1. Introduction
Since its introduction in the early 1970s, Body Mass Index (BMI) has served as the cornerstone for defining and classifying obesity in clinical and public health contexts. Originating from the work of Ancel Keys and colleagues, BMI offered a convenient, population-level proxy for body fat content, enabling cross-national comparisons and epidemiological surveillance with minimal resources [
1]. The metric was rapidly adopted by global health authorities, most notably the World Health Organization (WHO), which formally endorsed BMI-based cut-offs for overweight (≥25 kg/m
2) and obesity (≥30 kg/m
2) in its 2000 technical report—cementing its status as the global standard [
2]. Owing to its simplicity and reproducibility, BMI has been widely used to monitor trends, guide interventions, and assess risk stratification across diverse populations. However, as pointed out in subsequent critiques, its utility at the individual level remains limited, as it fails to distinguish between lean and fat mass or account for ethnic and sex-based differences in body composition [
3]. These limitations are particularly pertinent in the context of modern precision medicine, which demands more nuanced and physiologically relevant markers for cardiometabolic risk assessment.
Despite its widespread adoption, BMI has increasingly come under scrutiny for its inability to reflect individual variability in cardiometabolic health. Individuals with identical BMI values may exhibit markedly different physiological profiles—ranging from insulin sensitivity and lipid levels to inflammatory markers and visceral fat accumulation—resulting in distinct levels of cardiovascular risk [
4]. This heterogeneity has led to the recognition of divergent obesity phenotypes, including the so-called metabolically healthy obese (MHO) and metabolically unhealthy normal weight (MUNW) individuals. While the former present with a high BMI but favorable metabolic profiles, the latter may have normal BMI values despite harboring significant metabolic dysfunction. Such phenotypes illustrate the disconnect between BMI and actual health status, emphasizing the metric’s limited discriminative capacity in both clinical and preventive cardiology [
5].
Emerging evidence from large-scale population studies supports the use of metabolic profiling and multi-omic analysis to uncover risk patterns that BMI alone cannot detect. These approaches have revealed that some individuals within the same BMI category may exhibit drastically different lipidomic and proteomic signatures, correlating with divergent cardiometabolic trajectories. Moreover, the reliance on BMI as a universal metric fails to account for population-specific factors, including ethnic differences in fat distribution, muscle mass, and inflammation—all of which modulate cardiovascular risk independently of total body weight [
6,
7]. Clinical guidelines have increasingly emphasized the importance of evaluating fat distribution—particularly visceral and ectopic fat—as these are more closely linked to cardiovascular outcomes than BMI-defined obesity alone [
8]. As such, a paradigm shift is underway toward more granular, phenotype-based models of obesity classification that better align with the goals of precision medicine.
Advances in precision medicine have highlighted the biological complexity hidden within BMI-defined obesity. Polygenic risk scores (PRSs), derived from genome-wide association studies, have shown that genetic predisposition to obesity involves distinct pathways: central nervous system genes often influence appetite regulation and overall adiposity, while adipose tissue-related genes shape fat distribution and metabolic outcomes [
9].
In parallel, metabolomic profiling reveals extensive biochemical variation among individuals with similar BMI. Up to one-third of the human plasma metabolome is altered in obesity, yet the direction and intensity of these changes differ across individuals, enabling the identification of metabolically distinct subtypes [
10]. Tools such as metabolomic BMI (mBMI) can differentiate between metabolically unhealthy normal-weight individuals and metabolically healthy obese individuals with higher clinical accuracy than anthropometrics alone. While PRSs offer limited predictive power in isolation, integrating genetic and metabolomic data enhances cardiometabolic risk stratification [
11].
The emergence of advanced imaging technologies has revealed that total body weight alone is a poor predictor of cardiometabolic risk. Instead, regional fat distribution—particularly the accumulation of visceral adipose tissue (VAT)—has been shown to be more strongly associated with adverse cardiovascular outcomes than subcutaneous fat or BMI-defined obesity. VAT is a metabolically active depot, characterized by a higher density of proinflammatory immune cells and increased insulin resistance, contributing to an endocrine environment conducive to atherogenesis, hypertension, and insulin resistance [
12].
Cross-sectional modalities such as MRI, CT, and DEXA scans allow for precise quantification of VAT and other ectopic fat depots, offering superior risk stratification in both obese and normal-weight individuals [
13]. Notably, VAT drains into the portal circulation, directly affecting hepatic metabolism and promoting dyslipidemia, glucose intolerance, and systemic inflammation. This direct link accentuates its pathophysiological relevance in the progression of cardiovascular disease (CVD). Moreover, imaging studies have identified that VAT volume correlates more robustly with adverse outcomes—such as left ventricular hypertrophy, arterial stiffness, and coronary calcification—than BMI or waist circumference. Recent advances in artificial intelligence have enabled the development of deep learning models that combine chest radiographs with clinical variables to estimate VAT with high accuracy, potentially democratizing access to precision cardiometabolic risk profiling. Such approaches may outperform traditional anthropometric methods and provide actionable insight into patient-specific fat phenotypes [
14]. As precision medicine becomes increasingly central to cardiology, VAT quantification is poised to become a routine component of cardiovascular risk assessment.
In light of the limitations of BMI and the growing body of evidence supporting a biologically grounded classification of obesity, this review aims to re-examine how adiposity is defined and measured—particularly in the context of cardiovascular risk. We explore emerging alternatives to BMI, including multi-omic profiling, polygenic risk scoring, and imaging-based fat distribution metrics, with a focus on their relevance to cardiology and precision medicine. Special attention is given to the paradoxes of metabolically healthy obesity and metabolically unhealthy normal weight, and how these phenotypes challenge traditional assumptions about weight-related cardiovascular risk. By synthesizing recent findings from genetic, metabolic, and imaging research, we advocate for a redefinition of obesity that better aligns with pathophysiological mechanisms and individualized risk stratification. The review concludes by highlighting future directions and the clinical implications of adopting a precision-based approach to obesity in cardiovascular care.
2. Materials and Methods
This narrative review was conducted to synthesize current perspectives on the limitations of BMI and the emerging role of alternative metrics in cardiometabolic risk assessment, particularly within the context of precision medicine. To identify relevant literature, a targeted search was carried out using PubMed, Scopus, and Web of Science databases. Additional sources were identified through manual screening of the reference lists of selected articles, ensuring that recent advances and influential studies not captured by automated searches were also considered. The search was conducted in September 2025 and covered literature published between January 2018 and September 2025.
The search strategy included terms and Boolean combinations such as “obesity phenotypes,” “BMI limitations,” “visceral adiposity,” “metabolically healthy obesity,” “polygenic risk score,” “metabolomics,” “body composition imaging,” “cardiometabolic risk,” and “precision cardiology.” These terms were chosen to reflect the review’s primary focus on redefining obesity through multi-parametric approaches that integrate imaging, molecular, and genetic data, as well as the clinical implications of obesity-related phenotypes in cardiovascular care.
Two reviewers independently screened titles and abstracts for relevance, followed by full-text assessment of eligible articles. Discrepancies were resolved through discussion and consensus. Although this is a narrative rather than a systematic review, efforts were made to enhance methodological transparency by applying selected elements of systematic review reporting frameworks. Given the narrative nature of this review, a formal PRISMA framework was not applied, and no standardized risk of bias assessment tools were used. Formal quality appraisal tools were not appropriate due to the conceptual and exploratory aims of this synthesis, which integrates heterogeneous evidence types (e.g., omics studies, imaging protocols, and mechanistic models). Instead, emphasis was placed on conceptual integration, recency of evidence, and translational relevance to cardiovascular and precision medicine.
