Unveiling Primary Bone Tumors of the Spine: A Review of Essential Imaging Clues
Abstract
1. Introduction
2. Imaging Findings
2.1. Osteogenic
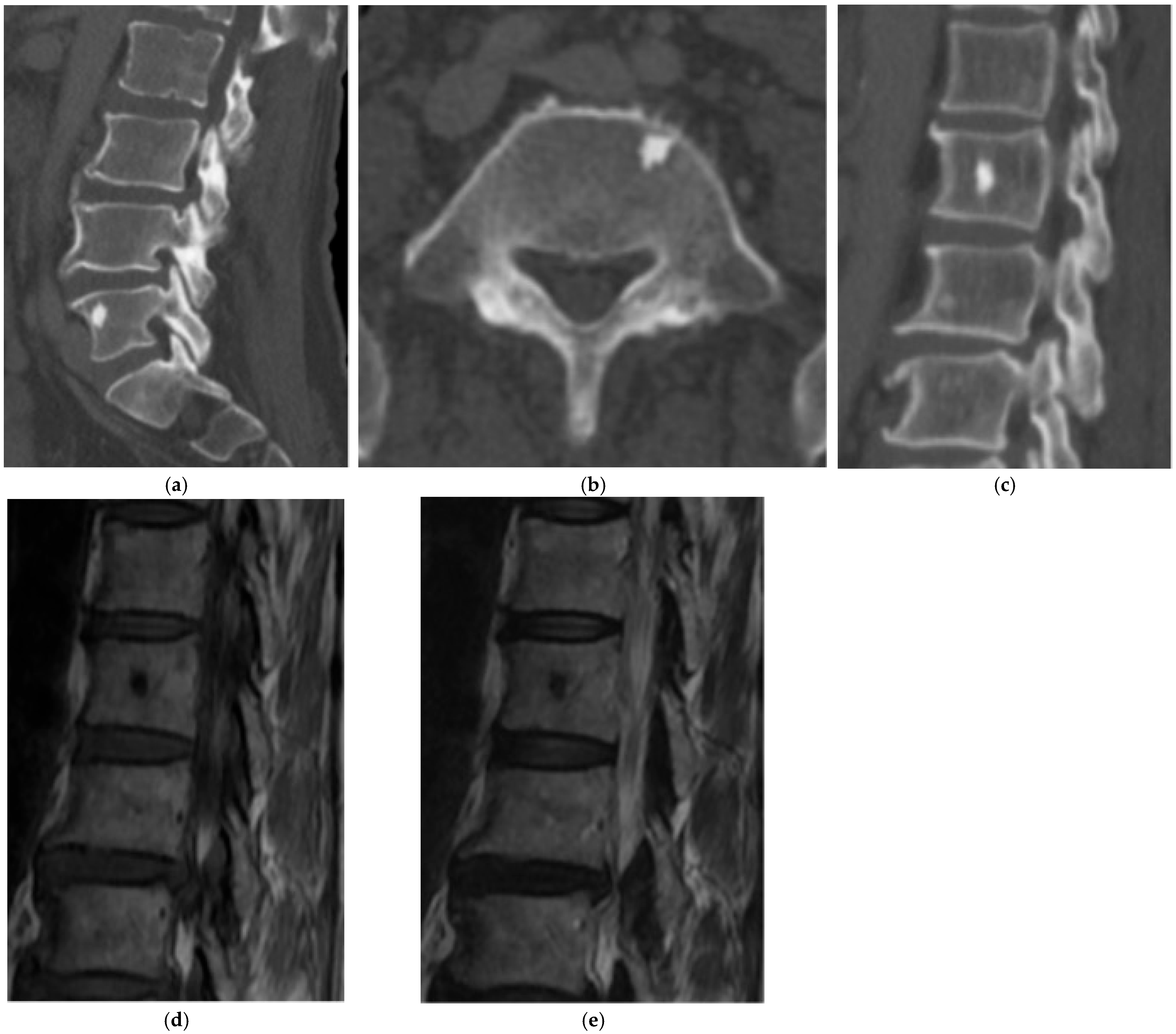
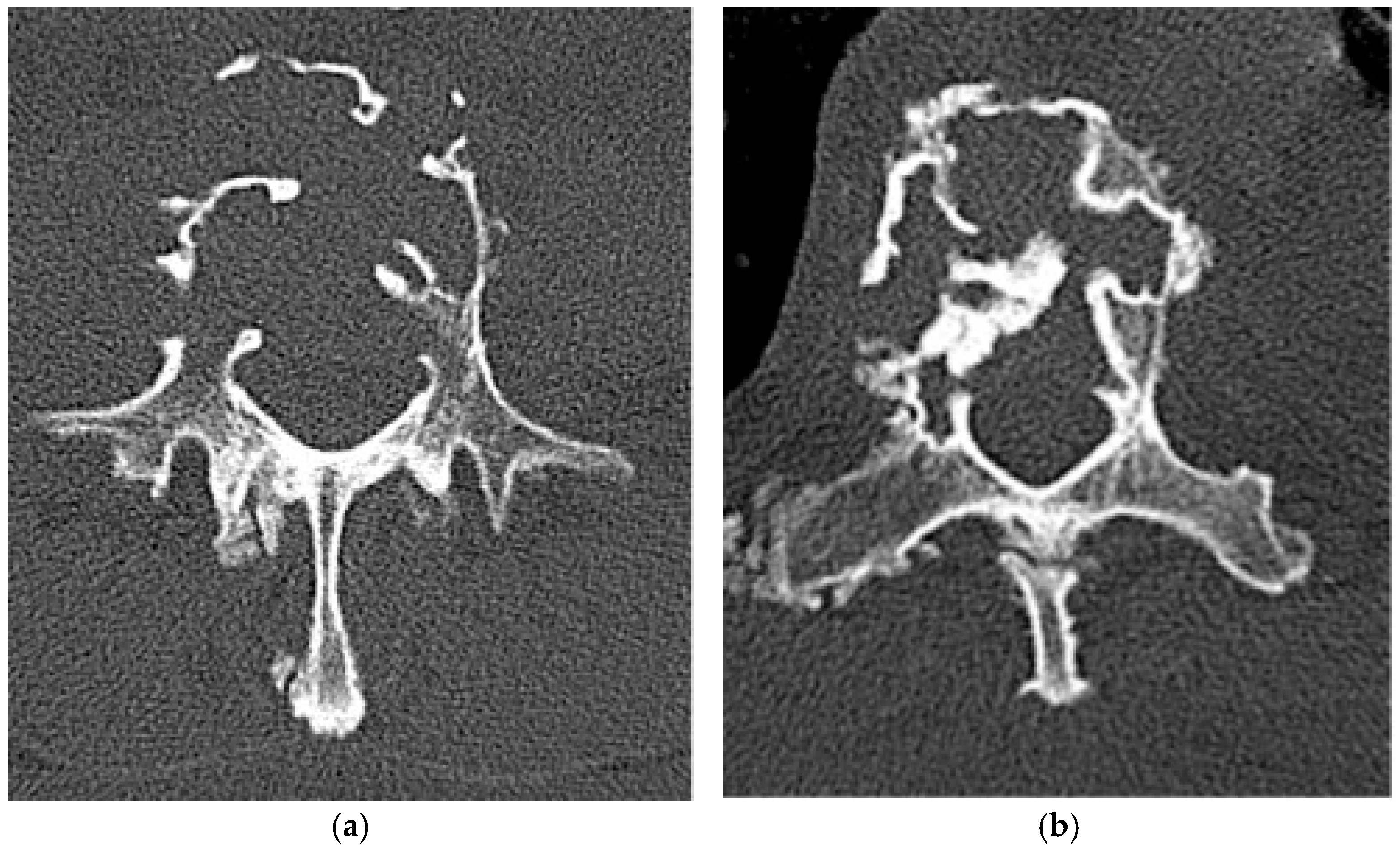
2.1.1. Axial Enostosis (Bone Island)
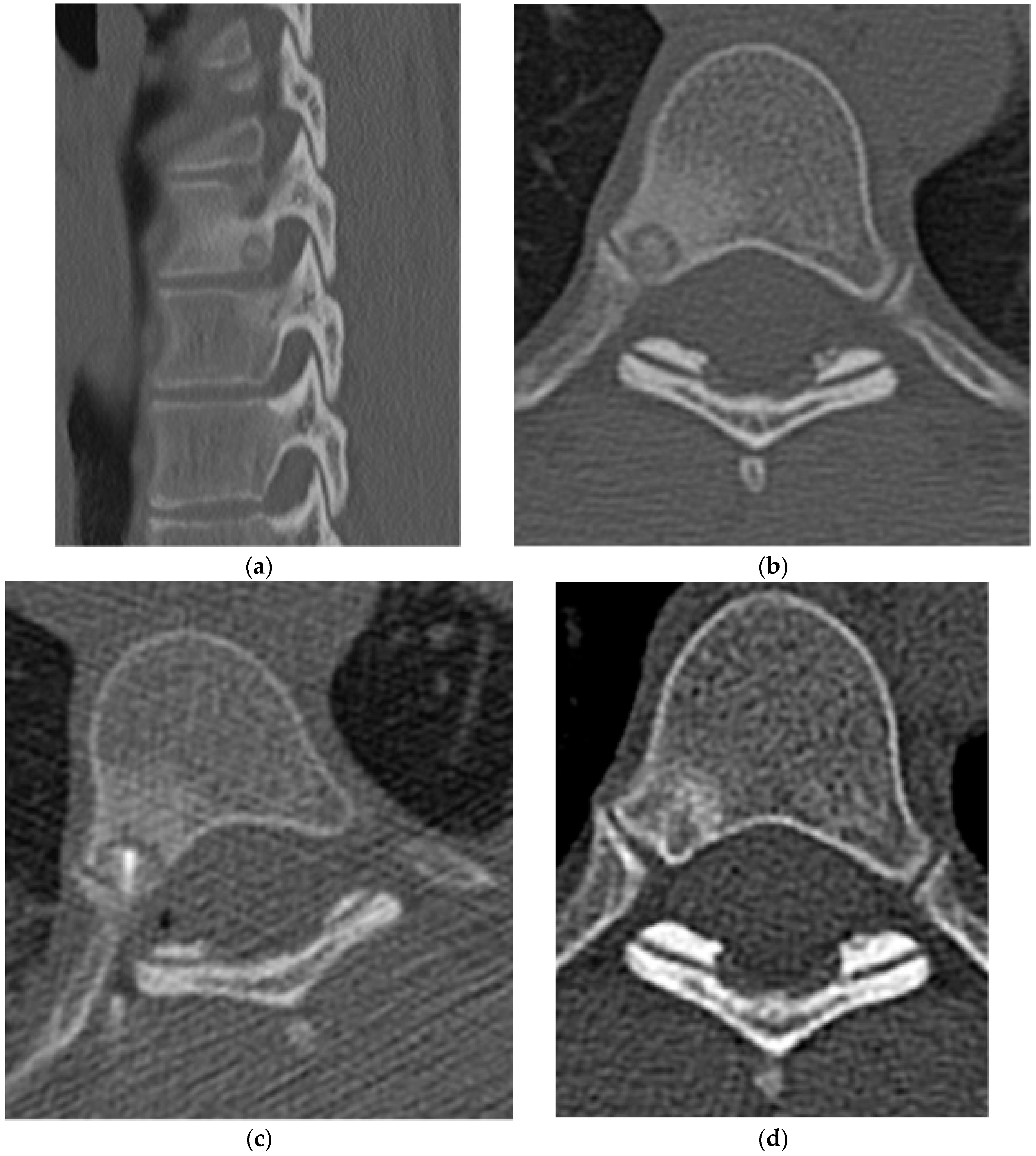
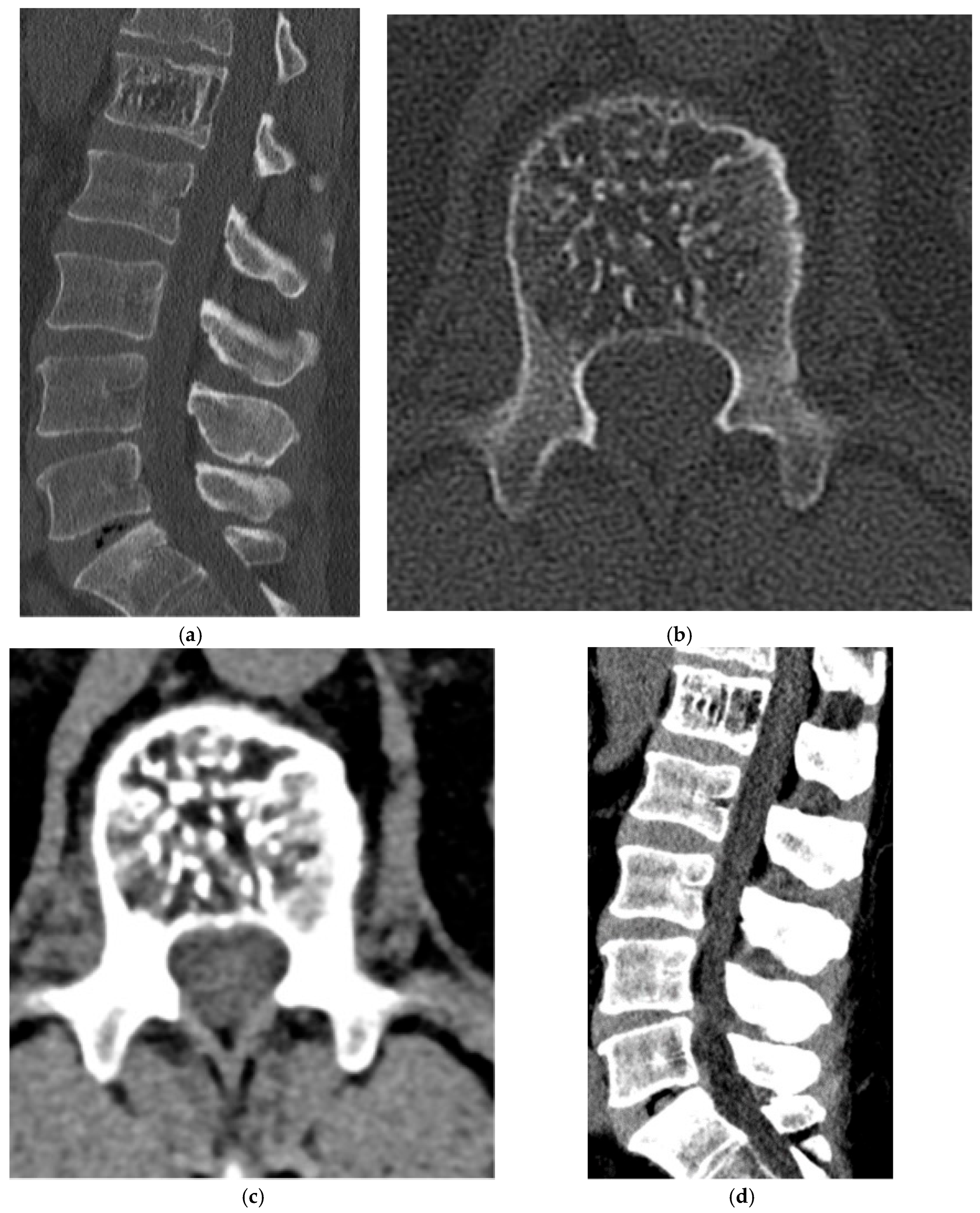
2.1.2. Osteoid Osteoma
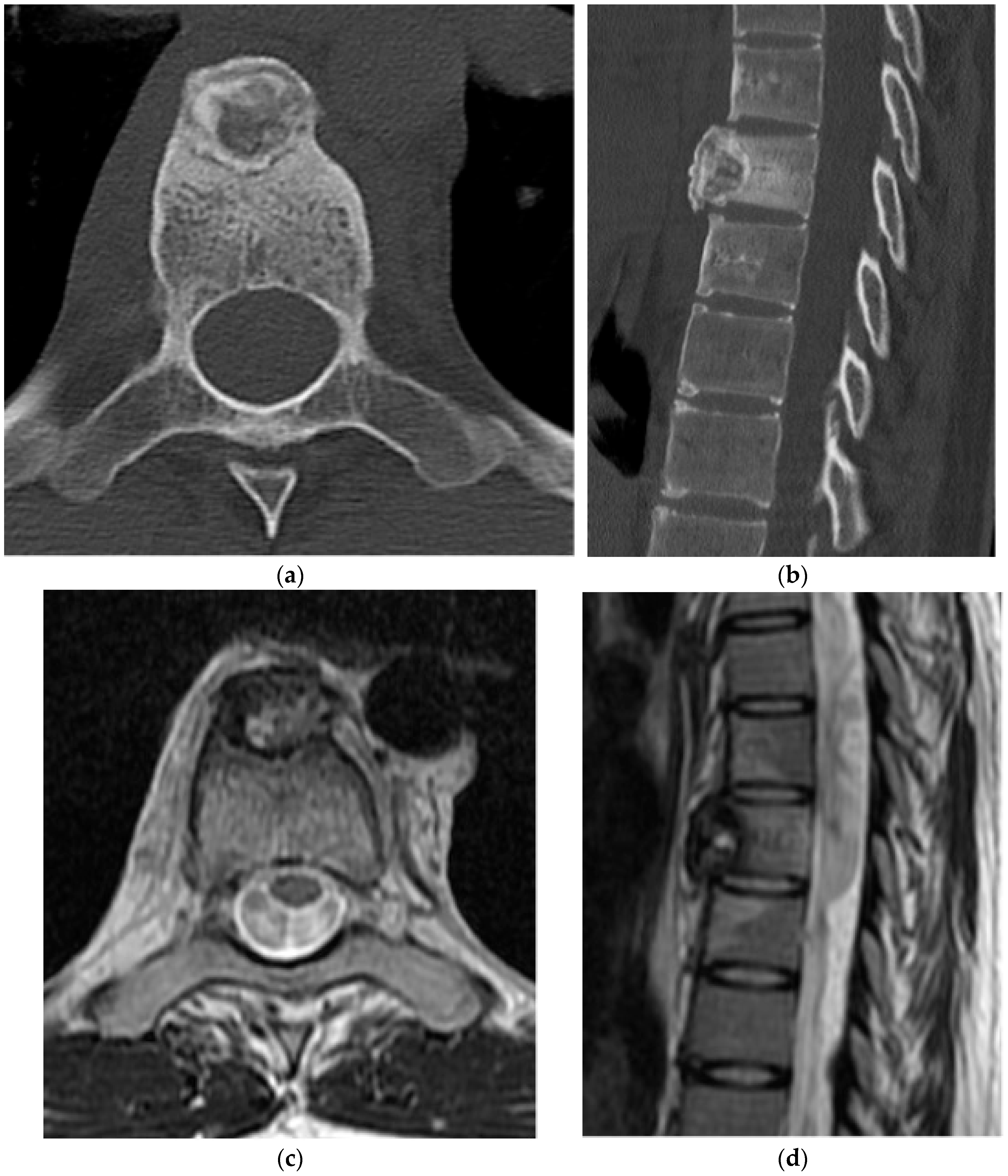
2.1.3. Osteoblastoma
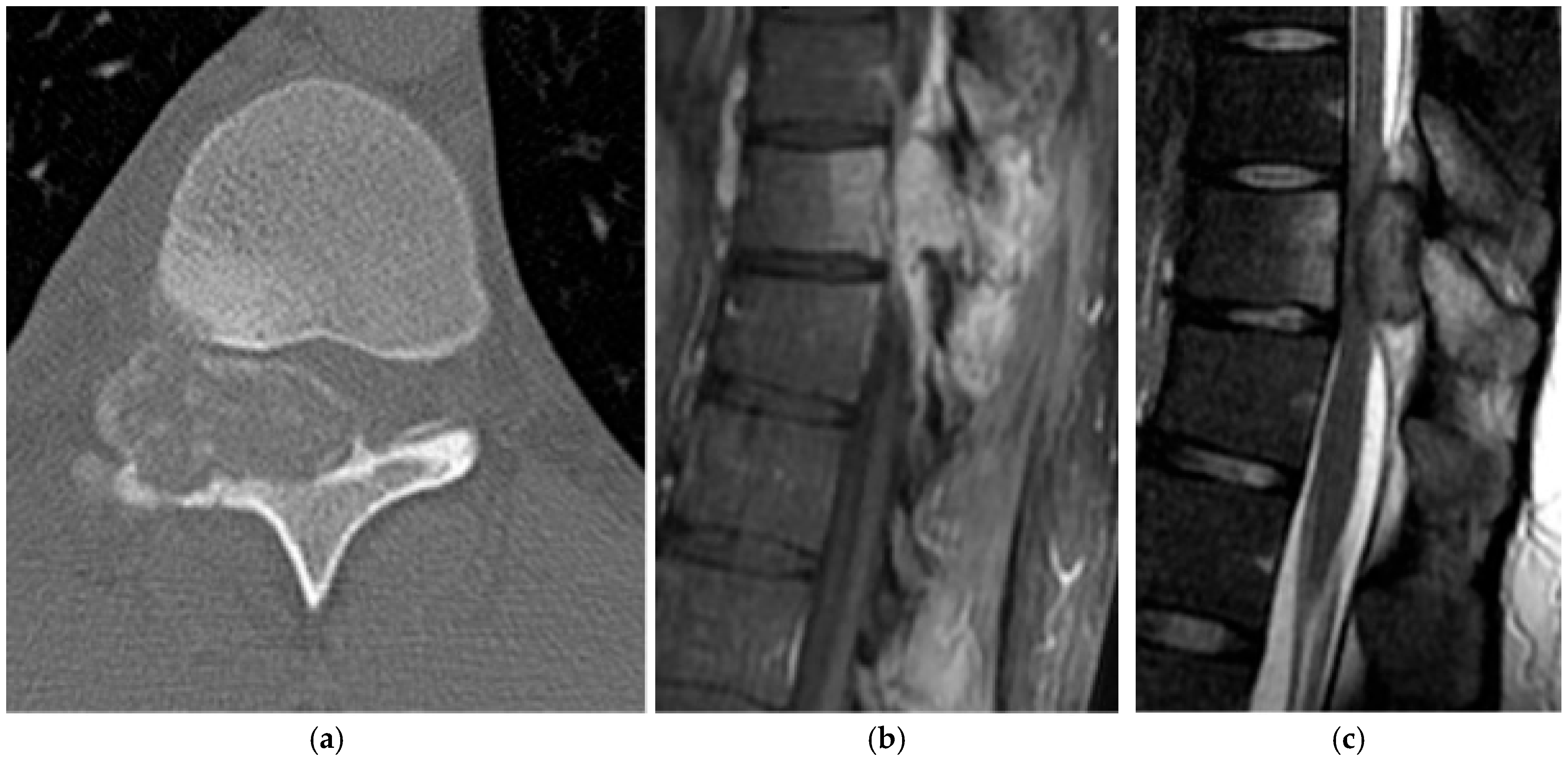
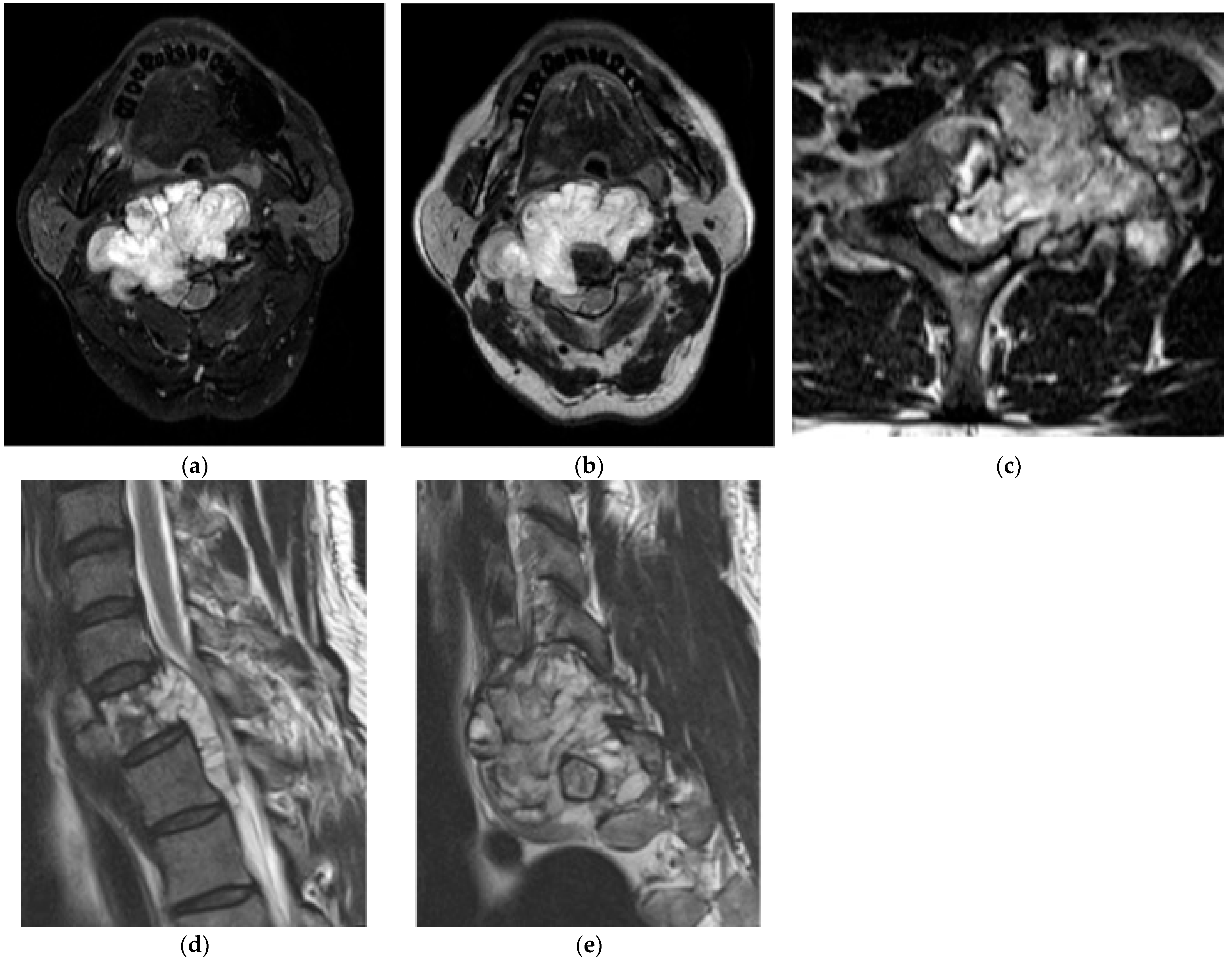
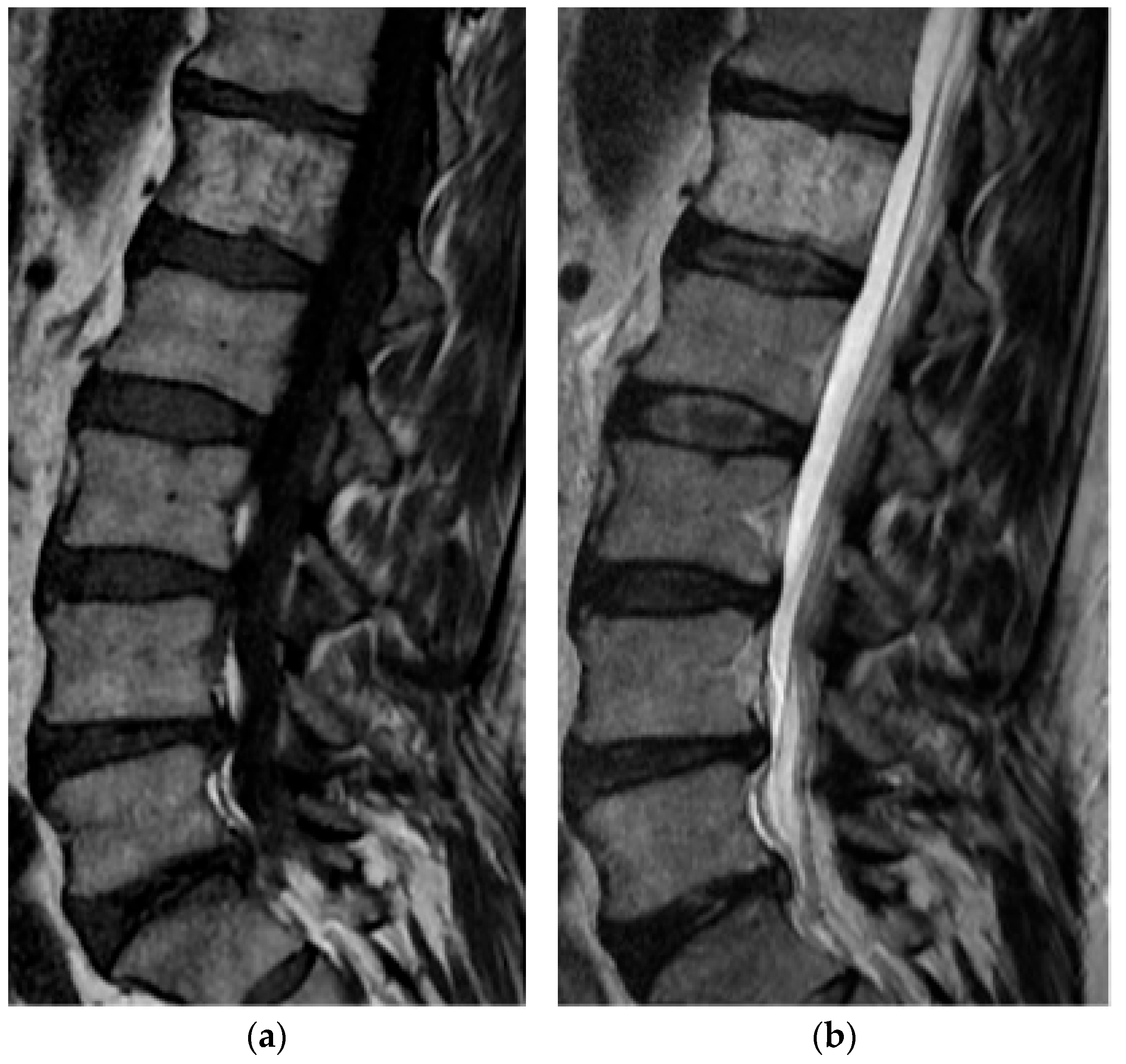
2.1.4. Osteosarcoma
2.2. Chondrogenic
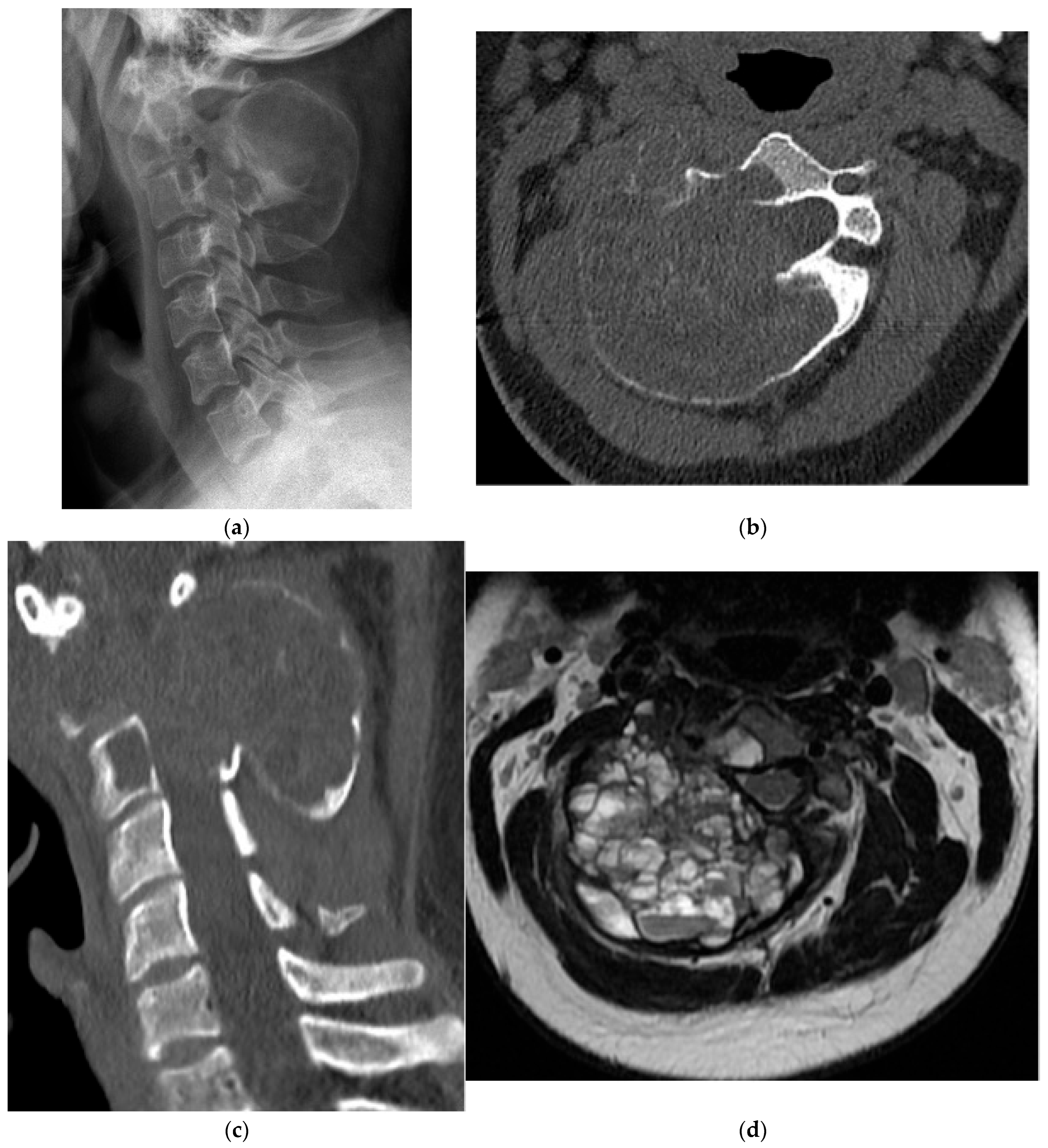
2.2.1. Osteochondroma
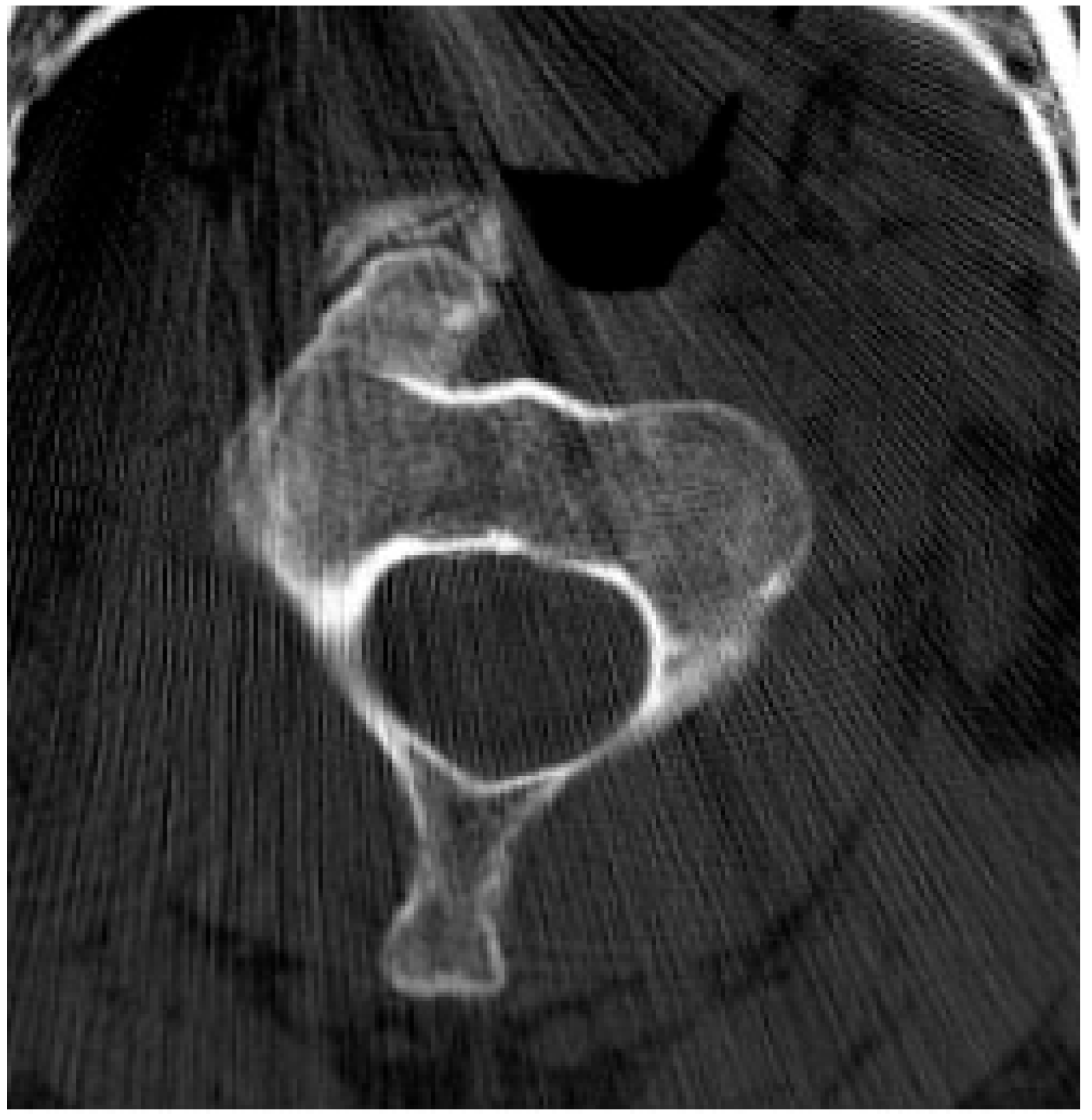
2.2.2. Chondroblastoma
2.2.3. Chondrosarcoma
2.3. Notochordal
2.3.1. Benign Notochordal Cell Tumors (BNCT)
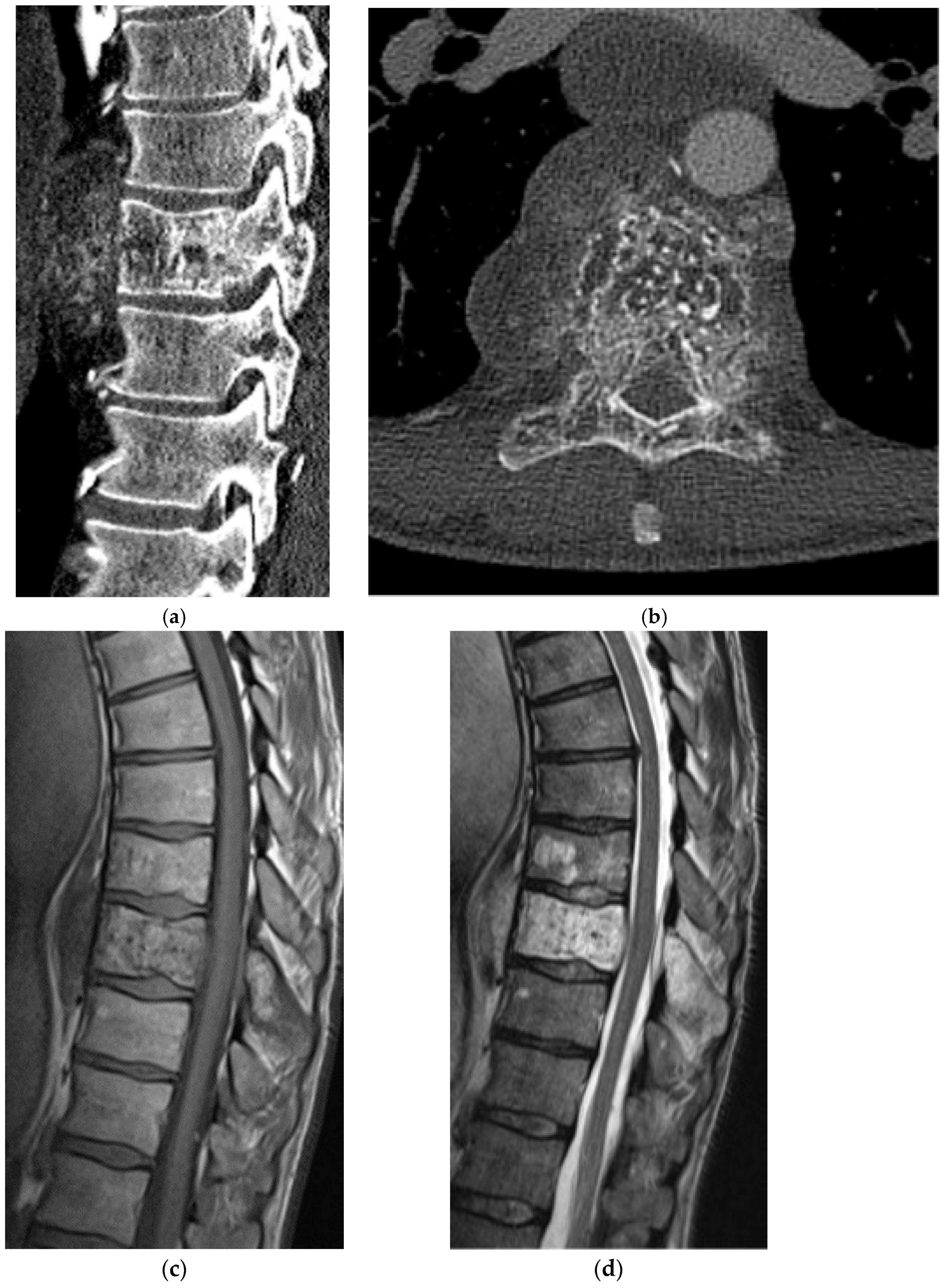
2.3.2. Chordoma
2.4. Hematolymphoid
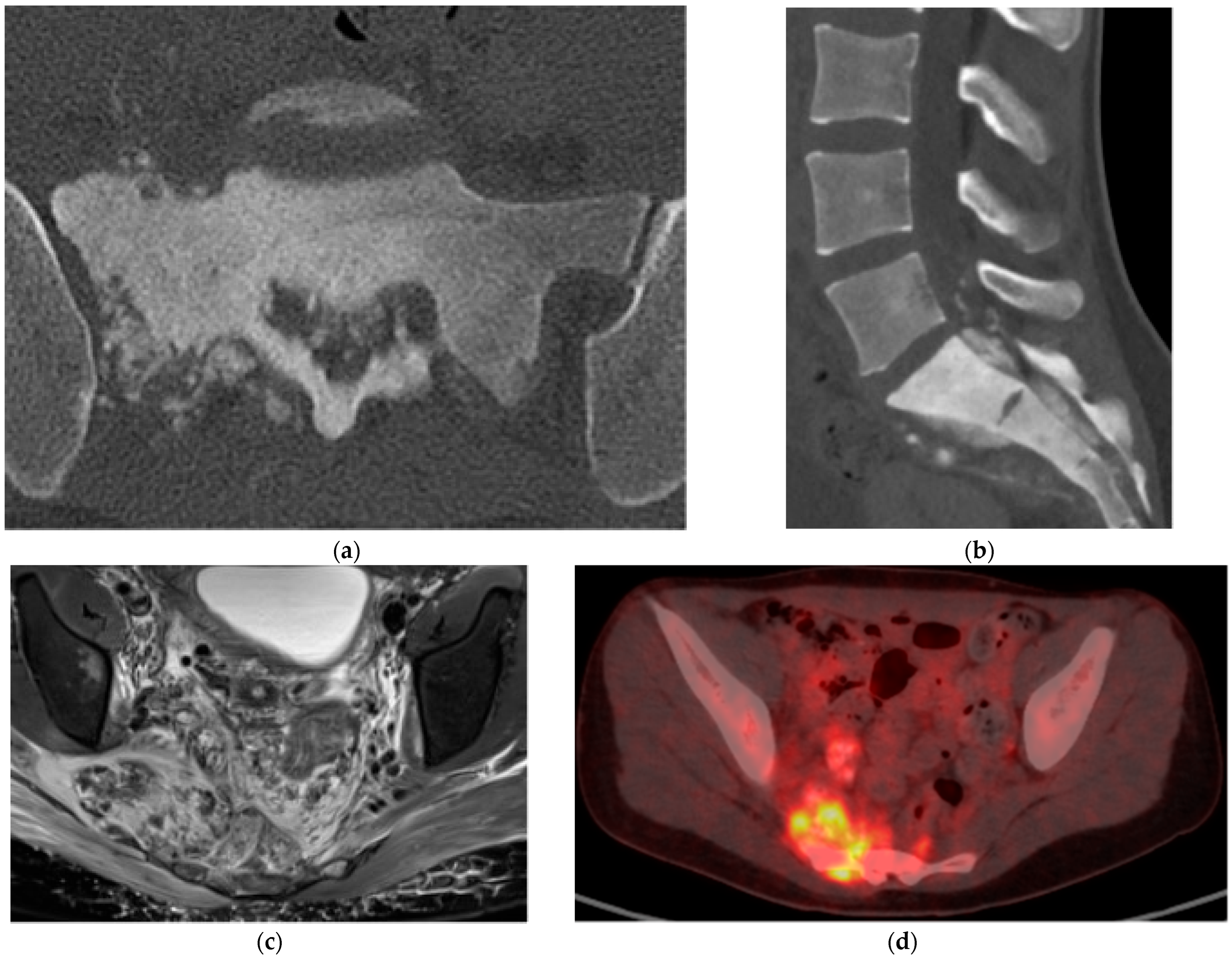
2.4.1. Plasmacytoma
2.4.2. Lymphoma
2.5. Vascular/Cystic
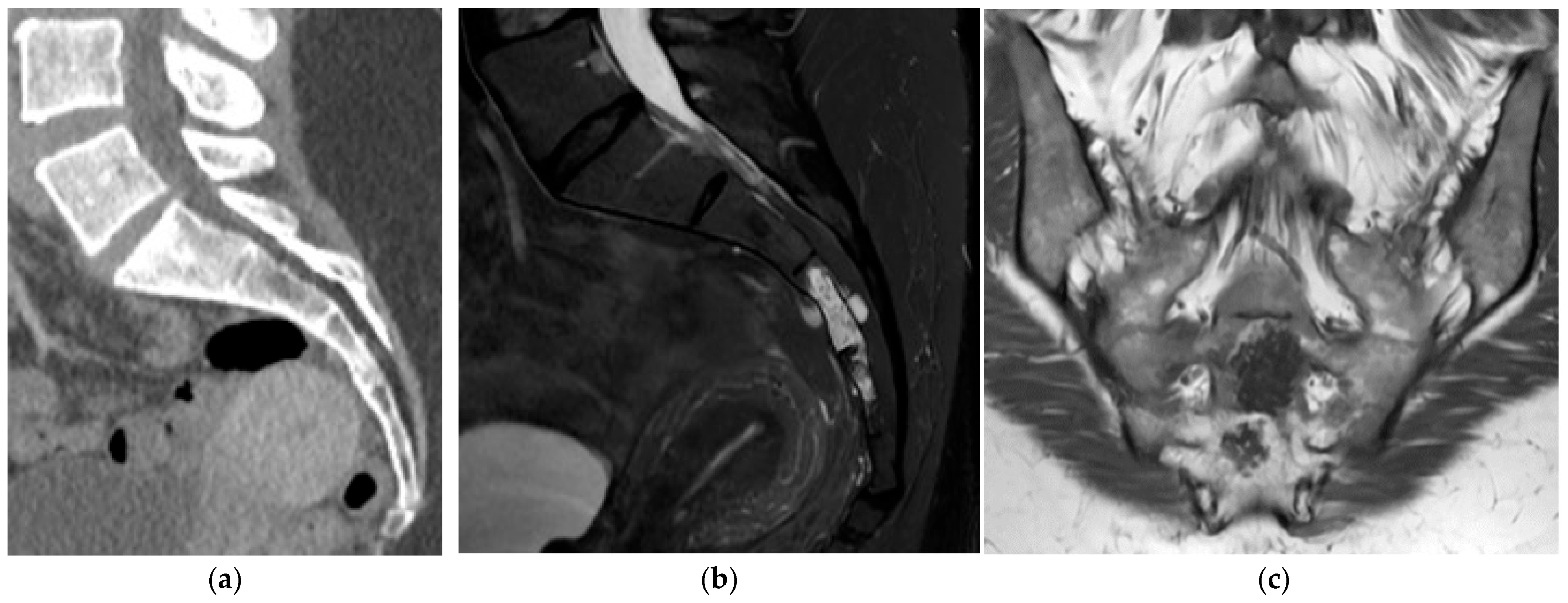
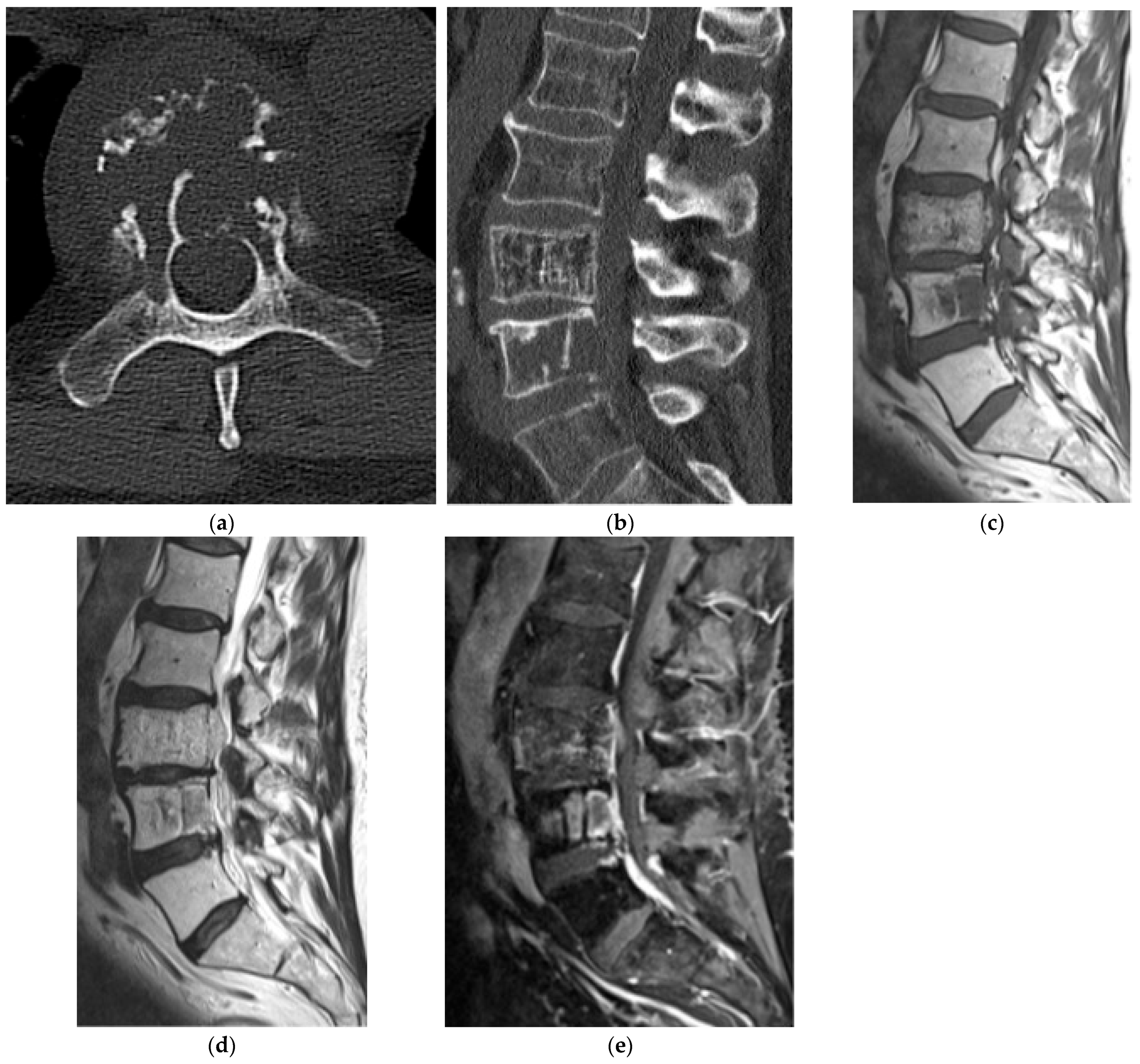
2.5.1. Hemangioma
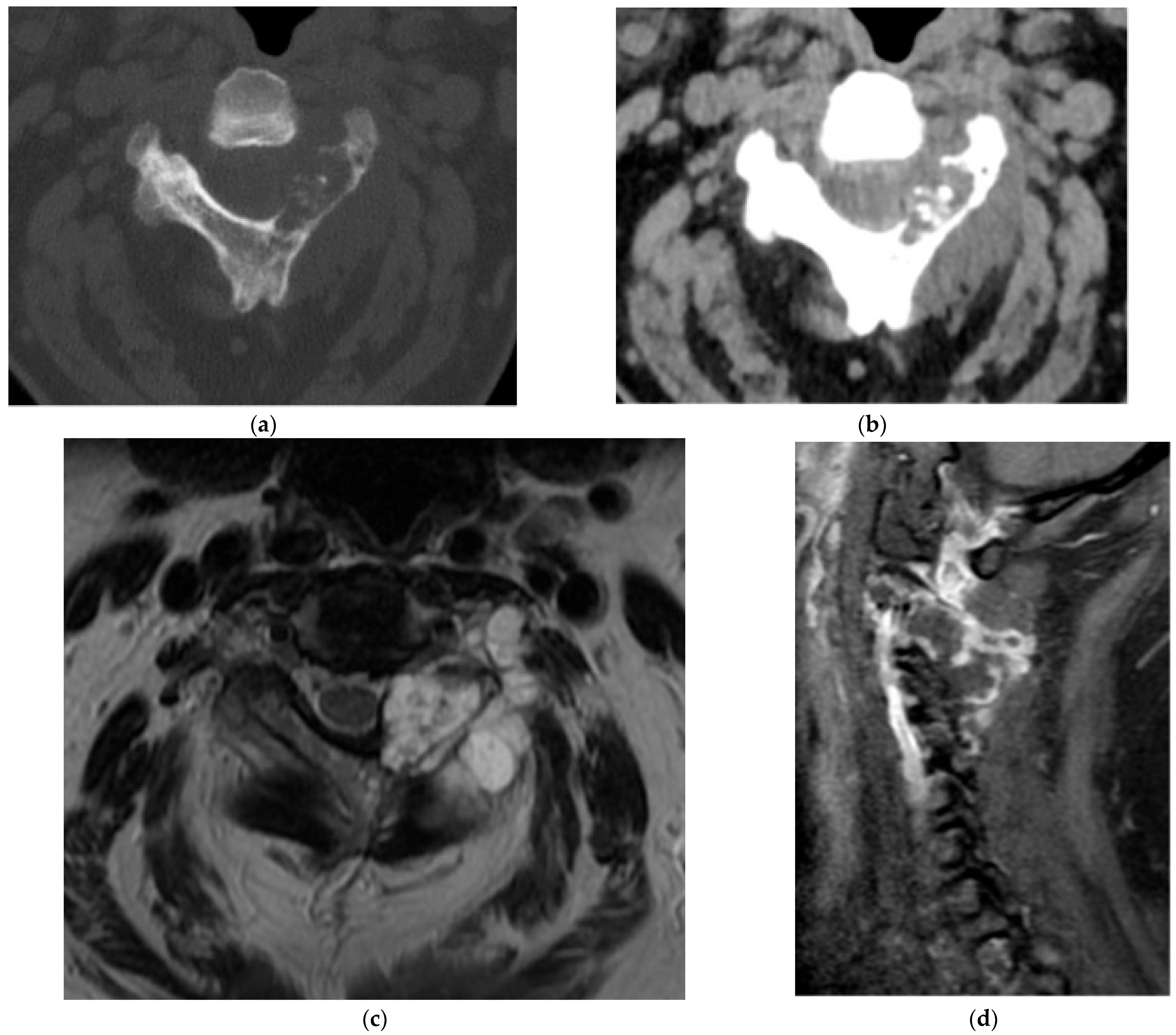
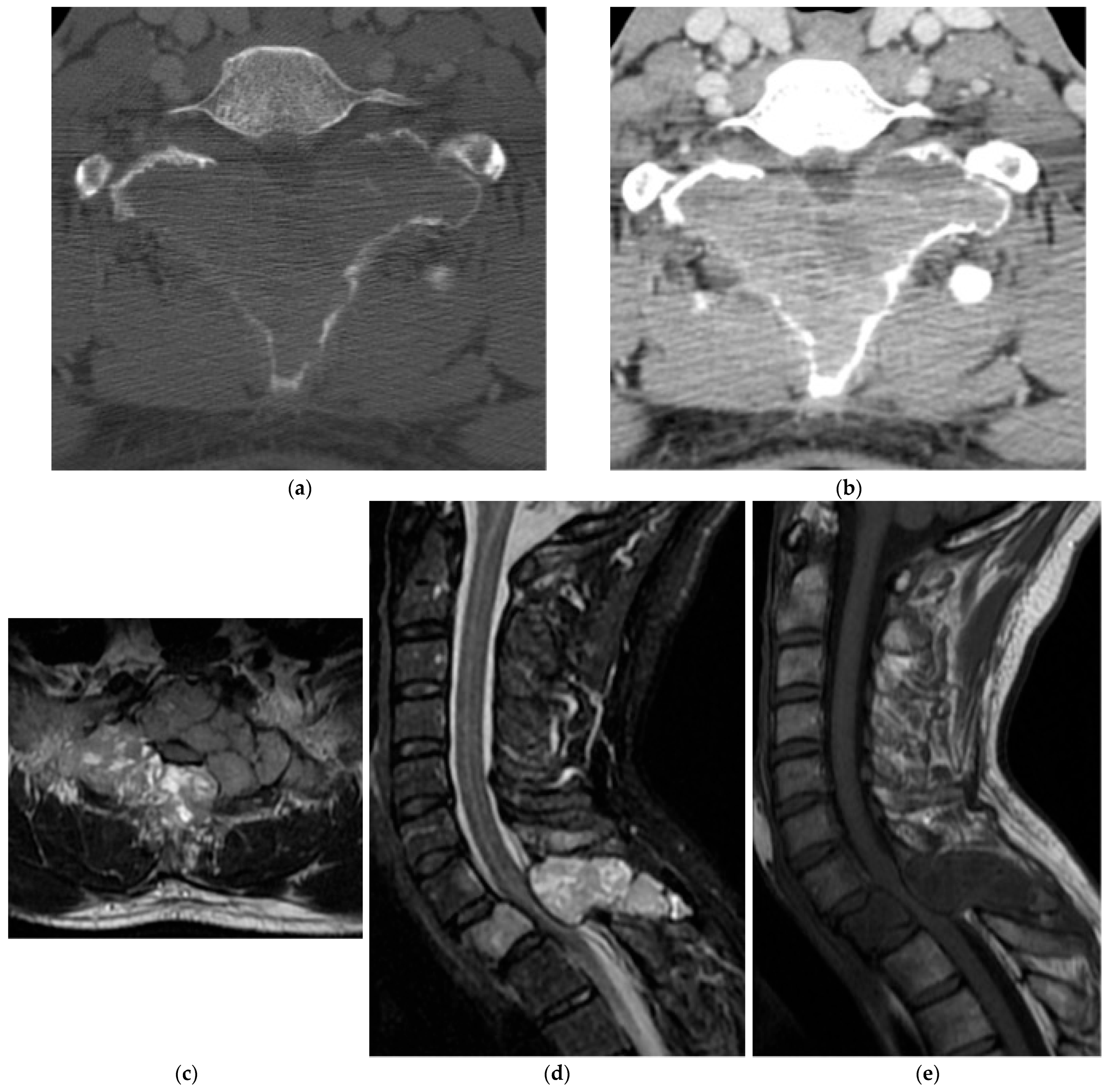
2.5.2. Aneurysmal Bone Cyst
2.6. Fibro-Osseous
Fibrous Dysplasia
2.7. Osteoclast-Rich Stromal
Giant Cell Tumor
2.8. Adipocytic
Lipoma
2.9. Bone Remodeling Disorder (Tumor-like)
Paget’s Disease
2.10. Primitive Small Round Cell
Ewing Sarcoma
2.11. Histiocytic
Langerhans Cell Histiocytosis
3. Discussion
3.1. Mechanical Considerations and Fracture Risk
3.2. Treatment
3.3. Advanced and Adjunct Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Aneurysmal bone cyst |
| BNCT | Benign notochordal cell tumor |
| CT | Computed tomography |
| DCE-MRI | Dynamic contrast-enhanced MRI |
| FD | Fibrous dysplasia |
| FDG | Fluorodeoxyglucose |
| LCH | Langerhans cell histiocytosis |
| MRI | Magnetic resonance imaging |
| PET | Positron emission tomography |
| SPECT | Single-photon emission computed tomography |
| VH | Vertebral hemangioma |
References
- Fornetti, J.; Welm, A.L.; Stewart, S.A. Understanding the Bone in Cancer Metastasis. J. Bone Miner. Res. 2018, 33, 2099–2113. [Google Scholar] [CrossRef]
- Riihimäki, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Clinical Landscape of Cancer Metastases. Cancer Med. 2018, 7, 5534–5542. [Google Scholar] [CrossRef] [PubMed]
- Disibio, G.; French, S.W. Metastatic Patterns of Cancers: Results from a Large Autopsy Study. Arch. Pathol. Lab. Med. 2008, 132, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.P.; Ashford, R.U.; Rao, A.S.; Dickson, R.A. Primary Bone Tumours of the Spine: A 42-Year Survey from the Leeds Regional Bone Tumour Registry. Eur. Spine J. 2007, 16, 405–409. [Google Scholar] [CrossRef]
- Chi, J.H.; Bydon, A.; Hsieh, P.; Witham, T.; Wolinsky, J.P.; Gokaslan, Z.L. Epidemiology and Demographics for Primary Vertebral Tumors. Neurosurg. Clin. North Am. 2008, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Murphey, M.D.; Andrews, C.L.; Flemming, D.J.; Temple, H.T.; Smith, W.S.; Smirniotopoulos, J.G. From the Archives of the AFIP. Primary Tumors of the Spine: Radiologic-Pathologic Correlation. Radiographics 1996, 16, 1131–1158. [Google Scholar] [CrossRef]
