Immuno-Hematological Profiles of HIV-Positive Patients Stratified by CD4+ T-Cell Counts: Toward Identifying a Surrogate Hematological Marker for Immune Suppression Severity
Abstract
1. Introduction
2. Materials and Methods
- -
- Group I (severe immunosuppression)—CD4+ < 200 cells/mm3
- -
- Group II (moderate immunosuppression)—CD4+ 200–500 cells/mm3
- -
- Group III (preserved immunity)—CD4+ > 500 cells/mm3
- -
- Hematologic data, namely, Red Blood Cell (RBC) counts, Hb, Hct, platelet counts, mean platelet volume (MPV), total WBC counts, the erythrocyte sedimentation rate (ESR), and lymphocyte counts (absolute and relative);
- -
- Immunologic data, namely, CD3+, CD4+, CD8+, B lymphocytes, Natural Killer (NK) cells, and CD4/CD8 ratios.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immunodeficiency Syndrome |
| ALC | Absolute Lymphocyte Count |
| ANOVA | Analysis of Variance |
| ART | Antiretroviral Therapy |
| BMI | Body Mass Index |
| CD | Cluster of Differentiation |
| ESR | Erythrocyte Sedimentation Rate |
| HAART | Highly Active Antiretroviral Therapy |
| Hb | Hemoglobin |
| Hct | Hematocrit |
| HIV | Human Immunodeficiency Virus |
| IRB | Institutional Review Board |
| MENA | Middle East and North Africa |
| NK | Natural Killer (cells) |
| PLHIV | People Living with HIV |
| RBC | Red Blood Cell |
| TLC | Total Lymphocyte Count |
| WBC | White Blood Cell |
| WHO | World Health Organization |
References
- Opie, J.; Verburgh, E.; Bailly, J.; Mayne, E.; Louw, V. Hematological complications of human immunodeficiency virus (HIV) infection: An update from an HIV-endemic setting. Open Forum Infect. Dis. 2024, 11, ofae162. [Google Scholar] [CrossRef]
- Vu, T.T.; Rupasinghe, D.; Khol, V.; Chaiwarith, R.; Tanuma, J.; Kumarasamy, N.; Khusuwan, S.; Somia, I.A.; Pujari, S.; Lee, M.P.; et al. Temporal trends from HIV diagnosis to ART initiation among adults living with HIV in the Asia–Pacific (2013–2023). AIDS Res. Ther. 2025, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Al-Mozaini, M.A.; Mansour, M.K.; Al-Hokail, A.A.; Mohmed, M.A.; Daham, M.A.; Al-Abdely, H.M.; Frayha, H.H.; Al-Rabiah, F.A.; Alhajjar, S.H.; Keshavjee, S.; et al. HIV-Care Outcome in Saudi Arabia: A Longitudinal Cohort. J. AIDS Clin. Res. 2014, 5, 370. [Google Scholar] [CrossRef]
- Malapati, B.; Nadeem, S.M.; Shah, M.M. Analysis of blood parameters in HIV positive patients. Int. J. Clin. Biochem. Res. 2020, 7, 388–394. [Google Scholar] [CrossRef]
- Oladele, A. Assessment of Haematological and Biochemical indices of HIV Patients at Diagnosis and Last Visit at the Imo State University Teaching Hospital, Orlu (A 6-Year Retrospective Study; 2008–2013). Available online: https://www.academia.edu/50584705/Assessment_of_Haematological_and_Biochemical_indices_of_HIV_Patients_at_Diagnosis_and_Last_Visit_at_the_Imo_State_University_Teaching_Hospital_Orlu_A_6_year_retrospective_study_2008_2013_ (accessed on 20 January 2025).