The included studies were synthesized using a narrative, thematic approach. Articles were grouped based on conceptual overlap into four major domains: (1) limitations of BMI in cardiovascular risk stratification; (2) imaging-based assessments of adiposity; (3) omics-driven obesity classification (including genomics and metabolomics); and (4) cardiological implications of phenotype-based definitions. This allowed for integrative comparison of evidence across methods and clinical contexts.
Studies were selected based on their relevance to adult human populations and their contribution to understanding obesity’s role in cardiovascular disease beyond BMI classification. Peer-reviewed original research articles, narrative reviews, and systematic reviews were included, provided they were published in English and aligned with the clinical and conceptual domains of interest. Articles focusing exclusively on pediatric populations, surgical or bariatric interventions, or animal models were excluded, as were case reports, editorials, and non-peer-reviewed materials.
A total of 35 articles were ultimately included in the review. The selected articles were grouped thematically to reflect four overarching areas: the limitations of BMI in cardiovascular risk stratification, imaging-based assessments of adiposity, omics-driven obesity classification, and cardiological implications of phenotype-based definitions.
Because this review follows a narrative design, no formal meta-analysis or quality appraisal tools were applied. Instead, emphasis was placed on conceptual integration, recent evidence, and the translational relevance of findings to clinical cardiology. The review aims to provide a comprehensive, yet focused synthesis of current knowledge, offering a framework for redefining obesity in the era of precision medicine.
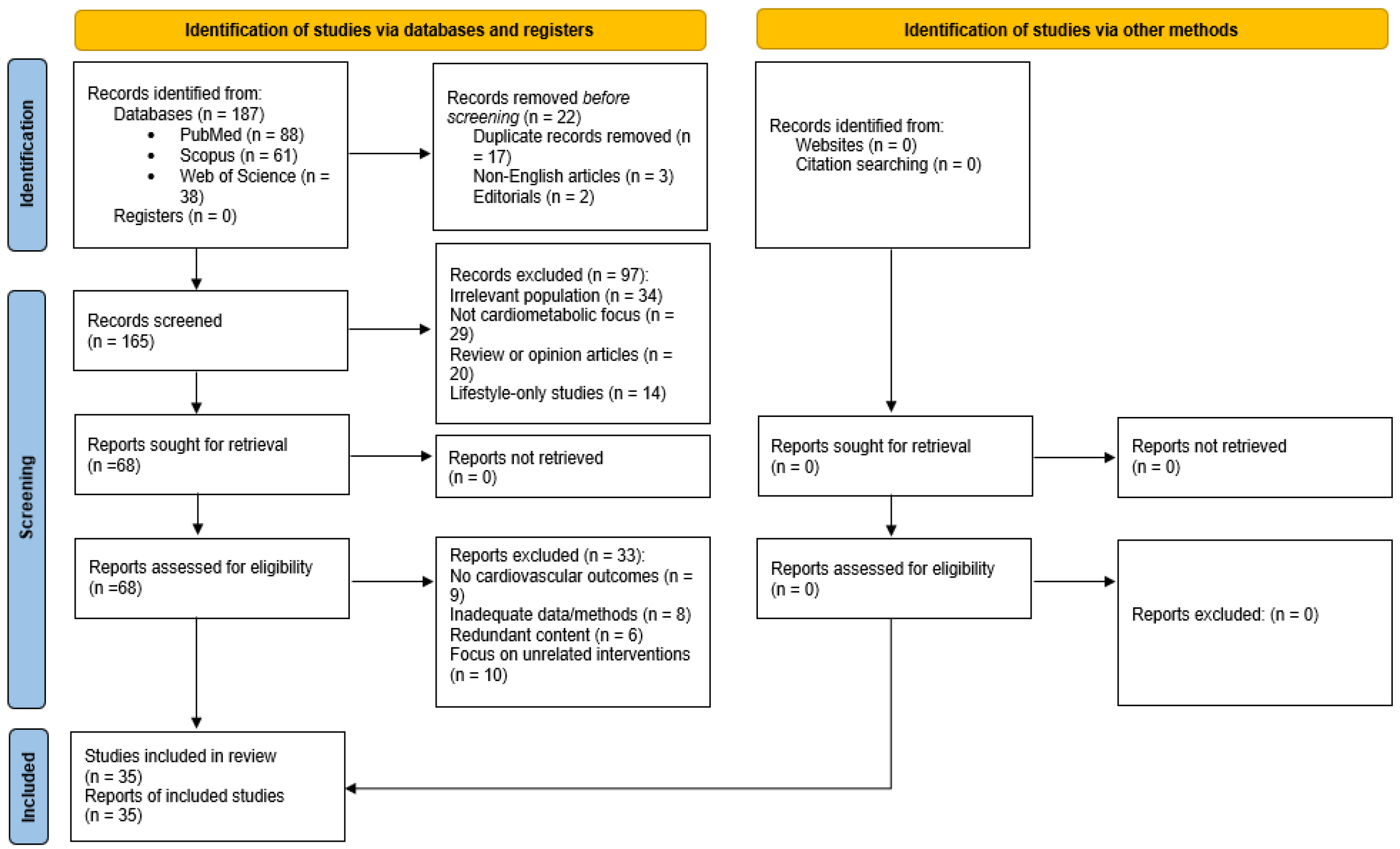
To enhance methodological transparency, a PRISMA 2020 flow diagram has been included (
Figure 1), summarizing the identification, screening, eligibility assessment, and inclusion of articles. This diagram is presented to increase transparency of the literature selection process, even though formal systematic procedures such as bias scoring and meta-analysis were not performed.
3. Results
3.1. Limitations of BMI in Cardiovascular Risk Prediction
Although BMI remains a cornerstone in defining obesity, it is a limited tool for assessing cardiometabolic risk. It does not differentiate between fat and lean mass, nor does it account for fat distribution—both of which are key contributors to the development of CVD. Recent research highlights substantial heterogeneity in obesity-related health outcomes, with some individuals exhibiting high BMI but low cardiometabolic risk. This phenomenon, often described as the “obesity paradox” or “fit-fat paradox,” challenges the assumption that excess body weight, as defined by BMI, uniformly predicts adverse cardiovascular outcomes [
15].
One of the clearest examples of this heterogeneity is the MHO phenotype. Individuals classified as MHO meet BMI criteria for obesity yet do not exhibit metabolic disturbances such as insulin resistance, dyslipidemia, or hypertension. Compared to their metabolically unhealthy obese (MUO) counterparts, MHO individuals typically show preserved insulin sensitivity, lower visceral and hepatic fat, and higher cardiorespiratory fitness [
16]. However, MHO is not a stable condition. Longitudinal evidence suggests that many individuals initially classified as MHO transition over time to a metabolically unhealthy state, with a concomitant increase in CVD risk [
16,
17].
Nevertheless, elevated cardiometabolic risk is not limited to individuals with obesity. Those with normal BMI but poor metabolic profiles—referred to as MUNW—may face comparable or even greater cardiovascular risk than MHO individuals. This phenotype is often characterized by increased visceral fat, impaired insulin sensitivity, and elevated proinflammatory markers despite a “normal” BMI [
17]. Thus, BMI alone cannot reliably differentiate between cardiometabolically benign and high-risk phenotypes.
A summary of the clinical and metabolic features associated with MHO, MUO, and MUNW is presented in
Table 1.
The limitations of BMI become particularly evident when considering its inability to differentiate between lean and fat mass or capture central adiposity. This is critical in cardiovascular risk prediction, where visceral fat, rather than total body weight, is a stronger determinant of adverse outcomes [
18]. Individuals with similar BMI values may differ substantially in their visceral fat content, which directly influences insulin resistance, lipid metabolism, and systemic inflammation.