- Sala, F.; Dapoto, A.; Morzenti, C.; Firetto, M.C.; Valle, C.; Tomasoni, A.; Sironi, S. Bone Islands Incidentally Detected on Computed Tomography: Frequency of Enostosis and Differentiation from Untreated Osteoblastic Metastases Based on CT Attenuation Value. Br. J. Radiol. 2019, 92, 20190249. [Google Scholar] [CrossRef]
- Onitsuka, H. Roentgenologic Aspects of Bone Islands. Radiology 1977, 123, 607–612. [Google Scholar] [CrossRef]
- Greenspan, A. Bone Island (Enostosis): Current Concept—A Review. Skelet. Radiol. 1995, 24, 111–115. [Google Scholar] [CrossRef]
- Luo, H.; Zou, L.; Yang, Q.; Yuan, C.; Ma, K.; Yang, S.; Luo, D.; Liu, C.; Liu, Z. Spectral CT Assists Differentiation of Osteoblastic Bone Metastasis from Bone Island in Newly Diagnosed Cancer Patients. Eur. Radiol. 2024, 34, 60–68. [Google Scholar] [CrossRef]
- Tepelenis, K.; Skandalakis, G.P.; Papathanakos, G.; Kefala, M.A.; Kitsouli, A.; Barbouti, A.; Tepelenis, N.; Varvarousis, D.; Vlachos, K.; Kanavaros, P.; et al. Osteoid Osteoma: An Updated Review of Epidemiology, Pathogenesis, Clinical Presentation, Radiological Features, and Treatment Option. In Vivo 2021, 35, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Atesok, K.I.; Alman, B.A.; Schemitsch, E.H.; Peyser, A.; Mankin, H. Osteoid Osteoma and Osteoblastoma. J. Am. Acad. Orthop. Surg. 2011, 19, 678–689. [Google Scholar] [CrossRef]
- Abdel Razek, A.A.K.; Castillo, M. Imaging Appearance of Primary Bony Tumors and Pseudo-Tumors of the Spine. J. Neuroradiol. 2010, 37, 37–50. [Google Scholar] [CrossRef]
- Marsh, B.W.; Bonfiglio, M.; Brady, L.P.; Enneking, W.F. Benign Osteoblastoma: Range of Manifestations. J. Bone Joint Surg. Am. 1975, 57, 1–9. [Google Scholar] [CrossRef]
- Berry, M.; Mankin, H.; Gebhardt, M.; Rosenberg, A.; Hornicek, F. Osteoblastoma: A 30-Year Study of 99 Cases. J. Surg. Oncol. 2008, 98, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.A.; Goulart, C.R.; Iwenofu, H.; Chin, L.S.; Lavelle, W.; Mendel, E. Osteoblastomas of the Spine: A Comprehensive Review. Neurosurg. Focus 2016, 41, E4. [Google Scholar] [CrossRef]
- Liu, J.; Han, S.; Li, J.; Yuan, Y.; Guo, W.; Yuan, H. Spinal Osteoblastoma: A Retrospective Study of 35 Patients’ Imaging Findings with an Emphasis on MRI. Insights Imaging 2020, 11, 122. [Google Scholar] [CrossRef]
- Huang, Z.; Fang, T.; Si, Z.; Li, Y.; Zhang, L.; Zheng, C.; Li, S.; Su, M.; Liu, X.; Li, X.; et al. Imaging Algorithm and Multimodality Evaluation of Spinal Osteoblastoma. BMC Musculoskelet. Disord. 2020, 21, 240. [Google Scholar] [CrossRef]
- Ahlawat, S.; Lenchik, L.; Baker, J.C.; Allen, H.; Banks, J.; Florou, V.; Garner, H.W.; Hammer, M.R.; Hiniker, S.M.; Kamel, S.I.; et al. ACR Appropriateness Criteria® Suspected Primary Bone Tumors: 2024 Update. J. Am. Coll. Radiol. 2025, 22, S440–S454. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma Incidence and Survival Rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Cole, S.; Gianferante, D.M.; Zhu, B.; Mirabello, L. Osteosarcoma: A Surveillance, Epidemiology, and End Results Program-Based Analysis from 1975 to 2017. Cancer 2022, 128, 2107–2118. [Google Scholar] [CrossRef]