- Vajpayee, M.; Kaushik, S.; Sreenivas, V.; Wig, N.; Seth, P. CDC staging based on absolute CD4 count and CD4 percentage in an HIV-1-infected Indian population: Treatment implications. Clin. Exp. Immunol. 2005, 141, 485–490. [Google Scholar] [CrossRef]
- Elghazaly, A.; AlSaeed, N.; Islam, S.; Alsharif, I.; Alharbi, L.; Al Ashagr, T.; Alshanifi, A.; Alrashoudi, R.; Alsharidi, A.; Alhokail, A.; et al. Assessing the knowledge and attitude towards HIV/AIDS among the general population and health care professionals in MENA region. PLoS ONE 2023, 18, e0288838. [Google Scholar] [CrossRef] [PubMed]
- Al-Mozaini, M.; Al-Rahabani, T.; Dirar, Q.; Alashgar, T.; Rabaan, A.A.; Murad, W.; Alotaibi, J.; Alrajhi, A. Human Immunodeficiency Virus in Saudi Arabia: Current and Future Challenges. J. Infect. Public Health 2023, 16, 1501–1509. [Google Scholar] [CrossRef]
- Al-Mazrou, Y.Y.; Al-Jeffri, M.H.; Fidail, A.I.; Al-Huzaim, N.; El-Gizouli, S.E. HIV/AIDS epidemic features and trends in Saudi Arabia. Ann. Saudi Med. 2005, 25, 100–104. [Google Scholar] [CrossRef]
- Wisaksana, R.; Sumantri, R.; Indrati, A.R.; Zwitser, A.; Jusuf, H.; de Mast, Q.; van Crevel, R.; van der Ven, A. Anemia and iron homeostasis in a cohort of HIV-infected patients in Indonesia. BMC Infect. Dis. 2011, 11, 213. [Google Scholar] [CrossRef]
- Wondimeneh, Y.; Ferede, G.; Yismaw, G.; Muluye, D. Total Lymphocyte Count as a surrogate marker for CD4 Cell Count in HIV-infected individuals in Gondar University Hospital, Northwest Ethiopia. AIDS Res. Ther. 2012, 9, 21. [Google Scholar] [CrossRef]
- Moore, D.M.; Awor, A.; Downing, R.S.; Were, W.; Solberg, P.; Tu, D.; Chan, K.; Hogg, R.S.; Mermin, J. Determining eligibility for antiretroviral therapy in resource-limited settings using total lymphocyte counts, hemoglobin, and body mass index. AIDS Res. Ther. 2007, 4, 1. [Google Scholar] [CrossRef]
- Shaukat, S.N.; Khan, S.; Raza, A.; Khanani, R.; Ghayaz, A.; Kazmi, S.U. Prognostic markers in HIV mono- and co-infected individuals: A study from Karachi-Pakistan. J. Infect. Public Health 2018, 11, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Rafatpanah, H.; Essmailian, L.; Hedayati-Moghaddam, M.R.; Vakili, R.; Norouzi, M.; Sarvghad, M.R.; Hosseinpour, A.M.; Sharebiani, H.; Rezaee, S.A.R. Evaluation of non-viral surrogate markers as predictive indicators for monitoring progression of human immunodeficiency virus infection: An eight-year analysis in a regional center. Jpn. J. Infect. Dis. 2016, 69, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Saudi Ministry of Health; The Saudi AIDS National Program (NAP). Saudi Guidelines for HIV Treatment, 3rd ed.; Ministry of Health: Riyadh, Saudi Arabia, 2024. Available online: https://www.moh.gov.sa/ (accessed on 20 January 2025).