A large-scale meta-analysis evaluating the discriminatory capacity of anthropometric indices for CVD found that both waist circumference (WC) and waist-to-hip ratio (WHR) outperformed BMI in predicting cardiovascular events. The pooled area under the curve (AUC) for BMI was 0.66, whereas WC and WHR had AUCs of 0.69 and 0.71, respectively—particularly among women—indicating superior predictive capacity for central adiposity markers [
19].
BMI’s application across diverse populations is further complicated by ethnic and sex-based differences in body composition. For instance, individuals of Asian descent may develop obesity-related complications at lower BMI thresholds, while those with greater muscle mass, such as athletes, may be misclassified as obese. In older adults, age-related muscle loss may lead to underestimation of adiposity using BMI alone, thereby obscuring risk [
18].
Beyond these technical limitations, framing obesity solely through BMI has broader clinical implications. BMI neither measures fat distribution nor accurately estimates body fat percentage and fails to capture the metabolic, genetic, and physiological diversity of obesity [
20]. Consequently, BMI may overlook MUNW, while misclassifying some MHO individuals as high-risk despite a favorable metabolic profile.
In response to these shortcomings, updated diagnostic frameworks have emerged. The Commission on Clinical Obesity proposes categorizing obesity into “preclinical” and “clinical” stages based on the presence of functional impairments and organ-specific dysfunction. In this model, BMI serves only as an initial screening tool. Diagnostic confirmation requires further assessment through direct adiposity measurements—such as dual-energy X-ray absorptiometry (DEXA) or bioelectrical impedance analysis (BIA)—or waist-based anthropometric indices [
21]. This shift prioritizes physiological relevance over rigid weight-based thresholds.
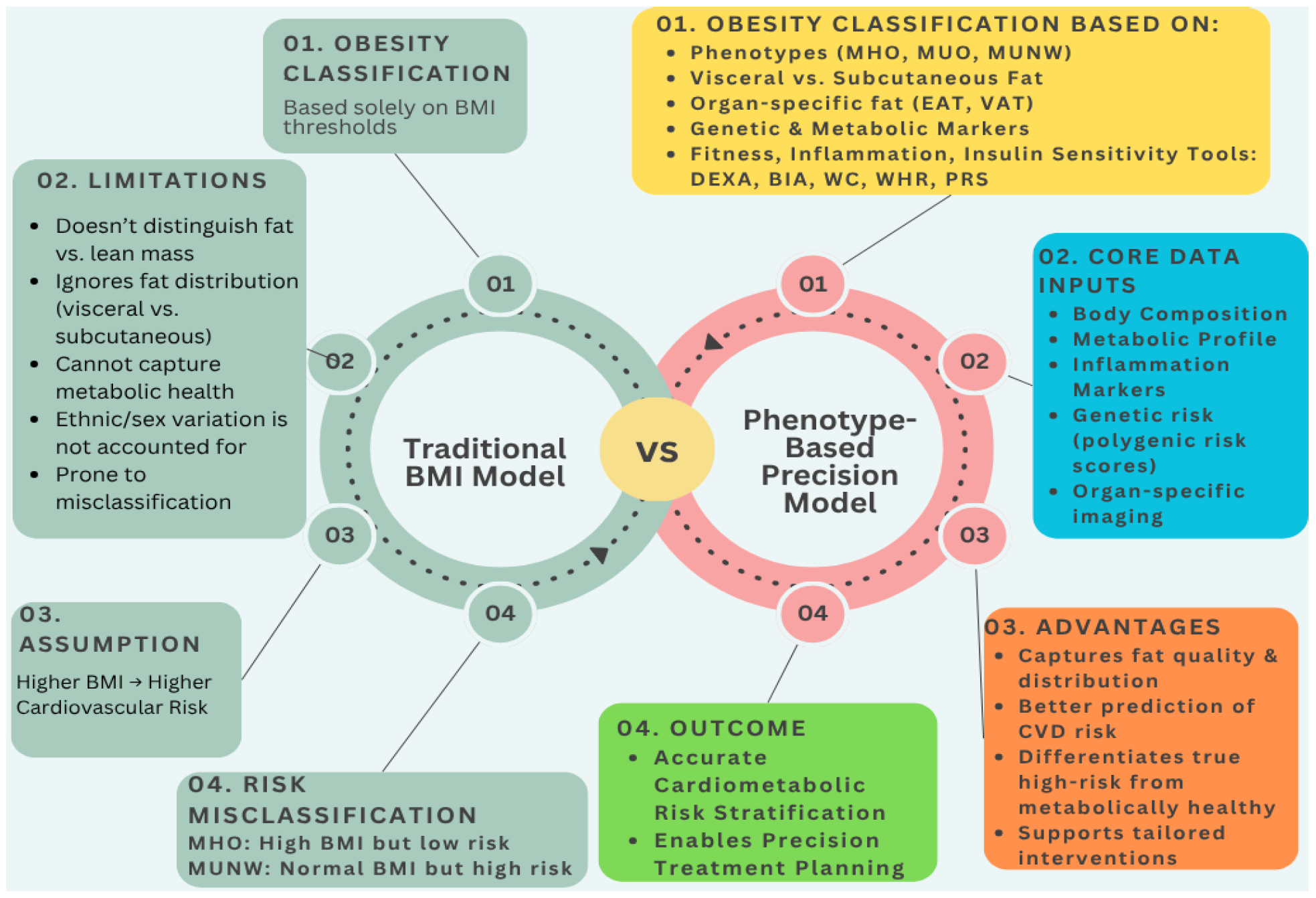
In order to illustrate the conceptual shift from conventional obesity assessment to a precision-based approach,
Figure 2 compares the traditional BMI model with a multi-dimensional, phenotype-driven framework for cardiometabolic risk evaluation.
3.2. Alternative Metrics of Obesity in Precision Medicine
WHR has emerged as a superior anthropometric marker of cardiometabolic risk, surpassing BMI and waist circumference in predictive power. Large cohort studies, including the Northern Finland Birth Cohort and UK Biobank, demonstrated that WHR more effectively captures risk related to β-cell dysfunction, insulin resistance, and type 2 diabetes, even when controlling for BMI and lifestyle factors [
22,
23,
24]. Furthermore, WHR correlates with metabolomic markers of inflammation, dyslipidemia, and hepatic steatosis, reinforcing its relevance in early detection and risk stratification [
25,
26].
It has to be noted that WHR offers enhanced discriminatory ability across sexes due to its sensitivity to sex-specific adipose distribution patterns: gluteofemoral dominance in women and abdominal accumulation in men [
27]. In female cohorts with cardiovascular disease, WHR-based phenotyping identified distinct metabolic profiles not reflected in BMI, underlining its added clinical value [
28].
VAT, distinguishable from subcutaneous adipose tissue (SAT), has an important role in metabolic dysregulation, contributing to inflammation, insulin resistance, and atherogenesis [
29,
30]. While WHR serves as a practical surrogate for central adiposity in large populations, imaging modalities such as CT and MRI offer direct quantification of VAT and SAT, enabling more precise phenotyping.
CT, due to its high resolution and Hounsfield unit-based tissue contrast, remains the reference standard for VAT quantification but is limited by radiation exposure. MRI provides radiation-free imaging with excellent anatomical detail, though its utility is constrained by lower signal contrast and segmentation complexity. DEXA, while useful for whole-body composition estimates, lacks the spatial resolution to differentiate VAT from SAT. These technical distinctions should guide their use in research versus clinical settings.