- Rojas, G.A.; Hubbard, A.K.; Diessner, B.J.; Ribeiro, K.B.; Spector, L.G. International Trends in Incidence of Osteosarcoma (1988–2012). Int. J. Cancer 2021, 149, 1044–1053. [Google Scholar] [CrossRef]
- Khin, Y.T.; Peh, W.C.G.; Chang, K.T.E.; Mya, S.N. High-Grade Primary Osteosarcoma of the Thoracic Spine Presenting as an Ivory Vertebra. Int. J. Cancer Clin. Res. 2018, 5, 2378–3419. [Google Scholar] [CrossRef]
- Motamedi, K.; Seeger, L.L. Benign Bone Tumors. Radiol. Clin. N. Am. 2011, 49, 1115–1134. [Google Scholar] [CrossRef] [PubMed]
- Kitsoulis, P.; Galani, V.; Stefanaki, K.; Paraskevas, G.; Karatzias, G.; Agnantis, N.J.; Bai, M. Osteochondromas: Review of the Clinical, Radiological and Pathological Features. In Vivo 2008, 22, 633–646. [Google Scholar] [PubMed]
- Sinelnikov, A.; Kale, H. Osteochondromas of the Spine. Clin. Radiol. 2014, 69, e584–e590. [Google Scholar] [CrossRef] [PubMed]
- Rajakulasingam, R.; Murphy, J.; Botchu, R.; James, S.L. Osteochondromas of the Cervical Spine: Case Series and Review. J. Clin. Orthop. Trauma 2020, 11, 905–909. [Google Scholar] [CrossRef]
- Patnaik, S.; Jyotsnarani, Y.; Uppin, S.G.; Susarla, R. Imaging Features of Primary Bone Tumors of the Spine: A Pictorial Essay. Indian J. Radiol. Imaging 2016, 26, 279–289. [Google Scholar] [CrossRef]
- Yakkanti, R.; Onyekwelu, I.; Carreon, L.Y.; Dimar, J.R. Solitary Osteochondroma of the Spine-A Case Series: Review of Solitary Osteochondroma with Myelopathic Symptoms. Global Spine J. 2018, 8, 323–339. [Google Scholar] [CrossRef]
- Brunet, L.; Torner Rubies, F.; Suñol, M.; Martínez, J.; Gracia, I.; Peiró, A.; Machado, P. Chondroblastomas in Children and Young Adults: Revision of 55 Cases. J. Pediatr. Orthop. 2024, 44, e184–e191. [Google Scholar] [CrossRef]
- Xu, H.; Nugent, D.; Monforte, H.L.; Binitie, O.T.; Ding, Y.; Letson, G.D.; Cheong, D.; Niu, X. Chondroblastoma of Bone in the Extremities: A Multicenter Retrospective Study. J. Bone Joint Surg. Am. 2015, 97, 925–931. [Google Scholar] [CrossRef]
- Chen, W.; DiFrancesco, L.M. Chondroblastoma: An Update. Arch. Pathol. Lab. Med. 2017, 141, 867–871. [Google Scholar] [CrossRef]
- Ilaslan, H.; Sundaram, M.; Unni, K.K. Vertebral Chondroblastoma. Skelet. Radiol. 2003, 32, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Cho, K.-J.; Park, Y.K.; Lee, J.S.; Kwon, H.J.; Chung, H.; Kim, M.J. Chondroblastoma of the Lumbar Spine: A Case Report and Review of the Literature. Korean J. Pathol. 2011, 45, 532–536. [Google Scholar] [CrossRef]
- Venkatasamy, A.; Chenard, M.P.; Massard, G.; Steib, J.-P.; Bierry, G. Chondroblastoma of the Thoracic Spine: A Rare Location. Case Report With Radiologic-Pathologic Correlation. Skelet. Radiol. 2017, 46, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Boehme, K.A.; Schleicher, S.B.; Traub, F.; Rolauffs, B. Chondrosarcoma: A Rare Misfortune in Aging Human Cartilage? The Role of Stem and Progenitor Cells in Proliferation, Malignant Degeneration and Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 311. [Google Scholar] [CrossRef]
