Uterine Angiomyolipoma Presenting as a Rapidly Growing Uterine Mass in a Postmenopausal Woman
Abstract
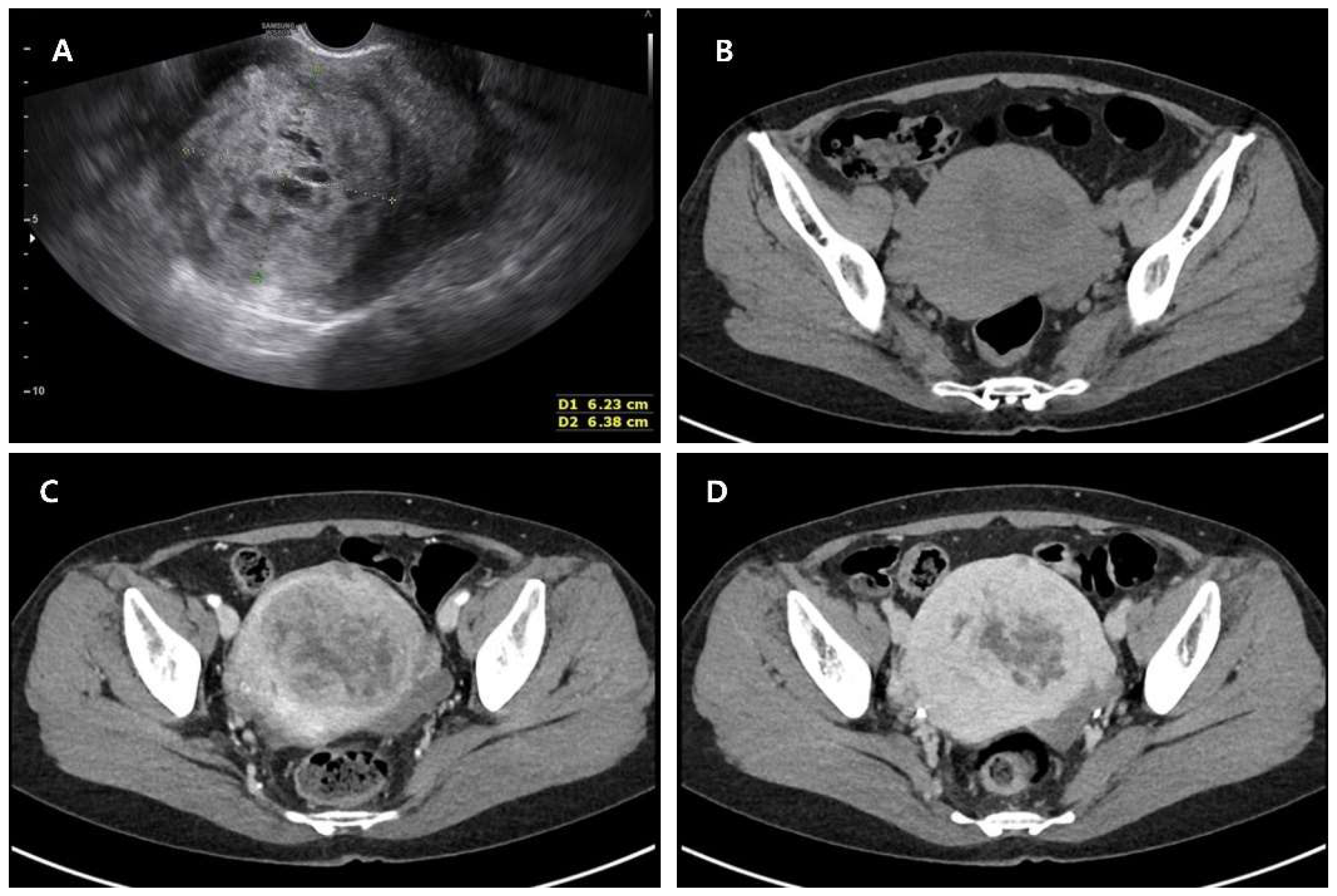
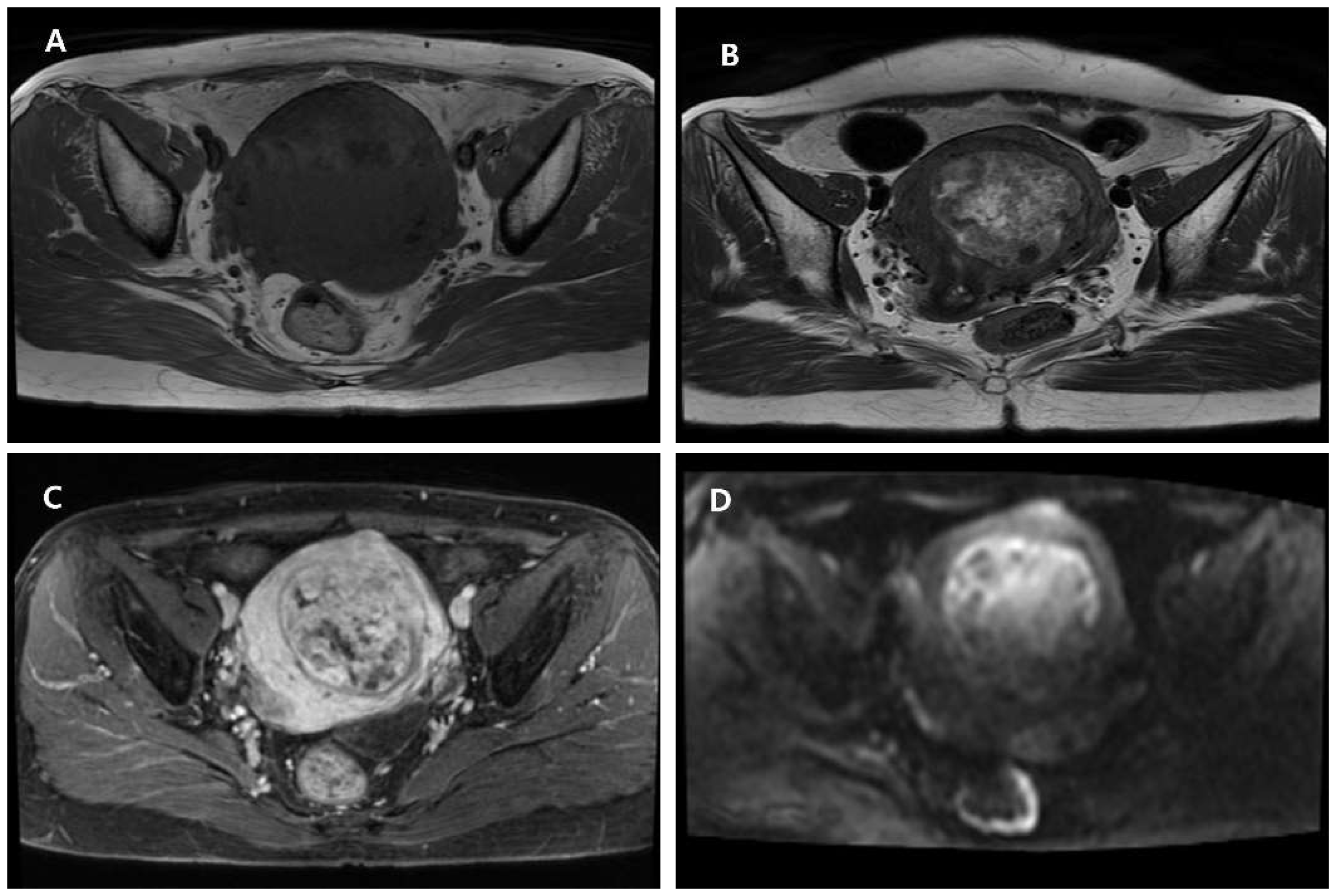
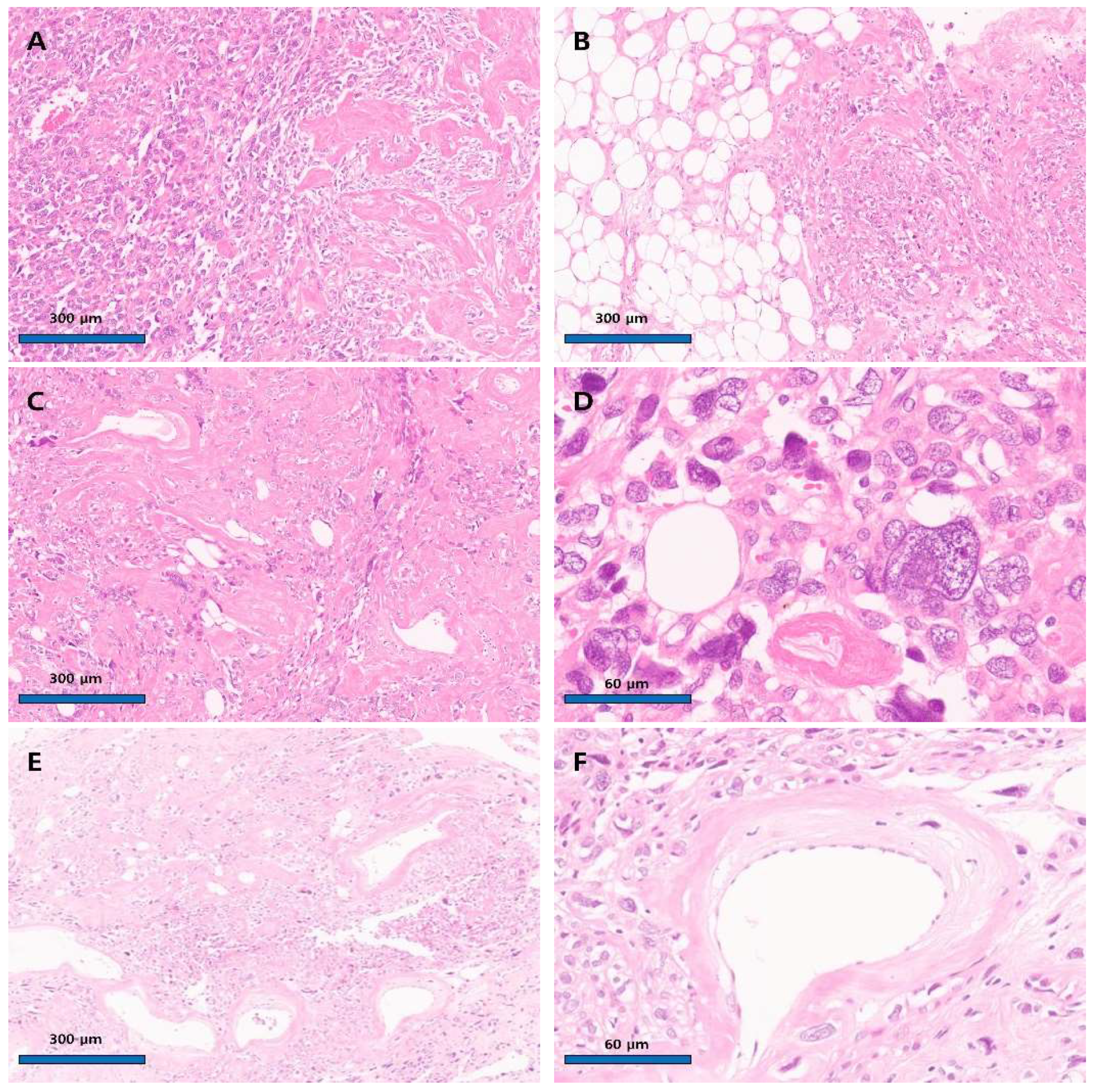
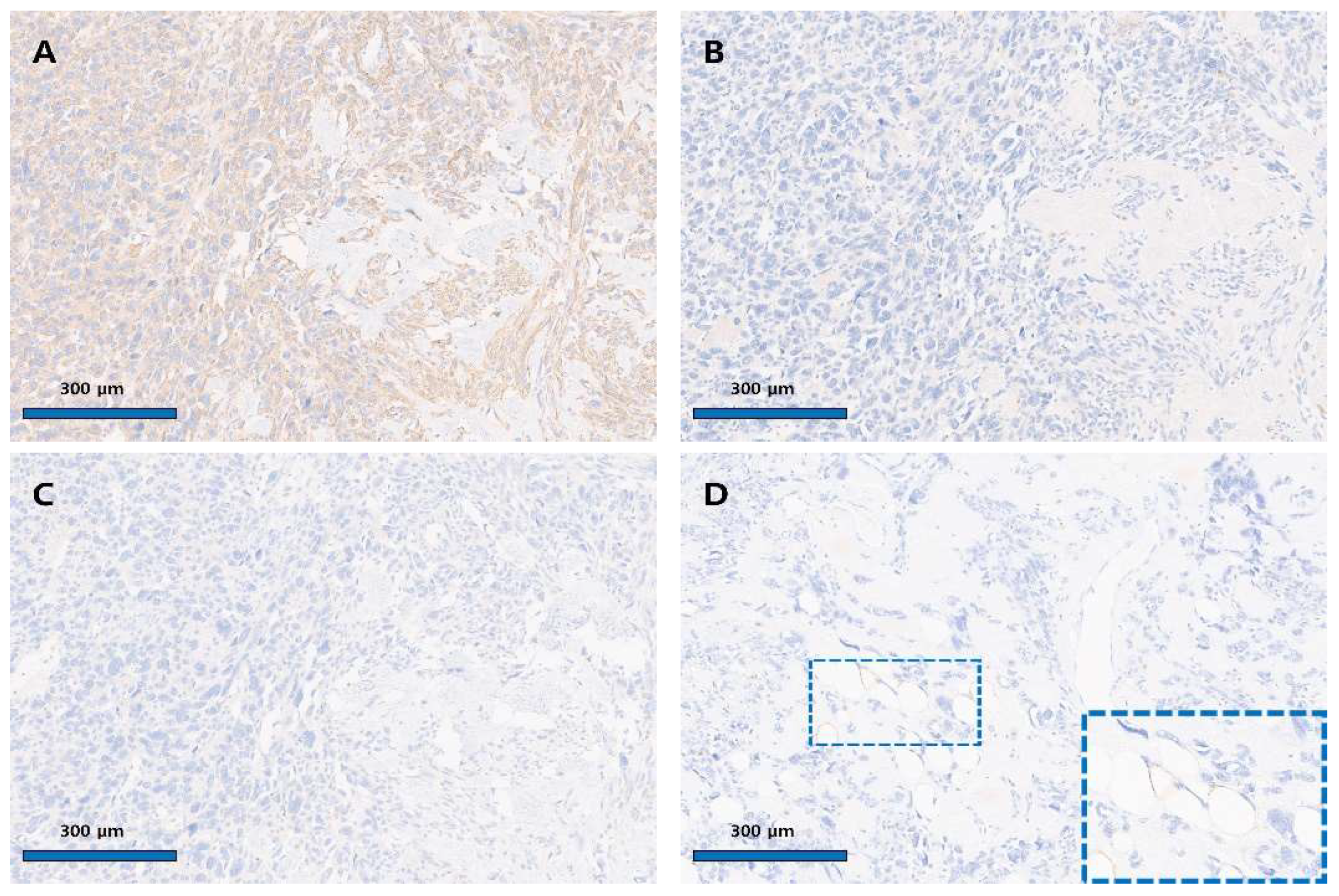
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Angiomyolipoma |
| PEComa | Perivascular epithelioid cell tumor |
| SMA | Smooth Muscle Actin |
| HMB-45 | Human Melanoma Black-45 |
References
- Anderson, A.E.; Yang, X.; Young, R.H. Epithelioid angiomyolipoma of the ovary: A case report and literature review. Int. J. Gynecol. Pathol. 2002, 21, 69–73. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cheville, J.C.; Sadow, P.M.; Gopalan, A.; Fine, S.W.; Al-Ahmadie, H.A.; Chen, Y.-B.; Oliva, E.; Russo, P.; Reuter, E.V.; et al. Epithelioid angiomyolipoma of the kidney: Pathological features and clinical outcome in a series of consecutively resected tumors. Mod. Pathol. 