Automated and semi-automated segmentation algorithms, particularly deep learning-based models such as convolutional neural networks (CNNs), have shown high accuracy in VAT and SAT segmentation. These approaches offer scalable solutions that reduce radiologist workload and improve reproducibility across sites. For example, a 2D U-Net applied to water–fat MRI demonstrated excellent performance across multiple centers, with Dice coefficients above 0.97 for VAT and SAT and quantification errors below 3% [
31]. In another study, a Cycle-GAN and U-Net pipeline enabled VAT segmentation from unannotated MRI data by generating synthetic CT images, validated through radiologist ratings [
32]. CT-based phenotyping using AI also showed that VAT distribution correlates more strongly with type 2 diabetes risk than BMI, even among individuals with normal weight [
33]. Comparison of VAT segmentation tools demonstrated high inter-model agreement (Cohen’s κ = 0.856), supporting the use of validated pipelines in clinical research workflows [
34].
A detailed comparison of these AI-based adipose tissue segmentation studies is presented in
Table 2.
DEXA has been increasingly recognized as a more precise alternative to BMI in the assessment of obesity and associated cardiometabolic risk. Evidence indicates that BMI significantly underestimates obesity prevalence when compared to DEXA-derived body fat percentage (BF%), particularly in populations with distinct ethnic and physiological profiles [
35]. It has been demonstrated that the BMI threshold of 30 kg/m
2 fails to accurately reflect adiposity in some groups, and a lower BMI cut-off may be more appropriate when DEXA is used as the reference standard. DEXA has also been validated for its ability to reliably quantify VAT. Studies have confirmed that VAT measured via DEXA correlates strongly with cardiometabolic biomarkers and provides reproducible estimates across diverse body sizes [
36]. Moreover, DEXA has proven useful in identifying phenotypes such as NWO, characterized by normal BMI but excess VAT, which would otherwise go undetected through traditional anthropometric indices [
37]. Comparative studies between DEXA and CT have demonstrated that while CT remains the gold standard for regional adiposity, DEXA provides clinically acceptable estimates of VAT and lean mass with lower cost, radiation exposure, and greater accessibility [
38].
To contextualize the comparative utility of various obesity assessment tools in precision medicine,
Table 3 summarizes the general strengths, limitations, and clinical applications of WHR, DEXA, CT, and MRI as phenotyping methods.
While numerous studies support the prognostic value of EAT volume, important methodological limitations remain. Imaging protocols vary considerably across studies—CT, MRI, and echocardiography each introduce unique biases, limiting comparability. Cut-off values for “high-risk” EAT or VAT differ widely and are not yet standardized or validated across diverse populations. Moreover, echocardiographic assessment of EAT is highly operator-dependent and lacks consistent measurement protocols, leading to limited reproducibility. Additionally, the predictive performance of VAT and EAT may vary by age, sex, ethnicity, and comorbid conditions, which are often underrepresented in existing cohorts. These factors highlight the need for greater harmonization in imaging methodology and more inclusive validation efforts.
3.3. Integrating Genetics and Multi-Omics
The integration of multi-omics approaches has provided critical insights into the complex molecular architecture of obesity. While genome-wide association studies (GWAS) have identified numerous loci associated with BMI, the functional interpretation and clinical translation of these findings remain limited. Multi-omics strategies combining genetic, transcriptomic, epigenomic, and proteomic data offer a more comprehensive framework for identifying molecular determinants of obesity and refining cardiometabolic risk stratification.
Recent advances in multi-omics research are redefining our understanding of the molecular architecture underlying obesity. Rather than treating obesity as a homogenous condition defined solely by BMI, integrative genomic approaches now allow for the dissection of tissue-specific and pathway-specific mechanisms. For instance, a large genome-wide multi-omics meta-analysis by Tang et al. [
39], which combined GWAS, transcriptome wide (TWAS), and epigenome wide (EWAS) data from over 350,000 individuals, identified 195 genes significantly associated with BMI. Among these, 21 were linked to adipose tissue networks and 53 to brain-specific networks, with 11 genes overlapping both. This dual enrichment pinpoints the role of the brain–adipose axis in regulating energy balance and body weight.
Immune and inflammatory signaling within adipose tissue have also emerged as key contributors to obesity pathogenesis. Li et al. [
40] employed transcriptomics and single-cell RNA sequencing (scRNA-seq) to identify TREM2 and CXCR4 as central regulators of macrophage-driven inflammation and fibrosis in obese adipose tissue. Their work demonstrated that TREM2 is selectively expressed in lipid-associated macrophages and was reproducibly validated across both murine and human datasets, implicating it in obesity-induced immune dysfunction.
Beyond identifying single-gene drivers, multi-omics profiling is now uncovering molecular phenotypes that diverge from traditional anthropometric indicators. As highlighted by Hu and Jia [
41], individuals with similar BMI may exhibit markedly different molecular signatures, particularly in lipid metabolism, inflammatory tone, and insulin responsiveness. This observation has been further supported by Aleksandrova et al. [
42], who argued that omics-based classification strategies can reveal metabolically unhealthy individuals who are misclassified as low-risk based on BMI alone. Their findings also accentuate the limitations of BMI as a universal risk metric and highlight the need for individualized, biology-driven assessments.
The translational potential of these insights is perhaps most evident in obesity-related comorbidities such as non-alcoholic fatty liver disease (NAFLD). Using a targeted multi-omics approach, Diels et al. [
43] identified paraoxonase-1 (PON1) as a key determinant of hepatic steatosis severity. Reduced expression and activity of PON1 were linked to oxidative stress, lipid dysregulation, and liver inflammation. As their results imply, integrating proteomic and imaging data improved early detection of NAFLD and suggested that PON1 may serve as both a biomarker and therapeutic target in obesity-associated liver disease.
Epigenetic regulation also appears to play a dynamic role in obesity risk and progression. In a multi-omics analysis, Zhang et al. [
44] showed that histone methylation patterns in genes such as PPARG, LEPTIN, and TNF are closely associated with adiposity, adipocyte hypertrophy, and inflammation. These modifications were found to be tissue- and context-specific, suggesting their utility in disease staging and metabolic risk stratification.
At a systems level, obesity is increasingly being conceptualized not as a single phenotype but as a spectrum defined by distinct biological axes. Odoemelam et al. [
45] proposed a four-axis model—encompassing general adiposity, hepatic fat, systemic inflammation, and muscle composition—derived from integrative imaging and multi-omics data. Each axis reflects unique molecular signatures that align with specific cardiometabolic risk trajectories, offering a more granular alternative to BMI-based classification.
All of these insights are not limited to risk stratification but are beginning to inform personalized intervention strategies. Woldemariam et al. [
46] demonstrated that integrating genomic, transcriptomic, metabolomic, and microbiomic profiles can stratify individuals into discrete molecular endotypes. These endotypes, characterized by features such as mitochondrial dysfunction, branched-chain amino acid metabolism, and chronic inflammation, are now being explored as the basis for tailoring lifestyle and pharmacologic interventions to the patient’s unique molecular profile.
All the studies mentioned illustrate the promise of multi-omics approaches in redefining obesity as a heterogeneous, biologically stratified condition—moving beyond weight-centric paradigms toward a framework grounded in metabolic function and molecular pathophysiology.