- Weinschenk, R.C.; Wang, W.-L.; Lewis, V.O. Chondrosarcoma. J. Am. Acad. Orthop. Surg. 2021, 29, 553–562. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.K. Classification of Chondrosarcoma: From Characteristic to Challenging Imaging Findings. Cancers 2023, 15, 1703. [Google Scholar] [CrossRef] [PubMed]
- Katonis, P.; Alpantaki, K.; Michail, K.; Lianoudakis, S.; Christoforakis, Z.; Tzanakakis, G.; Karantanas, A. Spinal Chondrosarcoma: A Review. Sarcoma 2011, 2011, 378957. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Thelen, J.C.; Bhatt, A.A. Bone Up on Spinal Osseous Lesions: A Case Review Series. Insights Imaging 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, L.; Vanel, D.; Leclère, J. Imaging of Chondrosarcomas. Cancer Imaging 2003, 4, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, J.; Guo, L.; Yin, Z.; He, H. Large Enchondroma of the Thoracic Spine: A Rare Case Report and Review of the Literature. BMC Musculoskelet. Disord. 2017, 18, 155. [Google Scholar] [CrossRef]
- Ahmend, A.R.; Tan, T.S.; Unni, K.K.; Collins, M.S.; Wenger, D.E.; Sim, F.H. Secondary Chondrosarcoma in Osteochondroma: Report of 107 Patients. Clin. Orthop. Relat. Res. 2003, 411, 193–206. [Google Scholar] [CrossRef]
- Jin, B.; Yang, J.; Zhen, J.; Xu, Y.; Wang, C.; Jing, Q.; Shang, Y. Intravoxel Incoherent Motion and Dynamic Contrast-Enhanced MRI Can Differentiate Between Atypical Cartilaginous Tumors and High-Grade Chondrosarcoma: Correlation with Histological Vessel Characteristics. J. Comput. Assist. Tomogr. 2024, 48, 123–128. [Google Scholar] [CrossRef]
- Peña-Burgos, E.M.; Torena Lerchundi, N.; Fuentes-Sánchez, J.; Tapia-Viñe, M.; Fernández-Baíllo, N.; Pozo-Kreilinger, J.J. Notochordal Cell-Derived Lesions: A 55-Year Casuistic Analysis of 50 Cases with Radiologic-Pathologic Correlation in a Tertiary Referral Hospital, and Literature Review. Eur. Spine J. 2024, 33, 3315–3323. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Mochizuki, K.; Ohsawa, M.; Inoue, T.; Kageyama, K.; Suzuki, A.; Takami, T.; Miki, Y. Differentiating Benign Notochordal Cell Tumors from Chordomas: Radiographic Features on MRI, CT, and Tomography. AJR Am. J. Roentgenol. 2011, 196, 644–650. [Google Scholar] [CrossRef]
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma—Current Understanding and Modern Treatment Paradigms. J. Clin. Med. 2021, 10, 1054. [Google Scholar] [CrossRef]
- Jawad, M.U.; Schneiderbauer, M.M.; Min, E.S.; Cheung, M.C.; Koniaris, L.G.; Scully, S.P. Primary Lymphoma of Bone in Adult Patients. Cancer 2010, 116, 871–879. [Google Scholar] [CrossRef]
