7 |
| Crust | 45 (35.4) | 14 (11.0) | 64.6 |
| Histopathology | Observer 1 | Observer 2 | Observer 3 | ||||
|---|---|---|---|---|---|---|---|
| LC-OCT | Concordance (%) | LC-OCT | Concordance (%) | LC-OCT | Concordance (%) | ||
| Lobule | 127 (100) | 127 (100) | 100 | 127 (100) | 100 | 127 (100) | 100 |
| Lobule composition | |||||||
| Core a | 127 (100) | 125 (98.4) | 98.4 | 120 (94.5) | 94.5 | 127 (100) | 100 |
| Palisading | 125 (98.4) | 3 (2.4) | 3.9 | 9 (7.1) | 8.7 | 32 (25.2) | 26.8 |
| Inner rim b | 94 (74.0) | 60 (47.2) | 52.8 | 20 (15.8) | 29.1 | 119 (93.7) | 69.3 |
| Outer rim c | 123 (96.9) | 111 (87.4) | 85.8 | 53 (41.7) | 43.3 | 88 (69.3) | 70.9 |
| Lobule location | |||||||
| Separated from epidermis | 16 (12.6) | 19 (15.0) | 18 (14.2) | 22 (17.3) | |||
| Connected to epidermis | 45 (35.4) | 34 (26.8) | 40 (31.5) | 45 (35.4) | |||
| Both | 66 (52.0) | 74 (58.3) | 89.8 | 69 (54.3) | 93.1 | 60 (47.2) | 91.7 |
| Lobule morphology | |||||||
| Round/ovoid | 107 (84.3) | 104 (81.9) | 81.9 | 87 (68.5) | 79.5 | 90 (70.9) | 81.9 |
| Hemispheric | 81 (63.8) | 77 (60.6) | 71.7 | 33 (26) | 59.1 | 79 (62.2) | 73.2 |
| Branched | 50 (39.4) | 41 (32.3) | 63 | 14 (11.0) | 62.2 | 19 (15.0) | 63.0 |
| Polymorphic | 102 (80.3) | 89 (70.1) | 66.1 | 6 (4.7) | 22.8 | 61 (48.0) | 59.8 |
| Blood vessels | 127 (100) | 127 (100) | 100 | 127 (100) | 100 | 127 (100) | 100 |
| Stroma involvement | |||||||
| Stretching | 53 (41.7) | 59 (46.46) | 62.2 | 84 (66.1) | 56.7 | 64 (50.4) | 64.6 |
| Brightness d | 0 (100) | 35 (27.56) | 72.4 | 53 (41.7) | 58.3 | 87 (68.5) | 31.5 |
| Epidermal changes | |||||||
| Parakeratosis | 55 (43.3) | 27 (21.3) | 59.1 | 4 (3.2) | 56.7 | 29 (22.8) | 54.3 |
| Disorganized epidermis | 11 (8.7) | 20 (15.8) | 78.7 | 2 (1.6) | 89.8 | 14 (11.0) | 83.5 |
| Disrupted DEJ | 105 (82.7) | 81 (63.8) | 68.5 | 102 (80.3) | 81.9 | 93 (73.2) | 74.8 |
| Bright cells e | |||||||
| within epidermis | 127 (100) | 123 (96.9) | 96.9 | 123 (96.8) | 96.9 | 103 (81.1) | 81.1 |
| within lobules | 127 (100) | 84 (100) | 66.9 | 121 (95.3) | 94.5 | 109 (85.8) | 85.8 |
| Other | |||||||
| Erosion/ulceration | 40 (31.5) | 17 (13.4) | 69.3 | 3 (2.4) | 69.3 | 15 (11.8) | 67.7 |
| Crust | 45 (35.4) | 39 (30.7) | 65.4 | 0 (0) | 64.6 | 24 (18.9) | 64.6 |
| Observer 1 | Observer 2 | Observer 3 | κ | p-Value | |
|---|---|---|---|---|---|
| Subjective correlation | |||||
| Weak | 17 (13.4) | 2 (1.6) | 10 (7.9) | ||
| Strong | 110 (86.6) | 125 (98.4) | 117 (92.1) | ||
| All evaluations | 127 (100) | 127 (100) | 127 (100) | 0.10 | 0.02 |
| Observer 1 | Observer 2 | Observer 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Agreement (%) | κ | p-Value | Agreement (%) | κ | p-Value | Agreement (%) | κ | p-Value | |
| Observer 1 | 80.1 | 0.09 | 0.09 | 86.0 | 0.41 | <0.001 | |||
| Observer 2 | 80.1 | 0.09 | 0.09 | 85.4 | 0.09 | 0.13 | |||
| Observer 3 | 86.0 | 0.41 | <0.001 | 85.4 | 0.09 | 0.13 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boussingault, L.; Lenoir, C.; Stefani, A.D.; Cappilli, S.; Fontaine, M.; Diet, G.; Miyamoto, M.; Cinotti, E.; Tognetti, L.; Pérez-Anker, J.; et al. Line-Field Confocal Optical Coherence Tomography of Basal Cell Carcinoma: Systematic Correlation with Histopathology. Diagnostics 2025, 15, 3059. https://doi.org/10.3390/diagnostics15233059
Boussingault L, Lenoir C, Stefani AD, Cappilli S, Fontaine M, Diet G, Miyamoto M, Cinotti E, Tognetti L, Pérez-Anker J, et al. Line-Field Confocal Optical Coherence Tomography of Basal Cell Carcinoma: Systematic Correlation with Histopathology. Diagnostics. 2025; 15(23):3059. https://doi.org/10.3390/diagnostics15233059
Chicago/Turabian StyleBoussingault, Lucas, Clément Lenoir, Alessandro Di Stefani, Simone Cappilli, Margot Fontaine, Gwendoline Diet, Makiko Miyamoto, Elisa Cinotti, Linda Tognetti, Javiera Pérez-Anker, and et al. 2025. "Line-Field Confocal Optical Coherence Tomography of Basal Cell Carcinoma: Systematic Correlation with Histopathology" Diagnostics 15, no. 23: 3059. https://doi.org/10.3390/diagnostics15233059
APA StyleBoussingault, L., Lenoir, C., Stefani, A. D., Cappilli, S., Fontaine, M., Diet, G., Miyamoto, M., Cinotti, E., Tognetti, L., Pérez-Anker, J., Malvehy, J., Puig, S., Perrot, J.-L., Peris, K., del Marmol, V., & Suppa, M. (2025). Line-Field Confocal Optical Coherence Tomography of Basal Cell Carcinoma: Systematic Correlation with Histopathology. Diagnostics, 15(23), 3059. https://doi.org/10.3390/diagnostics15233059