3.4. Epicardial Adipose Tissue: A Cardiometabolic Biomarker in Obesity Redefinition
Recent findings suggest that the cardiovascular consequences of obesity extend beyond what is measurable by BMI, supporting a shift toward more nuanced definitions based on metabolic function and ectopic fat distribution. Adipose tissue, particularly when deposited viscerally and around the heart as epicardial adipose tissue, plays an active role in cardiovascular remodeling. In obesity, hypertrophied adipocytes become metabolically dysregulated, secreting elevated levels of inflammatory cytokines including TNF-α, IL-6, and MCP-1, which contribute to systemic insulin resistance, endothelial dysfunction, and myocardial fibrosis. These changes underpin the development of HFpEF, which is increasingly linked to metabolic obesity, even in the absence of overt hyperglycemia or elevated BMI. The role of lipotoxicity has also gained attention, with excessive accumulation of lipid intermediates such as ceramides and diacylglycerols within cardiomyocytes contributing to cellular stress, apoptosis, and impaired contractility. These cardiometabolic effects are further exacerbated in individuals with metabolically unhealthy phenotypes, regardless of body size. Imaging-based studies confirm that increased EAT volume is independently associated with left ventricular hypertrophy, subclinical diastolic dysfunction, and coronary atherosclerosis, and may better predict cardiovascular risk than BMI or waist circumference alone [
47]. As a surrogate of visceral adiposity and metabolic risk, EAT is also modifiable, and emerging pharmacologic strategies target this depot directly. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), widely used for obesity and type 2 diabetes, have demonstrated pleiotropic benefits that include reductions in systemic inflammation, improvement of myocardial energetics, and attenuation of adverse structural remodeling. In patients with HFpEF, GLP-1RAs were associated with improvements in symptoms, exercise tolerance, and selected biomarkers of cardiac stress. These cardioprotective effects appeared to be at least partially independent of weight loss or glycemic control, and were more pronounced in individuals with concurrent obesity and metabolic dysfunction [
48]. Recent evidence from the STEP-HFpEF trial has solidified their role in obesity-related cardiac dysfunction. In this randomized controlled study involving 529 patients with HFpEF and obesity, once-weekly semaglutide significantly improved symptoms, physical function, and biomarkers of cardiac stress—independent of glycemic status or weight loss alone. Patients receiving semaglutide experienced greater reductions in NT-proBNP (−21%), CRP levels, and improved KCCQ-CSS scores (+16.6 points) compared to placebo. These cardiometabolic improvements were more pronounced in those with visceral adiposity, supporting the hypothesis that therapies targeting ectopic fat depots such as epicardial adipose tissue may offer pleiotropic benefits in obesity-related HFpEF [
49]. Nevertheless, variability in study designs, heterogeneity in patient populations, and insufficient stratification by heart failure phenotypes limit definitive conclusions. The cardiometabolic benefits of GLP-1RAs are supported by outcome trials showing a reduction in major adverse cardiovascular events (MACE), particularly stroke and cardiovascular mortality, with modest effects on heart failure hospitalizations. While these therapies may not yet be routinely applied for HF management, their favorable profile in obese individuals at risk of cardiovascular events stresses the need to redefine obesity through a cardiocentric and phenotype-specific lens [
50]. This includes re-evaluating risk in those with metabolically healthy obesity or metabolically unhealthy normal weight, who may harbor unrecognized subclinical myocardial changes despite falling outside traditional BMI-based risk thresholds.
The role of EAT in cardiac structure and function is being redefined from a passive fat deposit to an active endocrine organ influencing myocardial remodeling and electrophysiological stability. Recent echocardiographic evidence in middle-aged adults shows that incremental increases in EAT thickness are associated with graded changes in left ventricular wall thickness, left atrial dilation, and impaired diastolic function, independent of BMI, waist circumference, or blood pressure. This suggests a continuous rather than threshold-based impact of EAT on cardiac morphology and function [
51]. It is important to note that these alterations occur even in younger populations, indicating that subclinical remodeling related to visceral fat may begin decades before overt cardiovascular disease becomes manifest. EAT appears to exert its effects via direct paracrine mechanisms due to its unique anatomic proximity to the myocardium, lacking any fascial separation. In elderly hypertensive patients, EAT thickness measured via transthoracic echocardiography was found to predict the 2-year incidence of atrial fibrillation (AF) with high reproducibility. Each 1 mm increase in EAT was associated with a 62% greater risk of incident AF, and a cut-off value of 6.5 mm effectively stratified high-risk individuals. This relationship remained significant even after adjusting for traditional risk factors such as BMI, blood pressure, diabetes, and atrial size, emphasizing the independent predictive value of EAT [
52]. Furthermore, adding EAT to clinical risk models significantly improved discrimination, calibration, and reclassification metrics. The presence of elevated EAT not only contributes to atrial remodeling but also aligns with elevated inflammatory mediators and diastolic filling abnormalities—early indicators of heart failure with HFpEF. Complementing this, editorial perspectives stress the need for longitudinal studies and multi-omic profiling to further elucidate the biological mechanisms linking EAT to myocardial disease, while also highlighting ethnic variability and the modifying effects of pharmacologic agents such as GLP-1 receptor agonists [
53]. Taken together, these insights support a redefinition of obesity that prioritizes fat distribution and metabolic behavior over crude anthropometric indices. Integrating EAT measurement into routine echocardiographic evaluations may represent a low-cost, high-yield strategy for early cardiovascular risk detection and precision-guided intervention in obese and metabolically vulnerable populations.
To complement the narrative findings,
Table 4 and
Table 5 provide an integrated summary of the key clinical correlates and imaging-based metrics of EAT in the context of obesity-related cardiac remodeling, as reported in recent studies [
47,
48,
49,
50,
51,
52,
53,
54].
4. Discussion
4.1. Summary of Key Findings
This review highlights the limitations of BMI as a universal marker of cardiometabolic risk and underscores the need for a paradigm shift toward more biologically grounded, phenotype-driven approaches. Emerging evidence consistently shows that metrics such as VAT, EAT, WHR, and multi-omic biomarkers offer significantly greater predictive power for cardiovascular outcomes than BMI alone [
15,
16,
17,
19,
47]. Furthermore, the delineation of obesity phenotypes—such as MHO and MUNW—demonstrates the inadequacy of BMI in capturing true metabolic risk [
4,
16]. Integrating advanced imaging, PRS, and metabolomic profiling enables more accurate stratification and paves the way for precision-guided interventions.
This narrative review expands upon the recent quantitative syntheses that support the superior predictive value of WHR and VAT over BMI. For example, a meta-analysis [
56] of over 700,000 participants found that WHR is significantly associated with increased myocardial infarction risk, especially in women and Asian populations (OR = 1.98, 95% CI: 1.75–2.24). Similarly, a classical meta-regression [
57] demonstrated that each 0.01 unit increase in WHR is associated with a 5% higher risk of cardiovascular events, independent of BMI. A more recent population-based imaging study [
58] showed that VAT, but not abdominal subcutaneous adipose tissue, was independently predictive of coronary heart disease (HR per 1-SD VAT = 1.15, 95% CI: 1.09–1.22), and this association persisted even after adjustment for waist circumference.
While the reviewed studies generally support the superiority of VAT, EAT, and omics-based metrics over BMI, the evidence is not uniformly consistent. Some cohorts report weaker associations in elderly or ethnically diverse populations, raising concerns about generalizability. In addition, while certain studies emphasize the predictive strength of EAT volume via CT, others using echocardiographic thickness report more modest associations, reflecting methodological variability. These inconsistencies call for standardization across imaging modalities and validation across demographic groups. Despite these differences, the emerging consensus supports a shift away from BMI-centric frameworks toward a more nuanced, phenotype-based understanding of cardiometabolic risk.