- Pennington, Z.; Ehresman, J.; McCarthy, E.F.; Ahmed, A.K.; Pittman, P.D.; Lubelski, D.; Goodwin, C.R.; Sciubba, D.M. Chordoma of the Sacrum and Mobile Spine: A Narrative Review. Spine J. 2021, 21, 500–517. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, S.L.; Bilsky, M.H.; Laufer, I. Chordomas of the Skull Base, Mobile Spine, and Sacrum: An Epidemiologic Investigation of Presentation, Treatment, and Survival. World Neurosurg. 2018, 113, e618–e627. [Google Scholar] [CrossRef]
- Das, P.; Soni, P.; Jones, J.; Habboub, G.; Barnholtz-Sloan, J.S.; Recinos, P.F.; Kshetry, V.R. Descriptive Epidemiology of Chordomas in the United States. J. Neurooncol. 2020, 148, 173–178. [Google Scholar] [CrossRef]
- Hashim, H.; Rosman, A.K.; Abdul Aziz, A.; Roqiah, A.K.; Bakar, N.S. Atypical Clival Chordoma in an Adolescent Without Imaging Evidence of Bone Involvement. Malays. J. Med. Sci. 2014, 21, 78–82. [Google Scholar] [PubMed]
- Lee, S.H.; Kwok, K.Y.; Wong, S.M.; Chan, C.X.J.; Wong, Y.T.; Tsang, M.L. Chordoma at the Skull Base, Spine, and Sacrum: A Pictorial Essay. J. Clin. Imaging Sci. 2022, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Liu, C.; Chen, C.; Yan, J. Solitary Plasmacytoma of Bone of the Spine: Results from the SEER Registry. Spine 2019, 44, E117–E125. [Google Scholar] [CrossRef]
- Major, N.M.; Helms, C.A.; Richardson, W.J. The “Mini-Brain”: Plasmacytoma in a Vertebral Body on MR Imaging. AJR Am. J. Roentgenol. 2000, 175, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.G.O.; Carneiro, B.C.; Pastore, D.; Silva, I.P.; Yamashita, S.R.; Consolo, F.D.; Hungria, V.T.M.; Sandes, A.F.; Rizzatti, E.G.; Nico, M.A.C. Whole-Body Imaging of Multiple Myeloma: Diagnostic Criteria. Radiographics 2019, 39, 1077–1097. [Google Scholar] [CrossRef]
- Bhagavathi, S.; Fu, K. Primary Bone Lymphoma. Arch. Pathol. Lab. Med. 2009, 133, 1868–1871. [Google Scholar] [CrossRef]
- Beal, K.; Allen, L.; Yahalom, J. Primary Bone Lymphoma: Treatment Results and Prognostic Factors with Long-Term Follow-Up of 82 Patients. Anticancer Res. 2006, 26, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.; Ong, K.O. Imaging of Musculoskeletal Lymphoma. Cancer Imaging 2013, 13, 448–457. [Google Scholar] [CrossRef]
- Thomas, A.G.; Vaidhyanath, R.; Kirke, R.; Rajesh, A. Extranodal Lymphoma from Head to Toe: Part 1, the Head and Spine. AJR Am. J. Roentgenol. 2011, 197, 350–356. [Google Scholar] [CrossRef]
- Slon, V.; Stein, D.; Cohen, H.; Sella-Tunis, T.; May, H.; Hershkovitz, I. Vertebral hemangiomas: Their demographical characteristics, location along the spine and position within the vertebral body. Eur. Spine J. 2015, 24, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Teferi, N.; Chowdhury, A.J.; Mehdi, Z.; Challa, M.; Eschbacher, K.; Bathla, G.; Hitchon, P. Surgical Management of Symptomatic Vertebral Hemangiomas: A Single Institution Experience and Literature Review. Spine J. 2023, 23, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Taihi, L.; Viry, F.; Bossard, P.; Polivka, M.; Bousson, V. Aggressive vertebral hemangioma: A post-bioptic finding, the gas web sign-report of two cases. BJR Case Rep. 2020, 6, 20190091. [Google Scholar] [CrossRef] [PubMed]
