Determinants of Eligibility and Timing of Autologous Transplantation in Multiple Myeloma: The Role of R-MCI and Diagnostic Plasma Cell Burden
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Comparison Between Transplanted and Non-Transplanted Patients
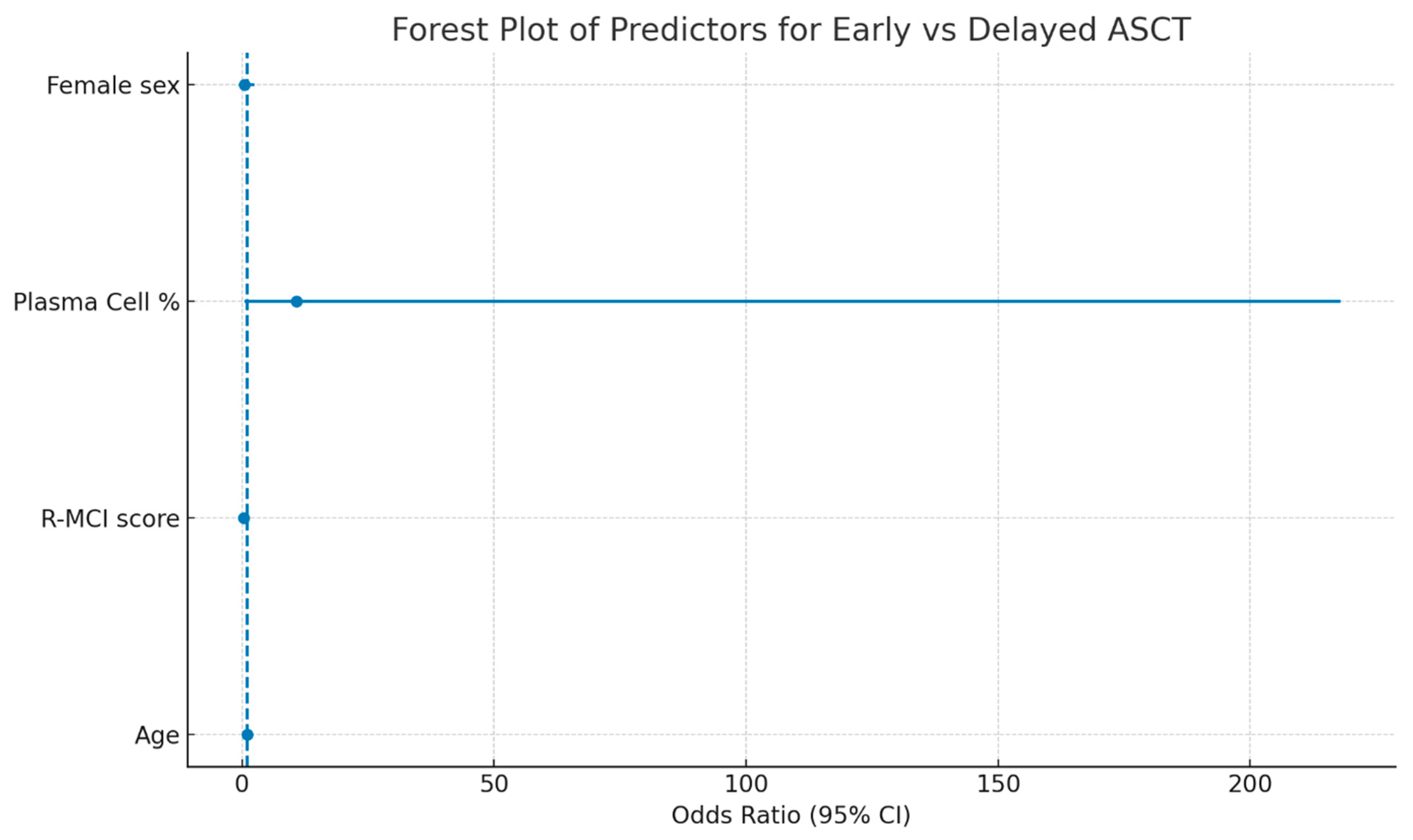
3.3. Transplant Timing: Early vs. Delayed ASCT
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohty, M.; Facon, T.; Malard, F.; Harousseau, J.-L. A roadmap towards improving outcomes in multiple myeloma. Blood Cancer J. 2024, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M. Triplet therapy, transplantation, and maintenance until progression in myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef]
- Blackburn, L.; Mansour, A.; Zhao, Q.; Cottini, F.; Khan, A.; Bumma, N.; Devarakonda, S.; Umyarova, E.; Rosko, A.E.; Vaughn, J. Real World Predictors, Timing, and Outcomes of Autologous Stem Cell Transplantation in Patients with Multiple Myeloma. Hemato 2024, 5, 407–419. [Google Scholar] [CrossRef]
- Lupak, O.; Xiaoxia, H.; Xie, P.; Thanikachalam, K.; Jabbour-Aida, H.; Farhan, S.; Emole, J. Disparities in utilization of autologous stem cell transplantation as consolidative therapy for multiple myeloma: A single institution retrospective review. Clin. Lymphoma Myeloma Leuk. 2021, 21, e680–e685. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Moreau, P.; Mateos, M.-V.; Zweegman, S.; Cook, G.; Engelhardt, M.; Delforge, M.; Hajek, R. EHA–EMN Evidence-Based Guidelines for diagnosis, treatment and follow-up of patients with multiple myeloma. Nat. Rev. Clin. Oncol. 2025, 22, 680–700. [Google Scholar] [CrossRef]
- Facon, T.; Dimopoulos, M.A.; Meuleman, N.; Belch, A.; Mohty, M.; Chen, W.-M.; Kim, K.; Zamagni, E.; Rodriguez-Otero, P.; Renwick, W. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia 2020, 34, 224–233. [Google Scholar] [CrossRef]
- Engelhardt, M.; Domm, A.-S.; Dold, S.M.; Ihorst, G.; Reinhardt, H.; Zober, A.; Hieke, S.; Baayen, C.; Müller, S.J.; Einsele, H. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017, 102, 910. [Google Scholar] [CrossRef]
- Mian, H.; Wildes, T.M.; Vij, R.; Pianko, M.J.; Major, A.; Fiala, M.A. Dynamic frailty risk assessment among older adults with multiple myeloma: A population-based cohort study. Blood Cancer J. 2023, 13, 76. [Google Scholar] [CrossRef]
- Al Saleh, A.S.; Parmar, H.V.; Visram, A.; Muchtar, E.; Buadi, F.K.; Go, R.S.; Dispenzieri, A.; Kapoor, P.; Warsame, R.; Lacy, M.Q. Increased bone marrow plasma-cell percentage predicts outcomes in newly diagnosed multiple myeloma patients. Clin. Lymphoma Myeloma Leuk. 2020, 20, 596–601. [Google Scholar] [CrossRef]