4.2. Clinical and Translational Implications
The clinical implications of these findings are substantial. Cardiologists and primary care providers must recognize that two patients with similar BMI may have different risk trajectories due to differences in fat distribution, inflammation, and metabolic flexibility [
18,
47]. Imaging-based assessments of VAT and EAT provide actionable, non-invasive biomarkers that correlate more strongly with subclinical cardiac remodeling, atherosclerosis, and heart failure progression than traditional anthropometric indices [
12,
50]. Importantly, pharmacologic agents like GLP-1 receptor agonists appear to influence these fat depots independently of weight loss, offering new therapeutic angles that transcend BMI-defined treatment pathways [
48,
49]. A recent meta-analysis [
59] of 29 studies including 19,709 patients, confirmed that higher EAT measurements, quantified either by CT-derived volume or echocardiographic thickness, are independently associated with an increased risk of MACE. In adjusted analyses, each 1-SD increase in EAT volume was associated with a 42% higher risk of MACE (aHR = 1.42; 95% CI, 1.22–1.65), and individuals in the highest EAT volume category had more than double the risk (aHR = 2.64; 95% CI, 1.23–5.67). CT-derived EAT volume was more predictive (aHR = 1.74) than EAT thickness by echocardiography (aHR = 1.20). Secondary outcomes also showed that elevated EAT was associated with significantly increased odds of cardiac death (OR = 2.53), myocardial infarction (OR = 2.63), coronary revascularization (OR = 2.99), and atrial fibrillation (aOR = 4.04). These important results reinforce the clinical utility of EAT quantification as a non-invasive biomarker for cardiovascular risk stratification.
These findings also have potential implications for public health frameworks and clinical policy. As evidence accumulates on the limitations of BMI in capturing metabolic risk, international bodies such as the WHO and cardiology societies like the ESC and ACC may consider revising obesity definitions and risk stratification tools to incorporate phenotype-specific markers, including WHR, VAT quantification, and omics-based classifiers. It is important to note that regulatory interest in obesity-related metrics is growing. For instance, recent FDA label expansions and clinical guidance have acknowledged the cardioprotective effects of GLP-1 receptor agonists in patients with HFpEF, independent of weight loss. This shows a broader shift in regulatory thinking—from weight-centric to cardiometabolic-centric endpoints—which supports integrating more precise, mechanistically grounded obesity metrics into clinical guidelines.
4.3. Limitations of Current Evidence
Despite these promising developments, several limitations persist in the existing literature. Many omics-based studies lack long-term follow-up, limiting their predictive value for hard cardiovascular outcomes. Ethnic, sex-based, and age-related differences in fat distribution are often underrepresented or underpowered in cohort studies, raising concerns about generalizability [
7,
28]. Additionally, while deep learning–based tools for VAT and EAT quantification show high accuracy in research settings, clinical implementation remains constrained by cost, infrastructure, and expertise [
31,
32]. The integration of multi-modal data into electronic health records is another major bottleneck in translating precision obesity metrics into everyday practice.
4.4. Challenges in Clinical Adoption
Adopting a phenotype-based model in clinical cardiology faces structural and educational hurdles. Clinicians remain trained in BMI-centric paradigms, and practice guidelines have yet to fully endorse alternative metrics such as VAT, WHR, or PRS. In lower-resource settings, access to MRI or DEXA may be limited, and omics data may not be readily available or interpretable. To mitigate these challenges, simplified clinical tools that approximate VAT or EAT using accessible surrogates—like advanced echocardiography or AI-enhanced radiographs—could serve as transitional steps toward full precision implementation [
14,
52].
4.5. Future Research and Policy Directions
Future studies should aim to standardize the measurement and interpretation of alternative obesity metrics across populations and care settings. Longitudinal, multi-ethnic cohort studies are needed to evaluate how combinations of imaging, omics, and anthropometric data predict major adverse cardiovascular events over time. Additionally, the development of integrated risk scores that include EAT thickness, metabolomic BMI, and inflammatory markers could enhance risk stratification, particularly for MUNW individuals who are often overlooked in traditional frameworks [
10,
45]. At a policy level, redefining obesity to include functional and distributional measures of adiposity could prompt a shift in diagnostic thresholds and treatment eligibility criteria.
5. Conclusions
The continued reliance on BMI as a primary marker of obesity-related cardiovascular risk is increasingly misaligned with advances in precision medicine. This review emphasizes the inadequacy of BMI in capturing individual differences in fat distribution, metabolic health, and genetic susceptibility. Phenotypes such as MHO and MUNW exemplify the disconnect between body size and cardiovascular risk, accentuating the urgent need to redefine obesity beyond weight-based cutoffs.
Alternative metrics—WHR, VAT, EAT, and multi-omic biomarkers—offer more physiologically relevant, predictive, and individualized assessments. Imaging modalities and molecular profiling tools have demonstrated the ability to stratify cardiometabolic risk more accurately, enabling early detection of subclinical disease and informing personalized interventions.
As cardiology increasingly embraces precision approaches, the clinical paradigm must shift from a “one-size-fits-all” BMI model to an integrated framework that considers adipose tissue distribution, function, and molecular signatures. Moving forward, redefining obesity through a cardiocentric, multi-parametric lens will be essential to improving risk prediction, optimizing treatment strategies, and aligning clinical practice with the biological realities of metabolic disease. To bridge the gap between precision research and real-world cardiology, future obesity assessment should integrate VAT and EAT quantification into standard cardiovascular risk evaluations. Similarly, incorporating metabolic profiling—including lipidomics, inflammatory markers, and polygenic risk scores—can enhance individualized risk prediction. Emerging digital health tools and artificial intelligence-based imaging platforms offer scalable solutions for phenotyping adiposity with greater accuracy and lower cost. As these technologies become more accessible, clinicians will be better equipped to implement phenotype-based obesity management strategies that move beyond BMI, enabling earlier intervention and more targeted cardiometabolic care.
6. Strengths and Limitations
This review offers a timely and comprehensive synthesis of emerging obesity classification models that transcend traditional BMI-based frameworks. By integrating findings from multi-omics, imaging modalities, and population-level phenotype studies, the review provides a cohesive, translational narrative that bridges metabolic research and cardiovascular practice. It incorporates recent high-impact literature (2018–2025), including genome-wide association studies, advanced imaging analyses, and metabolomic profiling, making the review particularly relevant for clinicians and researchers engaged in precision cardiology.
As a narrative review, this work does not apply formal meta-analytic techniques or systematic bias assessments, which may limit the reproducibility of literature inclusion and synthesis. Furthermore, narrative approaches are susceptible to selection and confirmation biases, and may inadvertently reflect prevailing paradigms in the field. While omics-based metrics hold great potential for refining cardiometabolic risk prediction, they remain limited by methodological and translational challenges. These include small and often homogeneous sample sizes, high inter-study heterogeneity, lack of assay and platform standardization, elevated costs, and underdeveloped clinical pipelines for data interpretation.
The review primarily focuses on adult populations, with limited discussion of pediatric obesity phenotypes or age-specific trajectories of metabolic risk. While it draws on diverse global data sources, representation from low- and middle-income countries remains limited, potentially constraining generalizability. Additionally, rapid technological developments in omics and imaging may render certain conclusions provisional, pending broader clinical validation and standardization.
Author Contributions
Conceptualization, A.-M.R., A.M., M.-M.G. and D.I.; methodology, A.-L.R., D.T. and M.-D.T.; software, D.T., D.I. and A.-M.R.; validation, A.-M.R. and M.-D.T.; formal analysis, A.-M.R., M.-D.T. and A.-L.R.; investigation, A.M. and D.I.; resources, M.-M.G., M.-D.T. and A.-L.R.; data curation, D.I., M.-D.T. and A.-M.R.; writing—original draft preparation, A.-M.R., M.-D.T. and A.-L.R.; writing—review and editing, D.I. and D.T.; visualization, A.-M.R. and A.-L.R.; supervision, A.-L.R., M.-D.T. and A.-M.R.; project administration, A.-M.R.; funding acquisition, A.-M.R. and M.-D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors used an AI language model (ScholarGPT v4.1, based on OpenAI’s ChatGPT) to assist with grammar correction and language refinement. All revisions were reviewed and approved by the authors to ensure accuracy and integrity.