- Cloran, F.; Pukenas, B.; Loevner, L.; Aquino, C.; Schuster, J.; Mohan, S. Aggressive spinal haemangiomas: Imaging correlates to clinical presentation with analysis of treatment algorithm and clinical outcomes. Br. J. Radiol. 2015, 88, 20140771. [Google Scholar] [CrossRef]
- Restrepo, R.; Zahrah, D.; Pelaez, L.; Temple, H.T.; Murakami, J.W. Update on Aneurysmal Bone Cyst: Pathophysiology, Histology, Imaging, and Treatment. Pediatr. Radiol. 2022, 52, 1601–1614. [Google Scholar] [CrossRef]
- Palmisciano, P.; Hunter, M.; Lokesh, N.; Bin Alamer, O.; Scalia, G.; Giammalva, G.R.; Maugeri, R.; Iacopino, D.G.; Umana, G.E.; Haider, A.S. Aneurysmal Bone Cyst of the Spine in Adult Patients: A Systematic Review and Comparison of Primary vs Secondary Lesions. J. Clin. Neurosci. 2022, 100, 15–22. [Google Scholar] [CrossRef]
- Czervionke, L.F. Aneurysmal Bone Cyst. In Imaging in Spine Surgery, 1st ed.; Ross, J.S., Bendok, B.R., McClendon, J., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 54–59. [Google Scholar]
- Wright, J.F.C.; Stoker, D.J. Fibrous Dysplasia of the Spine. Clin. Radiol. 1988, 39, 523–527. [Google Scholar] [CrossRef]
- Marshman, L.A.G.; David, K.M.; O’Donovan, D.G.; Chawda, S.J. Fibrous dysplasia of the cervical spine presenting as a pathological fracture. Br. J. Neurosurg. 2004, 18, 527–533. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, I.S.; Choi, J.-Y.; Cho, K.H.; Suh, K.J.; Lee, J.W.; Song, J.W. CT and MRI of Fibrous Dysplasia of the Spine. Br. J. Radiol. 2012, 85, 996–1001. [Google Scholar] [CrossRef]
- Hatano, H.; Morita, T.; Ariizumi, T.; Kawashima, H.; Ogose, A. Malignant Transformation of Fibrous Dysplasia: A Case Report. Oncol. Lett. 2014, 8, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Chiu, R.G.; Rosinski, C.L.; Ansari, D.; Chaker, A.N.; Nunna, R.S.; Behbahani, M.; Mehta, A.I. Incidence, Management, and Outcomes of Spinal Giant Cell Tumor of Bone in Adult Patients: A National Cancer Database Analysis. World Neurosurg. 2020, 144, e296–e305. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.S.; Li, Y.Q.; Wu, W.J.; Zhang, Z.K.; Gao, F.; Latif, M. Imaging Appearance of Giant Cell Tumor of the Spine Above the Sacrum. Br. J. Radiol. 2015, 88, 20140566. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Chung, H.W.; Cho, E.Y.; Hong, S.H.; Choi, S.-H.; Yoon, Y.C.; Yi, S.K. MRI Findings of Giant Cell Tumors of the Spine. AJR Am. J. Roentgenol. 2007, 189, 246–250. [Google Scholar] [CrossRef]
- Finn, M.A.; Walker, M.L. Spinal Lipomas: Clinical Spectrum, Embryology, and Treatment. Neurosurg. Focus 2007, 23, E10. [Google Scholar] [CrossRef]
- Pruthi, N.; Devi, B.I. Nondysraphic Cervical and Thoracic Intraspinal Lipomas: A Review. Br. J. Neurosurg. 2010, 24, 228–232. [Google Scholar] [CrossRef]
- Blount, J.P.; Elton, S. Spinal Lipomas. Neurosurg. Focus 2001, 10, e3. [Google Scholar] [CrossRef]
- Sen, D.; Satija, L.; Chatterji, S.; Majumder, A.; Singh, M.; Gupta, A. Vertebral intraosseous lipoma. Med. J. Armed Forces India 2015, 71, 293–296. [Google Scholar] [CrossRef]