- Bashir, Q.; Braunstein, M.; Buck, T.; Chmielewski, C.; Hartmann, B.; Janakiram, M.; McMahon, M.A.; Romundstad, L.; Steele, L.; Usmani, S.Z. Overcoming barriers to autologous stem cell transplantation in multiple myeloma: Recommendations from a multidisciplinary roundtable discussion. Transplant. Cell. Ther. 2023, 29, 666–673. [Google Scholar] [CrossRef]
- Tang, H.K.K.; Fung, C.Y.; Hwang, Y.Y.; Lee, H.; Lau, G.; Yip, S.F.; Kho, B.; Lau, C.K.; Leung, K.H.; Au, E. Prognostic factors in 448 newly diagnosed multiple myeloma receiving bortezomib-based induction: Impact of ASCT, transplant refusal and high-risk MM. Bone Marrow Transplant. 2024, 59, 660–669. [Google Scholar] [CrossRef]
- Kumar, L.; Hussain, M.M.; Chethan, R.; Sahoo, R.K.; Malik, P.S.; Sharma, O.D.; Mathew, A.; Jha, A.; Gupta, R.; Sharma, A. Multiple myeloma: Impact of time to transplant on the outcome. Clin. Lymphoma Myeloma Leuk. 2022, 22, e826–e835. [Google Scholar] [CrossRef]
- Engelhardt, M.; Ihorst, G.; Duque-Afonso, J.; Wedding, U.; Spät-Schwalbe, E.; Goede, V.; Kolb, G.; Stauder, R.; Wäsch, R. Structured assessment of frailty in multiple myeloma as a paradigm of individualized treatment algorithms in cancer patients at advanced age. Haematologica 2020, 105, 1183. [Google Scholar] [CrossRef] [PubMed]
- Sverrisdóttir, I.S.; Rögnvaldsson, S.; Thorsteinsdottir, S.; Gíslason, G.K.; Aspelund, T.; Turesson, I.; Björkholm, M.; Gregersen, H.; Hveding Blimark, C.; Landgren, O. Comorbidities in multiple myeloma and implications on survival: A population-based study. Eur. J. Haematol. 2021, 106, 774–782. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Ludwig, H.; Dimopoulos, M.A.; Bladé, J.; Mateos, M.V.; Rosiñol, L.; Boccadoro, M.; Cavo, M.; Lokhorst, H. Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN). Blood J. Am. Soc. Hematol. 2011, 118, 4519–4529. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Szabo, A.; Chhabra, S.; Hamadani, M.; D’Souza, A.; Usmani, S.Z.; Sieracki, R.; Gyawali, B.; Jackson, J.L.; Asimakopoulos, F. Autologous transplantation for newly diagnosed multiple myeloma in the era of novel agent induction: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 343–350. [Google Scholar] [PubMed]
- Lee, N.; Moon, S.; Lee, J.; Park, H.; Kong, S.; Bang, S.; Lee, J.; Yoon, S.; Lee, D. Discrepancies between the percentage of plasma cells in bone marrow aspiration and BM biopsy: Impact on the revised IMWG diagnostic criteria of multiple myeloma. Blood Cancer J. 2017, 7, e530. [Google Scholar] [CrossRef]
- Schoeller, K.; Ihorst, G.; Scheubeck, S.; Holler, M.; Woerner, S.M.; Reinhardt, H.; Mueller, S.P.; Duyster, J.; Wäsch, R.; Engelhardt, M. The Revised Myeloma Comorbidity Index (R-MCI) as a promising approach for predicting overall (os)-and progression-free (pfs) survival and optimizing therapy strategies in multiple myeloma (mm) patients (pts)-comparative analysis of 5 comorbidity indices (ci), including retro-and prospective applicability. Blood 2019, 134, 3474. [Google Scholar]