Conflicts of Interest
The author Andreea-Liana Rosu is employees of BBraun. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| CNN | Convolutional Neural Network |
| CT | Computed Tomography |
| CVD | Cardiovascular Disease |
| DEXA | Dual-Energy X-ray Absorptiometry |
| EAT | Epicardial Adipose Tissue |
| EF | Ejection Fraction |
| HDL | High-Density Lipoprotein |
| HF | Heart Failure |
| HU | Hounsfield Unit |
| MACE | Major Adverse Cardiovascular Events |
| MHO | Metabolically Healthy Obese |
| Mbmi | Metabolomic Body Mass Index |
| MRI | Magnetic Resonance Imaging |
| MUNW | Metabolically Unhealthy Normal Weight |
| MUO | Metabolically Unhealthy Obese |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| PRS | Polygenic Risk Score |
| SAT | Subcutaneous Adipose Tissue |
| TNF | Tumor Necrosis Factor |
| VAT | Visceral Adipose Tissue |
| WC | Waist Circumference |
| WHR | Waist-to-Hip Ratio |
| 2D U-Net | Two-Dimensional U-shaped Convolutional Network |
References
- Keys, A.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; Taylor, H.L. Indices of relative weight and obesity. J. Chronic Dis. 1972, 25, 329–343. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar] [PubMed]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Beyene, H.B.; Giles, C.; Huynh, K.; Wang, T.; Cinel, M.; Mellett, N.A.; Olshansky, G.; Meikle, T.G.; Watts, G.F.; Hung, J.; et al. Metabolic phenotyping of BMI to characterize cardiometabolic risk: Evidence from large population-based cohorts. Nat. Commun. 2023, 14, 6280. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Wilmanski, T.; Diener, C.; Earls, J.C.; Zimmer, A.; Lincoln, B.; Hadlock, J.J.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T.; et al. Multiomic signatures of body mass index identify heterogeneous health phenotypes and responses to a lifestyle intervention. Nat. Med. 2023, 29, 996–1008. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Invest. 2019, 129, 3978–3989. [Google Scholar] [CrossRef]
- Doumatey, A.P.; Bentley, A.R.; Akinyemi, R.; Olanrewaju, T.O.; Adeyemo, A.; Rotimi, C. Genes, environment, and African ancestry in cardiometabolic disorders. Trends Endocrinol. Metab. 2023, 34, 601–621. [Google Scholar] [CrossRef]
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2017, 6, 223–236. [Google Scholar] [CrossRef]
- Cirulli, E.T.; Guo, L.; Leon Swisher, C.; Shah, N.; Huang, L.; Napier, L.A.; Kirkness, E.F.; Spector, T.D.; Caskey, C.T.; Thorens, B.; et al. Profound Perturbation of the Metabolome in Obesity Is Associated with Health Risk. Cell Metab. 2019, 29, 488–500.e2. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shetty, S.; Suvarna, R.; Bhattacharya, S.; Seetharaman, K. Visceral Adiposity and Cardiometabolic Risk: Clinical Insights and Assessment. Cardiol. Rev. 2025. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Wróblewski, M.; Nuszkiewicz, J.; Sutkowy, P.; Wróblewska, J.; Woźniak, A. The Role of Oxidative Stress Enhanced by Adiposity in Cardiometabolic Diseases. Int. J. Mol. Sci. 2023, 24, 6382. [Google Scholar] [CrossRef]
- Alipour, E.; Gratzl, S.; Algohary, A.; Lin, H.; Bapat, M.; Do, D.; Baker, C.; Rodriguez, T.; Cartwright, B.M.; Hadlock, J.; et al. XComposition: Multimodal Deep Learning Model to Measure Body Composition Using Chest Radiographs and Clinical Data. Radiol. Adv. 2025, 2, umaf035. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B. Metabolic Health in Normal-Weight and Obese Individuals. Diabetologia 2019, 62, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, D.; Vermund, S.H. Advantages and Limitations of the Body Mass Index (BMI) to Assess Adult Obesity. Int. J. Environ. Res. Public Health 2024, 21, 757. [Google Scholar] [CrossRef]
- Darbandi, M.; Pasdar, Y.; Moradi, S.; Mohamed, H.J.J.; Hamzeh, B.; Salimi, Y. Discriminatory Capacity of Anthropometric Indices for Cardiovascular Disease in Adults: A Systematic Review and Meta-Analysis. Prev. Chronic Dis. 2020, 17, 200112. [Google Scholar] [CrossRef]
- Bray, G.A. Beyond BMI. Nutrients 2023, 15, 2254. [Google Scholar] [CrossRef]
- Hong, S.; Park, C.-Y. From Old to New: A Comprehensive Review of Obesity Diagnostic Criteria and Their Implications. Endocrinol. Metab. 2025, 40, 517–522. [Google Scholar] [CrossRef]
- Liu, N.; Wang, B.; Zhang, G.; Shen, M.; Cheng, P.; Guo, Z.; Zuo, L.; Yang, J.; Guo, M.; Wang, M.; et al. Waist-to-hip ratio better reflect beta-cell function and predicts diabetes risk in adult with overweight or obesity. Ann. Med. 2025, 57, 2462447. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, Y.; Liu, J.; Ye, F.; Hui, Q.; Chen, Y.; Liu, D.; Zhang, Q. Association of the Weight-Adjusted Waist Index with Hypertension in the Context of Predictive, Preventive, and Personalized Medicine. EPMA J. 2024, 15, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, V.-P.; Zhao, S.; Ihanus, A.; Tynkkynen, T.; Ala-Korpela, M. Epidemiological Associations between Obesity, Metabolism and Disease Risk: Are Body Mass Index and Waist-Hip Ratio All You Need? Int. J. Obes. 2025, 49, 2555–2560. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, L.; Hurtado, M.D.; Eckel-Passow, J.; Acosta, A. Precision Medicine for Obesity. Dig. Dis. Interv. 2021, 5, 239–248. [Google Scholar] [CrossRef]
- Lind, L.; Ahmad, S.; Elmståhl, S.; Fall, T. The metabolic profile of waist to hip ratio–A multi-cohort study. PLoS ONE 2023, 18, e0282433. [Google Scholar] [CrossRef]
- Lumish, H.S.; O’Reilly, M.; Reilly, M.P. Sex Differences in Genomic Drivers of Adipose Distribution and Related Cardiometabolic Disorders. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 45–60. [Google Scholar] [CrossRef]
- Leopold, J.A.; Antman, E.M. A precision medicine approach to sex-based differences in ideal cardiovascular health. Sci. Rep. 2021, 11, 14848. [Google Scholar] [CrossRef]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 6, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009, 9, e7038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langner, T.; Hedström, A.; Mörwald, K.; Weghuber, D.; Forslund, A.; Bergsten, P.; Ahlström, H.; Kullberg, J. Fully convolutional networks for automated segmentation of abdominal adipose tissue depots in multicenter water-fat MRI. Magn. Reson. Med. 2019, 81, 2736–2745. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, S.; Anwar, S.M.; Harmon, S.A.; Choyke, P.L.; Turkbey, B.; Bagci, U. Adipose tissue segmentation in unlabeled abdomen MRI using cross modality domain adaptation. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 1624–1628. [Google Scholar] [CrossRef]
