Temporal Triangular Alopecia—Clinical and Dermoscopic Features of a Rare Entity
Abstract

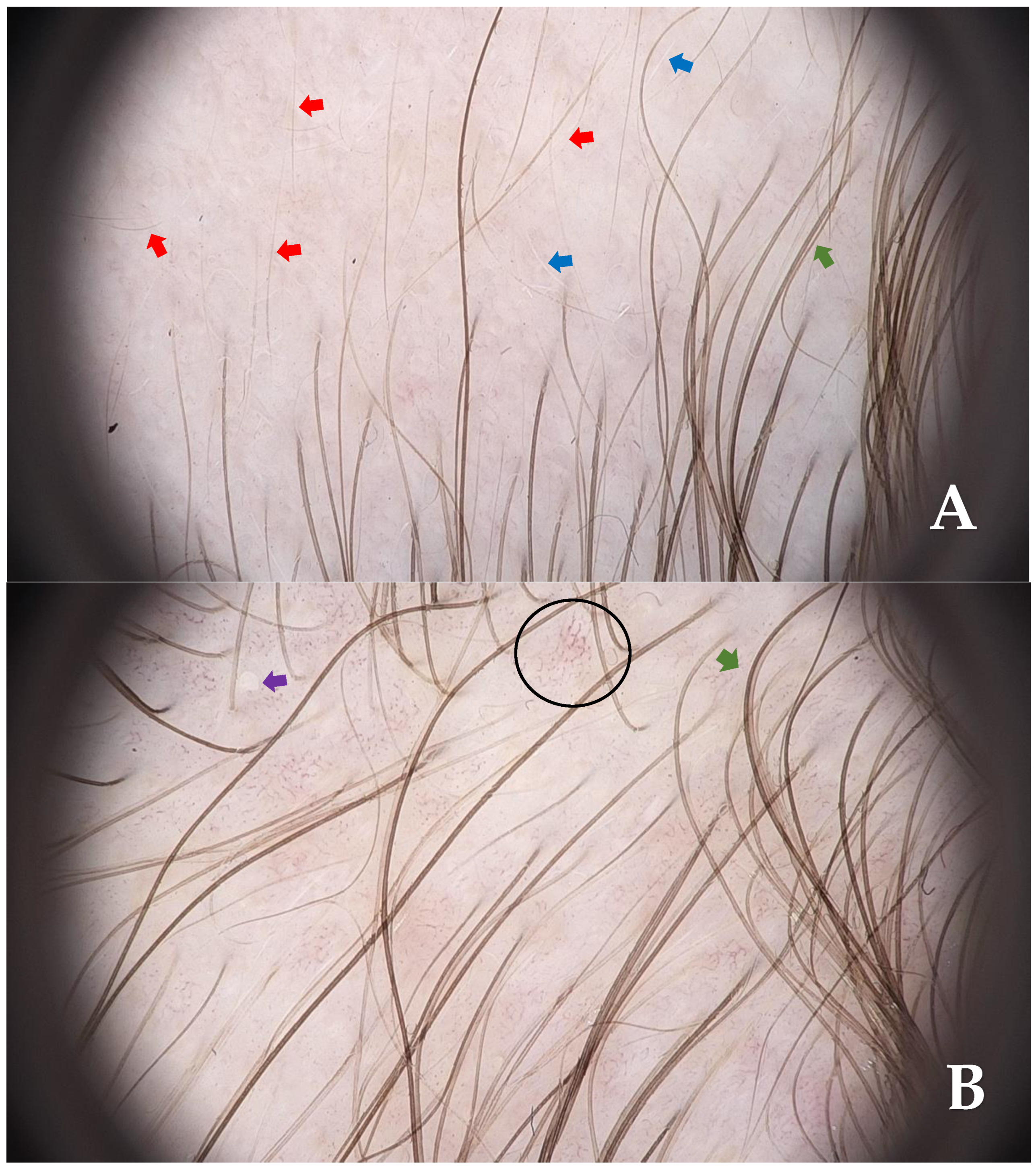
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-Crehuet, P.; Vaño-Galván, S.; Martorell-Calatayud, A.; Arias-Santiago, S.; Grimalt, R.; Camacho-Martínez, F.M. Clinical and trichoscopic characteristics of temporal triangular alopecia: A multicenter study. J. Am. Acad. Dermatol. 2016, 75, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Irisawa, R.; Tsuboi, R. Temporal triangular alopecia and a review of 52 past cases. J. Dermatol. 2010, 37, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.R.; Tandel, J.J.; Nair, P.A. Congenital triangular alopecia: A case report. Int. J. Trichology 2020, 12, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Yesudian, P.D. Congenital triangular alopecia. Int. J. Trichology 2015, 7, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Villa Lario, A.; Ferrando, J.; Moreno-Arrones, O.M.; Gómez-Zubiaur, A. Atypical congenital triangular alopecia (Brauer nevus): Case report and review of literature in occipital and mid-frontal localizations. Ski. Appendage Disord. 2021, 7, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.; Wilson, B.; Lefesvre, P.; Poorten, V.V.; Kirkham, N.; Mitra, D.; Verellen-Dumoulin, C.; Devriendt, K. Atypical findings in three patients with Pai syndrome and literature review. Am. J. Med. Genet. A 2012, 158A, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Nakajima, T.; Itami, S. Temporal triangular alopecia: Trichoscopic diagnosis. J. Dermatol. 2012, 39, 572–574. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, M.J.; Rodríguez-Pichardo, A.; Camacho, F. Congenital triangular alopecia (Brauer nevus). Pediatr. Dermatol. 1995, 12, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Pazzaglia, M.; Starace, M.; Militello, G.; Tosti, A. Videodermoscopy: A useful tool for diagnosing congenital triangular alopecia. Pediatr. Dermatol. 2008, 25, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Rakowska, A.; Maj, M.; Zadurska, M.; Czuwara, J.; Warszawik-Hetnzel, O.; Olszewska, M.; Rudnicka, L. Trichoscopy of Focal Alopecia in Children—New Trichoscopic Findings: Hair Bulbs Arranged Radially along Hair-Bearing Margins in Aplasia Cutis Congenita. Ski. Appendage Disord. 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Galluccio, G.; Oranges, T.; Pianigiani, E.; Ierardi, F.; Cinotti, E.; Rubegni, P. Line-Field Optical Coherence Tomography: Usefulness in the Non-Invasive Differential Diagnosis of Congenital Alopecia of Infancy. Dermatol. Pract. Concept 2024, 14, e2024142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ramam, M. Temporal Triangular Alopecia. Indian Dermatol. Online J. 2022, 14, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Acosta, F.; Ponce, I. Hair transplantation in triangular temporal alopecia. Actas Dermosifiliogr. 