- Dreyling, E.; Ihorst, G.; Reinhardt, H.; Räder, J.; Holler, M.; Herget, G.; Greil, C.; Wäsch, R.; Engelhardt, M. Optimizing individualized therapy decision-making in multiple myeloma (MM): Integration and impact of the Revised Myeloma Comorbidity Index in the MM-tumor board. Ann. Hematol. 2025, 104, 593–603. [Google Scholar] [CrossRef]
- Agarwal, M. Multiple myeloma: Treatment is getting individualized. Indian J. Hematol. Blood Transfus. 2016, 32, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pawlyn, C.; Davies, F.E. Toward personalized treatment in multiple myeloma based on molecular characteristics. Blood J. Am. Soc. Hematol. 2019, 133, 660–675. [Google Scholar] [CrossRef] [PubMed]
- Ebraheem, M.S.; Chakraborty, R.; Rochwerg, B.; Visram, A.; Mohyuddin, G.R.; Venner, C.P.; Sandhu, I.; McCurdy, A.; Facon, T.; Mateos, M.-V. Quadruplet regimens for patients with newly diagnosed multiple myeloma: A systematic review and meta-analysis. Blood Adv. 2024, 8, 5993–6002. [Google Scholar] [CrossRef]
- Shah, G.L.; Seier, K.; Devlin, S.M.; Chung, D.J.; Scordo, M.; Hultcrantz, M.; Korde, N.; Lendvai, N.; Lesokhin, A.M.; Mailankody, S. Depth of response and outcomes in patients with multiple myeloma undergoing autologous stem cell transplantation. Blood 2018, 132, 4619. [Google Scholar] [CrossRef]
- Ebraheem, M.; Kumar, S.K.; Dispenzieri, A.; Jevremovic, D.; Buadi, F.K.; Dingli, D.; Cook, J.; Lacy, M.Q.; Hayman, S.R.; Kapoor, P. Deepening responses after upfront autologous stem cell transplantation in patients with newly diagnosed multiple myeloma in the era of novel agent induction therapy. Transplant. Cell. Ther. 2022, 28, 760.e1–760.e5. [Google Scholar] [CrossRef]
- Vij, R.; Kumar, S.; Zhang, M.-J.; Zhong, X.; Huang, J.; Dispenzieri, A.; Abidi, M.H.; Bird, J.M.; Freytes, C.O.; Gale, R.P. Impact of pretransplant therapy and depth of disease response before autologous transplantation for multiple myeloma. Biol. Blood Marrow Transplant. 2015, 21, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Popat, R.; Counsell, N.; de Tute, R.; De-Silva, D.; Phillips, E.H.; Cavenagh, J.D.; Adedayo, T.; Braganca, N.; Roddie, C.; Streetly, M. Using depth of response to stratify patients to front line autologous stem cell transplant: Results of the phase II PADIMAC myeloma trial. Br. J. Haematol. 2021, 193, e19–e22. [Google Scholar] [CrossRef]

| Parameter | Condition | Score |
|---|---|---|
| Age | ≤50 years | 0 |
| 51–60 years | 1 | |
| 61–70 years | 2 | |
| >70 years | 3 | |
| ECOG performance status | 0–1 | 0 |
| 2 | 1 | |