- Singer, F.R.; Bone, H.G., III; Hosking, D.J.; Lyles, K.W.; Murad, M.H.; Reid, I.R.; Siris, E.S. Paget’s Disease of Bone: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014, 99, 4408–4422. [Google Scholar] [CrossRef]
- Dell’Atti, C.; Cassar-Pullicino, V.N.; Lalam, R.K.; Tins, B.J.; Tyrrell, P.N.M. The Spine in Paget’s Disease. Skelet. Radiol. 2007, 36, 609–626. [Google Scholar] [CrossRef]
- Banaganapalli, B.; Fallatah, I.; Alsubhi, F.; Jayasheela Shetty, P.; Awan, Z.; Elango, R.; Shaik, N.A. Paget’s Disease: A Review of the Epidemiology, Etiology, Genetics, and Treatment. Front. Genet. 2023, 14, 1131182. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, D.J.; Theodorou, S.J.; Kakitsubata, Y. Imaging of Paget Disease of Bone and Its Musculoskeletal Complications: Review. AJR Am. J. Roentgenol. 2011, 196, S64–S75. [Google Scholar] [CrossRef] [PubMed]
- Riggi, N.; Suvà, M.L.; Stamenkovic, I. Ewing’s Sarcoma. N. Engl. J. Med. 2021, 384, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Ilaslan, H.; Sundaram, M.; Unni, K.K.; Dekutoski, M.B. Primary Ewing’s Sarcoma of the Vertebral Column. Skelet. Radiol. 2004, 33, 506–513. [Google Scholar] [CrossRef]
- Vohar, V.G. Ewing’s Tumor: A Report on 27 Cases. Indian J. Radiol. Imaging 2008, 18, 106–112. [Google Scholar]
- Estes, D.N.; Magill, H.L.; Thompson, E.I.; Hayes, F.A. Primary Ewing Sarcoma: Follow-up with Ga-67 Scintigraphy. Radiology 1990, 177, 449–453. [Google Scholar] [CrossRef]
- Schaub, T.; Duber, C.; Anatoniadis, A.; Eissner, D.; Greinacher, I.; Gutjahr, P.; Thelen, M. The Place of Bone Scintigraphy in the Diagnosis and Follow-Up of Ewing’s Sarcoma. Rofo 1989, 150, 395–401. [Google Scholar] [CrossRef]
- Lim, C.S.; Yeoh, C. Spinal Epidural Involvement in Adult Langerhans Cell Histiocytosis: A Case Report. Medicine 2020, 99, e18794. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.G.; Zhong, W.Q.; Ma, Q.J.; Wei, F.; Yuan, H.S.; Dang, G.T.; Liu, Z.J. Langerhans Cell Histiocytosis with Multiple Spinal Involvement. Eur. Spine J. 2010, 20, 1961–1969. [Google Scholar] [CrossRef]
- Allen, C.E.; Merad, M.; McClain, K.L. Langerhans-Cell Histiocytosis. N. Engl. J. Med. 2018, 379, 856–868. [Google Scholar] [CrossRef]
- Guyot-Goubin, A.; Donadieu, J.; Barkaoui, M.; Bellec, S.; Thomas, C.; Clavel, J. Descriptive Epidemiology of Childhood Langerhans Cell Histiocytosis in France, 2000–2004. Pediatr. Blood Cancer 2008, 51, 71–75. [Google Scholar] [CrossRef]
- Garg, S.; Mehta, S.; Dormans, J.P. Langerhans Cell Histiocytosis of the Spine in Children: Long-Term Follow-Up. J. Bone Joint Surg. Am. 2004, 86, 1740–1750. [Google Scholar] [CrossRef]
- Huang, W.D.; Yang, H.X.; Wu, Z.P.; Huang, Q.; Xiao, J.R.; Yang, M.S.; Zhou, Z.H.; Yan, W.J.; Song, D.W.; Liu, T.L.; et al. Langerhans Cell Histiocytosis of the Spine: A Comparative Study of Clinical, Imaging Features, and Diagnosis in Children, Adolescents, and Adults. Spine J. 2013, 13, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Foote, M.; Deverall, H. Strategy in the Surgical Treatment of Primary Spinal Tumors. Glob. Spine J. 2012, 2, 249–266. [Google Scholar] [CrossRef]