- Al-Mughales, J.A. Development and validation of a three-parameter scoring system for monitoring HIV/AIDS patients in low-resource settings using hematological parameters. HIV/AIDS—Res. Palliat. Care 2023, 15, 599–610. [Google Scholar] [CrossRef]
- Kwantwi, L.B.; Tunu, B.K.; Boateng, D.; Quansah, D.Y. Body Mass Index, Haemoglobin, and Total Lymphocyte Count as a Surrogate for CD4 Count in Resource-Limited Settings. J. Biomark. 2017, 2017, 7907352. [Google Scholar] [CrossRef]
- Al-Mozaini, M.; Alrahbeni, T.; Dirar, Q.; Alotibi, J.; Alrajhi, A. HIV in the Kingdom of Saudi Arabia: Can we Change the Way We Deal with Co-Infections? Infect. Drug Resist. 2021, 14, 111–117. [Google Scholar] [CrossRef]
- Buseri, F.I.; Mark, D.; Jeremiah, Z.A. Evaluation of absolute lymphocyte count as a surrogate marker for CD4+ cell count for the initiation of antiretroviral therapy (ART) in resource-limited settings. Int. J. Biomed. Lab. Sci. 2012, 1, 44–49. [Google Scholar]
- Serrano-Villar, S.; Moreno, S.; Fuentes-Ferrer, M.; Sánchez-Marcos, C.; Ávila, M.; Sainz, T.; de Villar, N.; Fernández-Cruz, A.; Estrada, V. The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. HIV Med. 2014, 15, 40–49. [Google Scholar] [CrossRef]
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 20 January 2025).
- Damtie, S.; Workineh, L.; Kiros, T.; Eyayu, T.; Tiruneh, T. Hematological Abnormalities of Adult HIV-Infected Patients Before and After Initiation of Highly Active Antiretroviral Treatment at Debre Tabor Comprehensive Specialized Hospital, Northcentral Ethiopia: A Cross-Sectional Study. HIV AIDS 2021, 13, 477–484. [Google Scholar] [CrossRef]
- Sainz, T.; Serrano-Villar, S.; Díaz, L.; González Tomé, M.I.; Gurbindo, M.D.; de José, M.I.; Mellado, M.J.; Ramos, J.T.; Zamora, J.; Moreno, S.; et al. The CD4/CD8 Ratio as a Marker of T-Cell Activation, Senescence and Activation/Exhaustion in Treated HIV-Infected Children and Young Adults. AIDS 2013, 27, 1513–1516. [Google Scholar] [CrossRef]
- Ikeogu, M.O.; Okwara, J.E.; Okafor, H.U. Hematologic abnormalities in treatment-naïve HIV-infected children in Enugu, Nigeria. BMC Hematol. 2020, 20, 1. [Google Scholar]
- Nasir, E.F. HIV-/AIDS-Related Knowledge and Attitudes Among Saudi Health Professionals and Students: A Review Article. Eur. J. Gen. Dent. 2025; advance online publication. [Google Scholar]