| ≥3 | 2 | |
| Renal function (eGFR) | ≥60 mL/min/1.73 m2 | 0 |
| 30–59 mL/min/1.73 m2 | 1 | |
| <30 mL/min/1.73 m2 | 2 | |
| Pulmonary function (DLCO) | ≥65% predicted | 0 |
| 50–64% predicted | 1 | |
| <50% predicted | 2 | |
| Cytogenetic risk profile | Standard risk | 0 |
| High-risk abnormalities * | 1 |
| Variable | Transplanted (n = 61) | Non-Transplanted (n = 76) | p-Value |
|---|---|---|---|
| Age <65, n (%) | 50 (82.0%) | 19 (25.0%) | <0.001 |
| Female, n (%) | 20 (33%) | 38 (50%) | 0.064 |
| R-MCI, median (IQR) | 0 (0–1) | 1 (0–2) | <0.001 |
| Plasma Cell %, median (IQR) | 60 (36–80) | 57 (20–80) | 0.281 |
| ISS Stage I, n (%) | 12 (19.7%) | 14 (18.4%) | |
| ISS Stage II, n (%) | 16 (26.2%) | 20 (26.3%) | |
| ISS Stage III, n (%) | 27 (44.3%) | 36 (47.4%) | 0.958 |
| Variable | Univariate OR | Univariate 95% CI | Univariate p-Value | Multivariate OR | Multivariate 95% CI | Multivariate p-Value |
|---|---|---|---|---|---|---|
| Age | 0.987 | 0.915–1.064 | 0.726 | 1.028 | 0.939–1.126 | 0.549 |
| R-MCI score | 0.383 | 0.124–1.184 | 0.096 | 0.267 | 0.067–1.058 | 0.060 |
| Bone marrow plasma cell (%) | 1.549 | 0.170–14.129 | 0.698 | 10.797 | 0.535–217.883 | 0.121 |
| Female sex | 0.635 | 0.189–2.130 | 0.462 | 0.539 | 0.120–2.427 | 0.421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candan, O.; Bayar, A.; Naghizada, N.; Demirtas, D.; Yanik, A.M.; Yilmaz, A.F.; Tuglular, A.T.; Toptas, T.; Atagunduz, I. Determinants of Eligibility and Timing of Autologous Transplantation in Multiple Myeloma: The Role of R-MCI and Diagnostic Plasma Cell Burden. Diagnostics 2025, 15, 3038. https://doi.org/10.3390/diagnostics15233038
Candan O, Bayar A, Naghizada N, Demirtas D, Yanik AM, Yilmaz AF, Tuglular AT, Toptas T, Atagunduz I. Determinants of Eligibility and Timing of Autologous Transplantation in Multiple Myeloma: The Role of R-MCI and Diagnostic Plasma Cell Burden. Diagnostics. 2025; 15(23):3038. https://doi.org/10.3390/diagnostics15233038
Chicago/Turabian StyleCandan, Ozlem, Arda Bayar, Narmin Naghizada, Derya Demirtas, Ahmet Mert Yanik, Asu Fergun Yilmaz, Ayse Tulin Tuglular, Tayfur Toptas, and Isik Atagunduz. 2025. "Determinants of Eligibility and Timing of Autologous Transplantation in Multiple Myeloma: The Role of R-MCI and Diagnostic Plasma Cell Burden" Diagnostics 15, no. 23: 3038. https://doi.org/10.3390/diagnostics15233038
APA StyleCandan, O., Bayar, A., Naghizada, N., Demirtas, D., Yanik, A. M., Yilmaz, A. F., Tuglular, A. T., Toptas, T., & Atagunduz, I. (2025). Determinants of Eligibility and Timing of Autologous Transplantation in Multiple Myeloma: The Role of R-MCI and Diagnostic Plasma Cell Burden. Diagnostics, 15(23), 3038. https://doi.org/10.3390/diagnostics15233038