2009, 100, 913–915. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Otberg, N.; Kang, H.; Zanet, L.; Shapiro, J. Successful treatment of temporal triangular alopecia by hair restoration surgery using follicular unit transplantation. Dermatol. Surg. 2009, 35, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Sim, J.H.; Gye, J.; Namkoong, S.; Hong, S.P.; Kim, M.H.; Park, B.C. Successful hair transplantation for treatment of acquired temporal triangular alopecia. Dermatol. Surg. 2012, 38, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.E.; Jin, W.J.; Yun, J.Y.; Kim, H. An Unusual Case of Congenital Triangular Alopecia on Frontal Area Successfully Treated by Surgery. Int. J. Trichology 2020, 12, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Lueangarun, S.; Pacharapakornpong, S.; Tempark, T. Transient Treatment Response of Platelet-rich Plasma Injection for Temporal Triangular Alopecia: A Case Report with Dermoscopic Examination Follow-up. Int. J. Trichology 2020, 12, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.Y.; Byun, J.W.; Kang, M.J.; Yang, B.-H.; Song, H.-J.; Shin, J.; Choi, G.S. Successful treatment of temporal triangular alopecia with topical minoxidil. Ann. Dermatol. 2013, 25, 387. [Google Scholar] [CrossRef] [PubMed]
- Pathania, Y.S. Rapid response of topical minoxidil in congenital triangular alopecia. Dermatol. Ther. 2020, 33, e13371. [Google Scholar] [CrossRef] [PubMed]
- Sutisna, F.D.; Gondokaryono, S.P.; Dwiyana, R.F.; Effendi, R.M.R.A.; Tache, N.; Anandita, R. Congenital Triangular Alopecia: A Case of Effective Response with 5% Topical Minoxidil in a Male Adolescent. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagórska, B.; Żółkiewicz, J.; Maińska, U.; Sławińska, M. Temporal Triangular Alopecia—Clinical and Dermoscopic Features of a Rare Entity. Diagnostics 2025, 15, 2621. https://doi.org/10.3390/diagnostics15202621
Zagórska B, Żółkiewicz J, Maińska U, Sławińska M. Temporal Triangular Alopecia—Clinical and Dermoscopic Features of a Rare Entity. Diagnostics. 2025; 15(20):2621. https://doi.org/10.3390/diagnostics15202621
Chicago/Turabian StyleZagórska, Beata, Jakub Żółkiewicz, Urszula Maińska, and Martyna Sławińska. 2025. "Temporal Triangular Alopecia—Clinical and Dermoscopic Features of a Rare Entity" Diagnostics 15, no. 20: 2621. https://doi.org/10.3390/diagnostics15202621
APA StyleZagórska, B., Żółkiewicz, J., Maińska, U., & Sławińska, M. (2025). Temporal Triangular Alopecia—Clinical and Dermoscopic Features of a Rare Entity. Diagnostics, 15(20), 2621. https://doi.org/10.3390/diagnostics15202621