- Ministry of Health. Global AIDS Response Progress Report: Country Progress Report 2015; Ministry of Health: Riyadh, Saudi Arabia, 2015. [Google Scholar]
- Anyabolu, E.N.; Chukwuonye, I.I.; Anyabolu, A.E.; Enwere, O. A study of clinical and biochemical profiles of HIV subjects in Owerri, southeast Nigeria. J. Med. Sci. Clin. Res. 2015, 3, 8643–8650. [Google Scholar] [CrossRef]
- Shalaka, N.S.; Garred, N.A.; Zeglam, H.T.; Awasi, S.A.; Abukathir, L.A.; Altagdi, M.E.; Rayes, A.A. Clinical profile and factors associated with mortality in hospitalized patients with HIV/AIDS: A retrospective analysis from Tripoli Medical Centre, Libya, 2013. East. Mediterr. Health J. 2015, 21, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Ezeonwu, B.U.; Ikefuna, A.N.; Oguonu, T.; Okafor, H.U. Correlation between total lymphocyte count, Hb, hematocrit, and CD4 count in HIV patients in Nigeria. Pak. J. Biol. Sci. 2014, 17, 689–692. [Google Scholar] [CrossRef]
- Filemban, S.M.; Yasein, Y.A.; Abdalla, M.H.H.; Al-Hakeem, R.; Al-Tawfiq, J.A.; Memish, Z.A. Prevalence and behavioral risk factors for STIs/HIV among attendees of the Ministry of Health hospitals in Saudi Arabia. J. Infect. Dev. Ctries. 2015, 9, 402–408. [Google Scholar] [CrossRef]

| Parameter | Mean ± SD and Frequency (Percentage) |
|---|---|
| Age (Years) | 41.9 ± 11.9 |
| Gender | |
| Male | 79% |
| Female | 21% |
| RBC | 4.8 ± 0.8 × 106/µL |
| Normal | 71.6% |
| Deficiency | 28.4% |
| Hemoglobin (Hb) | 137.5 ± 22.6 g/dL |
| Normal | 74.7% |
| Deficiency | 25.3% |
| Hematocrit (Hct) | 0.4 ± 0.08 L/L |
| Normal | 62% |
| Deficiency | 38% |
| ESR | 49.5 ± 35.7 mm/h |
| Normal | 21.5% |
| Higher than normal | 78.5% |
| Platelets | 250.9 ± 76.1 |
| Lower than normal | 4.4% |
| Normal | 91.7% |
| Higher than normal | 3.9% |
| MVP | 9.9 ± 0.9 |
| Normal | 74.7% |
| Higher than normal | 25.3% |
| WBC | 6.2 ± 2.4 × 103/µL |
| Lower than normal | 16.6% |
| Normal | 80.5% |
| Higher than normal | 2.6% |
| Relative Lymphocytes % | 39 ± 12.9 |
| Lower than normal | 8.2% |
| Normal | 42.6% |
| Higher than normal | 49.2% |
| Lymphocytes (Absolute) | 2.4 ± 1 × 103/µL |
| Lower than normal | 8.3% |
| Normal | 65.4% |
| Higher than normal | 26.3% |