- Anwar, M.A.; El-Baba, C.; Elnaggar, M.H.; Elkholy, Y.O.; Mottawae, M.; Johar, D.; Al Shehabi, T.S.; Kobeissy, F.; Moussallem, C.; Massaad, E.; et al. Novel therapeutic strategies for spinal osteosarcomas. Semin. Cancer Biol. 2020, 64, 83–92. [Google Scholar] [CrossRef]
- Charest-Morin, R.; Fisher, C.G.; Sahgal, A.; Boriani, S.; Gokaslan, Z.L.; Lazary, A.; Reynolds, J.; Bettegowda, C.; Rhines, L.D.; Dea, N. Primary bone tumor of the spine—An evolving field: What a general spine surgeon should know. Glob. Spine J. 2019, 9 (Suppl. 1), 108S–116S. [Google Scholar] [CrossRef]
- Costa, F.; Restelli, F.; Innocenti, N.; Zygourakis, C.; Chen, Z.; Pojskić, M.; Yaman, O.; Gushcha, A.; Sharif, S.; Zileli, M. Management of benign primary vertebral tumors: WFNS spine committee recommendations. Interdiscip. Neurosurg. 2025, 41, 102114. [Google Scholar] [CrossRef]
- Dietz, M.S.; Al-Ibraheemi, A.; Davis, J.L.; Hawkins, C.M.; Craig, B.T.; Dasgupta, R.; Geller, D.S.; Shulman, D.S.; Cohen-Gogo, S.; Gupta, A.; et al. Optimizing Ewing sarcoma and osteosarcoma biopsy acquisition: A Children’s Oncology Group Bone Tumor Committee consensus statement. J. Natl. Compr. Cancer Netw. 2024, 23, e247063. [Google Scholar] [CrossRef] [PubMed]
- Lis, E.; Bilsky, M.H.; Pisisnki, L.; Boland, P.; Healey, J.H.; O’Malley, B.; Krol, G. Percutaneous CT-guided biopsy of osseous lesions of the spine in patients with known or suspected malignancy. AJNR Am. J. Neuroradiol. 2004, 25, 1583–1588. [Google Scholar] [PubMed]
- Liu, P.T.; Chivers, F.S.; Roberts, C.C.; Schultz, C.J.; Beauchamp, C.P. Imaging of Osteoid Osteoma with Dynamic Gadolinium-Enhanced MR Imaging. Radiology 2003, 227, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mukherjee, A.; Karunanithi, S.; Nadarajah, J.; Gamanagatti, S.; Khan, S.A.; Bal, C.; Kumar, R. 99mTc-Methylene Diphosphonate SPECT/CT as the One-Stop Imaging Modality for the Diagnosis of Osteoid Osteoma. Nucl. Med. Commun. 2014, 35, 876–883. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tregobov, N.; Krolikowski, M.; Dragoman, R.; Brakel, B.; Munk, P.L.; Heran, M.K.S. Unveiling Primary Bone Tumors of the Spine: A Review of Essential Imaging Clues. Diagnostics 2025, 15, 2970. https://doi.org/10.3390/diagnostics15232970
Tregobov N, Krolikowski M, Dragoman R, Brakel B, Munk PL, Heran MKS. Unveiling Primary Bone Tumors of the Spine: A Review of Essential Imaging Clues. Diagnostics. 2025; 15(23):2970. https://doi.org/10.3390/diagnostics15232970
Chicago/Turabian StyleTregobov, Noah, Michal Krolikowski, Ryan Dragoman, Benjamin Brakel, Peter L. Munk, and Manraj K. S. Heran. 2025. "Unveiling Primary Bone Tumors of the Spine: A Review of Essential Imaging Clues" Diagnostics 15, no. 23: 2970. https://doi.org/10.3390/diagnostics15232970
APA StyleTregobov, N., Krolikowski, M., Dragoman, R., Brakel, B., Munk, P. L., & Heran, M. K. S. (2025). Unveiling Primary Bone Tumors of the Spine: A Review of Essential Imaging Clues. Diagnostics, 15(23), 2970. https://doi.org/10.3390/diagnostics15232970