2013, 26, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, J.; Jinzaki, M.; Yao, M.; Nagashima, Y.; Mikami, S.; Yashiro, H.; Nozaki, M.; Mizuno, R.; Oya, M.; Kuribayashi, S. Epithelioid angiomyolipoma of the kidney: Radiological imaging. Int. J. Urol. 2013, 20, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Psomiadou, V.; Iavazzo, C.; Geramani, E.; Fotiou, A.; Karelis, L.; Valavanis, C.; Lekka, S.; Kokkali, K.; Vorgias, G. Uterine angiolipoleiomyoma. A case report and systematic literature review. Folia Med. 2022, 64, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Braga, A.C.; Pinto, A.; Van de Vijver, K.; Cornejo, K.; Pesci, A.; Zhang, L.; Vicente, M.-O.; Takako, K.; Gian, F.Z.; et al. Uterine PEComas: A Morphologic, Immunohistochemical, and Molecular Analysis of 32 Tumors. Am. J. Surg. Pathol. 2018, 42, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L.; Mentzel, T.; Lehr, H.A.; Fisher, C.; Balzer, B.L.; Weiss, S.W. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: A clinicopathologic study of 26 cases and review of the literature. Am. J. Surg. Pathol. 2005, 29, 1558–1575. [Google Scholar] [CrossRef] [PubMed]