| Lymphocyte Event | 3690.6 ± 1855.7 cells/µL |
| CD3 | 1502.4 ± 1105.1 × 103/µL |
| Lower than normal | 24.9% |
| Normal | 55.5% |
| Higher than normal | 19.7% |
| CD4 | 544.9 ± 506.7 cells/µL |
| Lower than normal | 44.5% |
| Normal | 52.8% |
| Higher than normal | 2.6% |
| CD8 | 906 ± 760.3 cells/µL |
| Lower than normal | 22.7% |
| Normal | 47.6% |
| Higher than normal | 29.7% |
| NK | 225.8 ± 216.4 cells/µL |
| Lower than normal | 27.5% |
| Normal | 59.8% |
| Higher than normal | 12.7% |
| B Lymphocyte | 217.4 ± 219.3 cells/µL |
| Lower than normal | 32.3% |
| Normal | 60.7% |
| Higher than normal | 7% |
| 4/8 Ratio | 0.6 ± 0.5 |
| Normal | 24.9% |
| Abnormal | 85.1% |
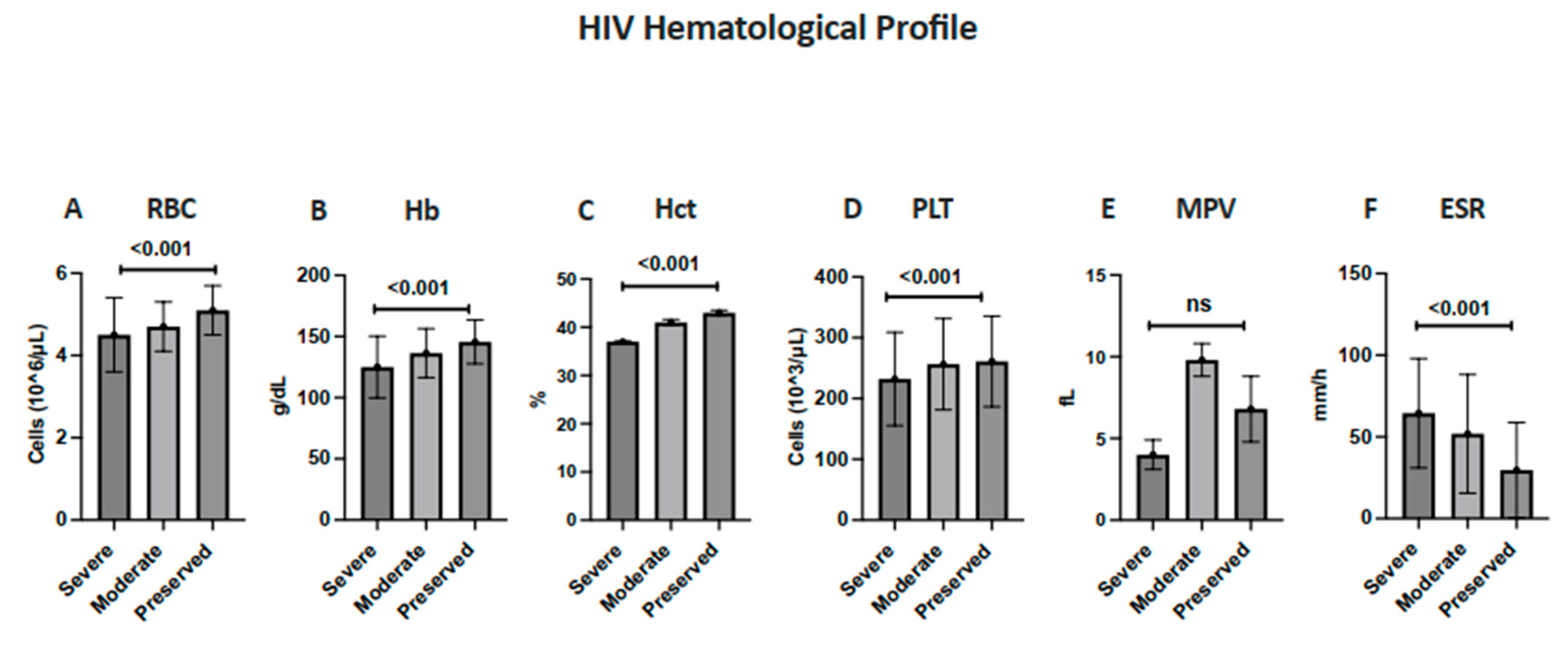
| Parameter | Severe Immunosuppression | Moderate Immunosuppression | Preserved Immunity | |||
|---|---|---|---|---|---|---|
| Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | |
| RBC | 44 (60.3%) | 29 (39.7%) | 25 (57.1%) | 19 (42.9%) | 96 (86.4%) | 16 (13.6%) |
| Hb | 40 (54.8%) | 33 (45.2%) | 31 (70.5%) | 13 (29.5%) | 100 (89.3%) | 12 (10.7%) |
| Hct | 31 (42.5%) | 42 (57.5%) | 25 (56.8%) | 19 (43.2%) | 86 (76.8%) | 26 (23.2%) |
| ESR | 2 (3.2%) | 71 (96.8%) | 10 (22.8%) | 34 (76.2%) | 46 (40.7%) | 66 (59.3%) |
| Platelets | 65 (89.0%) | 8 (11.0%) | 41 (93.2%) | 3 (6.8%) | 104 (92.9%) | 8 (7.1%) |
| MPV | 55 (75.3%) | 18 (24.7%) | 33 (75.0%) | 11 (25.0%) | 83 (74.1%) | 29 (25.9%) |
| WBC | 50 (68.5%) | 23 (31.5%) | 34 (77.3%) | 10 (22.7%) | 101 (90.2%) | 11 (9.8%) |
| Lymphocytes (Relative %) | 41 (56.6%) | 32 (43.4%) | 20 (46.2%) | 24 (53.8%) | 37 (33.0%) | 75 (67.0%) |
| Lymphocytes (absolute) | 43 (58.9%) | 30 (41.1%) | 37 (85.7%) | 7 (14.3%) | 69 (61.8%) | 43 (38.2%) |
| CD3 | 17 (23.3%) | 56 (76.7%) | 40 (90.9%) | 4 (9.1%) | 70 (62.5%) | 42 (37.5%) |
| CD4 | 0 (0.0%) | 73 (100%) | 15 (34.1%) | 29 (65.9%) | 106 (94.6%) | 6 (5.4%) |
| CD8 | 21 (28.8%) | 52 (71.2%) | 30 (68.2%) | 14 (31.8%) | 58 (51.8%) | 54 (48.2%) |
| NK | 18 (24.7%) | 55 (75.3%) | 32 (72.7%) | 12 (27.3%) | 87 (77.7%) | 25 (22.3%) |
| B-lymphocytes | 10 (13.7%) | 63 (86.3%) | 40 (90.9%) | 4 (9.1%) | 89 (79.5%) | 23 (20.5%) |
| CD4/CD8 ratio | 10 (13.7%) | 63 (86.3%) | 1 (2.3%) | 43 (97.7%) | 46 (41.1%) | 66 (58.9%) |