- Remedios, L.W.; Cho, C.; Schwartz, T.M.; Su, D.; Rudravaram, G.; Gao, C.; Krishnan, A.R.; Saunders, A.M.; Kim, M.E.; Bao, S.; et al. Data-Driven Abdominal Phenotypes of Type 2 Diabetes in Lean, Overweight, and Obese Cohorts. arXiv 2025, arXiv:2508.11063. [Google Scholar] [CrossRef]
- Hou, B.; Mathai, T.S.; Liu, J.; Parnell, C.; Summers, R.M. Enhanced Muscle and Fat Segmentation for CT-Based Body Composition Analysis: A Comparative Study. Int. J. Comput. Assist. Radiol. Surg. 2024, 19, 1589–1596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alammar, M.; Alsoghayer, S.; El-Abd, K.; Alkhenizan, A. Diagnostic Accuracy of Body Mass Index (BMI) When Diagnosing Obesity in a Saudi Adult Population in a Primary Care Setting. Diabetes Metab. Syndr. Obes. 2020, 13, 2515–2520. [Google Scholar] [CrossRef]
- Meredith-Jones, K.; Haszard, J.; Stanger, N.; Taylor, R. Precision of DXA-Derived Visceral Fat Measurements in a Large Sample of Adults of Varying Body Size. Obesity 2018, 26, 505–512. [Google Scholar] [CrossRef]
- Pluta, W.; Lubkowska, A.; Dudzińska, W. Diagnostic and Prognostic Value of Adipose Tissue Content and Distribution Indicators for Normal Weight Obesity in Young Women. Sci. Rep. 2025, 15, 12262. [Google Scholar] [CrossRef]
- Palmas, F.; Ciudin, A.; Guerra, R.; Eiroa, D.; Espinet, C.; Roson, N.; Burgos, R.; Simó, R. Comparison of Computed Tomography and Dual-Energy X-ray Absorptiometry in the Evaluation of Body Composition in Patients with Obesity. Front. Endocrinol. 2023, 14, 1161116. [Google Scholar] [CrossRef]
- Tang, J.; Xu, H.; Xin, Z.; Xin, Z.; Mei, Q.; Gao, M.; Yang, T.; Zhang, X.; Levy, D.; Liu, C.T. Identifying BMI-associated genes via a genome-wide multi-omics integrative approach using summary data. Hum. Mol. Genet. 2024, 33, 733–738. [Google Scholar] [CrossRef]
- Li, Y.; Nie, L.; Lv, T.; Zhou, J.; Ge, X.; Chen, C.; Yang, F.; Deng, Y.; Mao, J.; Wang, R.; et al. Investigation into the molecular mechanism of obesity: An integrated approach of multi-omics analysis, machine learning and experimental validation. J. Transl. Med. 2025, 23, 1123. [Google Scholar] [CrossRef]
- Hu, C.; Jia, W. Multi-omics profiling: The way toward precision medicine in metabolic diseases. J. Mol. Cell Biol. 2021, 13, 576–593. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Rodrigues, C.E.; Floegel, A.; Ahrens, W. Omics biomarkers in obesity: Novel etiological insights and targets for precision prevention. Curr. Obes. Rep. 2020, 9, 219–230. [Google Scholar] [CrossRef]
- Diels, S.; Cuypers, B.; Tvarijonaviciute, A.; Derudas, B.; Van Dijck, E.; Verrijken, A.; Van Gaal, L.F.; Laukens, K.; Lefebvre, P.; Ceron, J.J.; et al. A targeted multi-omics approach reveals paraoxonase-1 as a determinant of obesity-associated fatty liver disease. Clin. Epigenet 2021, 13, 142. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, X.H.; Qiu, C.; Shen, H.; Zhao, Q.; Zhao, L.J.; Tian, Q.; Sun, C.Q.; Deng, H.W. Integrative analysis of multi-omics data to detect the underlying molecular mechanisms for obesity in vivo in humans. Hum. Genom. 2022, 16, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Odoemelam, C.S.; Naz, A.; Thanaj, M.; Sorokin, E.P.; Whitcher, B.; Sattar, N.; Bell, J.D.; Thomas, E.L.; Cule, M.; Yaghootkar, H. Identifying four obesity axes through integrative multiomics and imaging analysis. Diabetes 2025, 74, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, S.; Dorner, T.E.; Wiesinger, T.; Stein, K.V. Multi-omics approaches for precision obesity management. Wien. Klin. Wochenschr. 2023, 135, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Edpuganti, S.; Thomas, K.S.; Ponnuri, S.A.; Tomar, R.K.; Kapuge, P.H. Obesity and metabolic syndrome: Pathophysiological mechanisms driving cardiovascular risk. Jr. Res. J. 2025, 5, 20–35. [Google Scholar] [CrossRef]
- Llongueras-Espí, P.; García-Romero, E.; Comín-Colet, J.; González-Costello, J. Role of Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs) in Patients with Chronic Heart Failure. Biomolecules 2025, 15, 1342. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Zou, R.; Zhang, M.; Lv, W.; Ren, J.; Fan, X. Role of epicardial adipose tissue in cardiac remodeling. Diabetes Res. Clin. Pr. 2024, 217, 111878. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, M.M.; Petersen, S.E. Epicardial adipose tissue: From cardiac wrapper to remodeller? Eur. J. Prev. Cardiol. 2025, 22, zwaf455. [Google Scholar] [CrossRef]
- Ma, M.; Zheng, X.; Wu, X.; Xie, Q. Epicardial adipose tissue thickness on transthoracic echocardiography predicts 2-year incident atrial fibrillation in elderly hypertensive patients. Front. Cardiovasc. Med. 2025, 12, 1650423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chi, J.; Wang, C.; Yang, Y.; Tian, R.; Chen, X. Epicardial Adipose Tissue in Patients with Coronary Artery Disease: A Meta-Analysis. J. Cardiovasc. Dev. Dis. 2022, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcao-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial Adipose Tissue Volume Assessed by Computed Tomography and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 490–497. [Google Scholar] [CrossRef]
- Mohamed, O.M.A.A.; Omer, S.S.B.; Mukhtar, M.; Mohmmed, T.; Mohamed, W.M.H.; Hassan, M.H.M.; Hassan, I.M. The Role of Epicardial Fat Thickness and B-type Natriuretic Peptide (BNP)/N-terminal Pro B-type Natriuretic Peptide (NT-proBNP) in Heart Failure Risk Stratification: A Systematic Review. Cureus 2025, 17, e84184. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Xiao, C.; Li, J.; Hu, T.; Li, L. Association between waist-to-hip ratio and risk of myocardial infarction: A systematic evaluation and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1438817. [Google Scholar] [CrossRef]
- de Koning, L.; Merchant, A.T.; Pogue, J.; Anand, S.S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur. Heart J. 2007, 28, 850–856. [Google Scholar] [CrossRef]
- Yi, D.; Tang, X.; Xing, Z. Visceral and subcutaneous adiposity and cardiovascular disease: Unravelling associations and prognostic value. Diabetes Obes. Metab. 2024, 15, 5819–5826. [Google Scholar] [CrossRef]
- Chong, B.; Jayabaskaran, J.; Ruban, J.; Goh, R.; Chin, Y.H.; Kong, G.; Ng, C.H.; Lin, C.; Loong, S.; Muthiah, M.D.; et al. Epicardial Adipose Tissue Assessed by Computed Tomography and Echocardiography Are Associated with Adverse Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2023, 16, e015159. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).