- Schoolmeester, J.K.; Howitt, B.E.; Hirsch, M.S.; Dal Cin, P.; Quade, B.J.; Nucci, M.R. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: Clinicopathologic and immunohistochemical characterization of 16 cases. Am. J. Surg. Pathol. 2014, 38, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Tanveer, N. Angiolipoleiomyoma or HMB-45 Negative Angiomyolipoma of Uterus: Need for Uniformity in Terminology. Indian J. Surg. Oncol. 2021, 12, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Laffargue, F.; Giacalone, P.L.; Charpin, C.; Lachard, A. Uterine angiomyolipoma associated with pregnancy. Gynecol. Oncol. 1993, 50, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, H.; Moriya, T.; Soeda, S.; Yonemoto, Y.; Nagura, H.; Sasano, H. Uterine angiomyolipoma: Case report and review of the literature. Pathol. Int. 2001, 51, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Cil, A.P.; Haberal, A.; Hucumenoglu, S.; Kovalak, E.E.; Gunes, M. Angiomyolipoma of the uterus associated with tuberous sclerosis: Case report and review of the literature. Gynecol. Oncol. 2004, 94, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoo, J.Y.; Yoo, S.H.; Seo, Y.H.; Yoon, J.H. Uterine angiomyolipoma with metastasis in a woman with tuberous sclerosis: A case report. Eur. J. Gynaecol. Oncol. 2013, 34, 339–342. [Google Scholar] [PubMed]
- Roy, I.; Jain, P.; Kaur, H. Angiomyolipoma of Uterus: A Case Report. J. Obstet. Gynaecol. India 2022, 72 (Suppl. 2), 418–420. [Google Scholar] [CrossRef] [PubMed]
- Verocq, C.; Noel, J.C.; Ouertani, S.; D’Haene, N.; Catteau, X. First Case Report of a Uterine Angiolipoleiomyoma with KRAS and KIT Mutations. Int. J. Gynecol. Pathol. 2022, 41, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Schoolmeester, J.K.; Dao, L.N.; Sukov, W.R.; Wang, L.; Park, K.J.; Murali, R.; Hameed, M.R.; Soslow, R.A. TFE3 translocation-associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: Morphology, immunophenotype, differential diagnosis. Am. J. Surg. Pathol. 2015, 39, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.C.; Chung, M.Y.; Ng, K.F.; Liu, C.Y.; Wang, J.S.; Chai, C.Y.; Huang, S.; Chen, P.; Ho, D. Constant allelic alteration on chromosome 16p (TSC2 gene) in perivascular epithelioid cell tumour (PEComa): Genetic evidence for the relationship of PEComa with angiomyolipoma. J. Pathol. 2008, 214, 387–393. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.H.; An, H.J.; Baek, J.C. Uterine Angiomyolipoma Presenting as a Rapidly Growing Uterine Mass in a Postmenopausal Woman. Diagnostics 2025, 15, 2995. https://doi.org/10.3390/diagnostics15232995
Song DH, An HJ, Baek JC. Uterine Angiomyolipoma Presenting as a Rapidly Growing Uterine Mass in a Postmenopausal Woman. Diagnostics. 2025; 15(23):2995. https://doi.org/10.3390/diagnostics15232995
Chicago/Turabian StyleSong, Dae Hyun, Hyo Jung An, and Jong Chul Baek. 2025. "Uterine Angiomyolipoma Presenting as a Rapidly Growing Uterine Mass in a Postmenopausal Woman" Diagnostics 15, no. 23: 2995. https://doi.org/10.3390/diagnostics15232995
APA StyleSong, D. H., An, H. J., & Baek, J. C. (2025). Uterine Angiomyolipoma Presenting as a Rapidly Growing Uterine Mass in a Postmenopausal Woman. Diagnostics, 15(23), 2995. https://doi.org/10.3390/diagnostics15232995