| Co-infection | Yes 19 (17%)/No 93 (83%) | Yes 6 (14%)/No 38 (86%) | Yes 11 (15%)/No 62 (85%) | |||
| Parameter | r (p-Value) |
|---|---|
| Age | 0.01 (0.88) |
| RBC | 0.32 (<0.001) |
| Hb | 0.318 (<0.001) |
| Hct | 0.338 (<0.001) |
| ESR | −0.369 (0.001) |
| Platelet | 0.198 (0.003) |
| MPV | −0.099 (0.136) |
| WBC | 0.289 (<0.001) |
| Lymphocyte relative % | 0.295 (<0.001) |
| Lymphocyte relative | 0.276 (<0.001) |
| Lymphocyte absolute | 0.505 (<0.001) |
| Lymphocyte event | 0.058 (0.385) |
| CD3+ | 0.762 (<0.001) |
| CD8+ | 0.411 (<0.001) |
| NK | 0.358 (<0.001) |
| B-lymphocytes | 0.63 (<0.001) |
| 4/8 ratio | 0.555 (<0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbaeen, A.F.; Iqbal, M.S.; Alsafi, R.; Al Masmoum, H.; Qadhi, A.; Alharbi, W.; Alharbi, A.M.; Kedwa, K.; Albeshri, M.H.; Alameer, M.S. Immuno-Hematological Profiles of HIV-Positive Patients Stratified by CD4+ T-Cell Counts: Toward Identifying a Surrogate Hematological Marker for Immune Suppression Severity. Diagnostics 2025, 15, 2976. https://doi.org/10.3390/diagnostics15232976
Arbaeen AF, Iqbal MS, Alsafi R, Al Masmoum H, Qadhi A, Alharbi W, Alharbi AM, Kedwa K, Albeshri MH, Alameer MS. Immuno-Hematological Profiles of HIV-Positive Patients Stratified by CD4+ T-Cell Counts: Toward Identifying a Surrogate Hematological Marker for Immune Suppression Severity. Diagnostics. 2025; 15(23):2976. https://doi.org/10.3390/diagnostics15232976
Chicago/Turabian StyleArbaeen, Ahmad F., Mohammad Shahid Iqbal, Radi Alsafi, Hibbah Al Masmoum, Alaa Qadhi, Waheeb Alharbi, Ahmad M. Alharbi, Khalid Kedwa, Mohammad H. Albeshri, and Mohammed S. Alameer. 2025. "Immuno-Hematological Profiles of HIV-Positive Patients Stratified by CD4+ T-Cell Counts: Toward Identifying a Surrogate Hematological Marker for Immune Suppression Severity" Diagnostics 15, no. 23: 2976. https://doi.org/10.3390/diagnostics15232976
APA StyleArbaeen, A. F., Iqbal, M. S., Alsafi, R., Al Masmoum, H., Qadhi, A., Alharbi, W., Alharbi, A. M., Kedwa, K., Albeshri, M. H., & Alameer, M. S. (2025). Immuno-Hematological Profiles of HIV-Positive Patients Stratified by CD4+ T-Cell Counts: Toward Identifying a Surrogate Hematological Marker for Immune Suppression Severity. Diagnostics, 15(23), 2976. https://doi.org/10.3390/diagnostics15232976






