Cardiovascular Disease in Pregnancy: When Two Hearts Beat as One
Abstract
1. Introduction
- Peripartum and pre-existing cardiomyopathies, focusing on mechanisms, prognosis, and evolving therapeutic strategies;
- Anticoagulation and prosthetic valve management, balancing maternal thrombotic protection and fetal safety;
- Hypertensive disorders of pregnancy, paradigmatic of endothelial and metabolic dysfunction linking obstetric and long-term cardiovascular disease.
2. Peripartum and Preexisting Cardiomyopathies
2.1. Pathophysiology and Mechanistic Insights
2.2. Diagnosis and Risk Stratification
2.3. Management and Evidence Gaps
2.4. Other Preexisting Cardiomyopathies
2.5. Postpartum and Long-Term Care
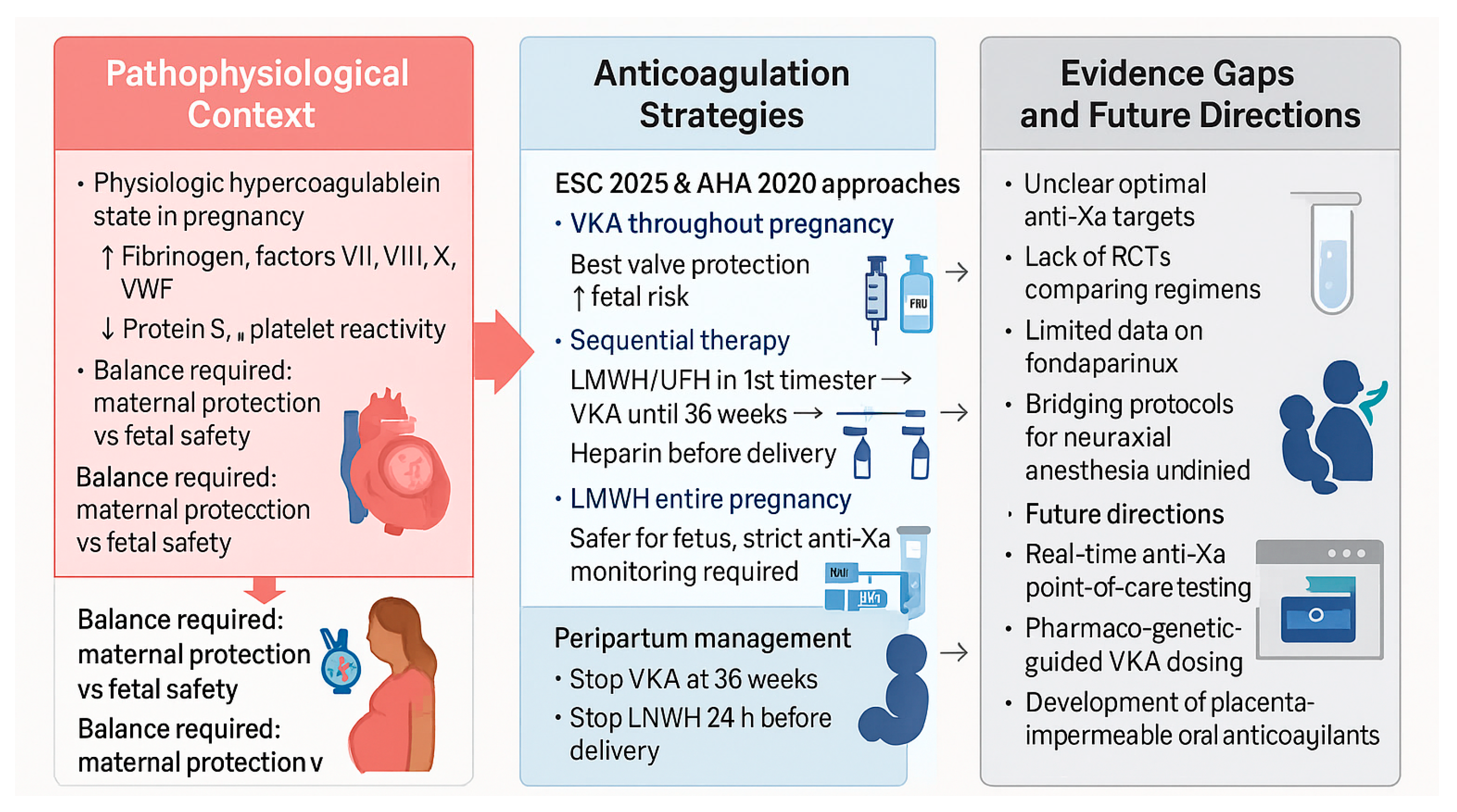
3. Anticoagulation and Mechanical Valve Management
3.1. Pathophysiological Basis
3.2. Evidence and Clinical Outcomes
3.3. Practical Management Strategies
- VKA throughout pregnancy except near delivery—ensures maximal valve protection but carries the greatest fetal risk, particularly at higher doses.
- Sequential therapy—LMWH or UFH during the first trimester (to avoid teratogenic exposure), followed by VKA until 36 weeks, then switching back to heparin near term.
- LMWH for the entire pregnancy—used when VKA avoidance is prioritized, requiring rigorous anti-Xa monitoring (target peak 0.8–1.2 U/mL, trough > 0.6 U/mL).
3.4. Evidence Gaps and Research Needs
3.5. Future Perspectives
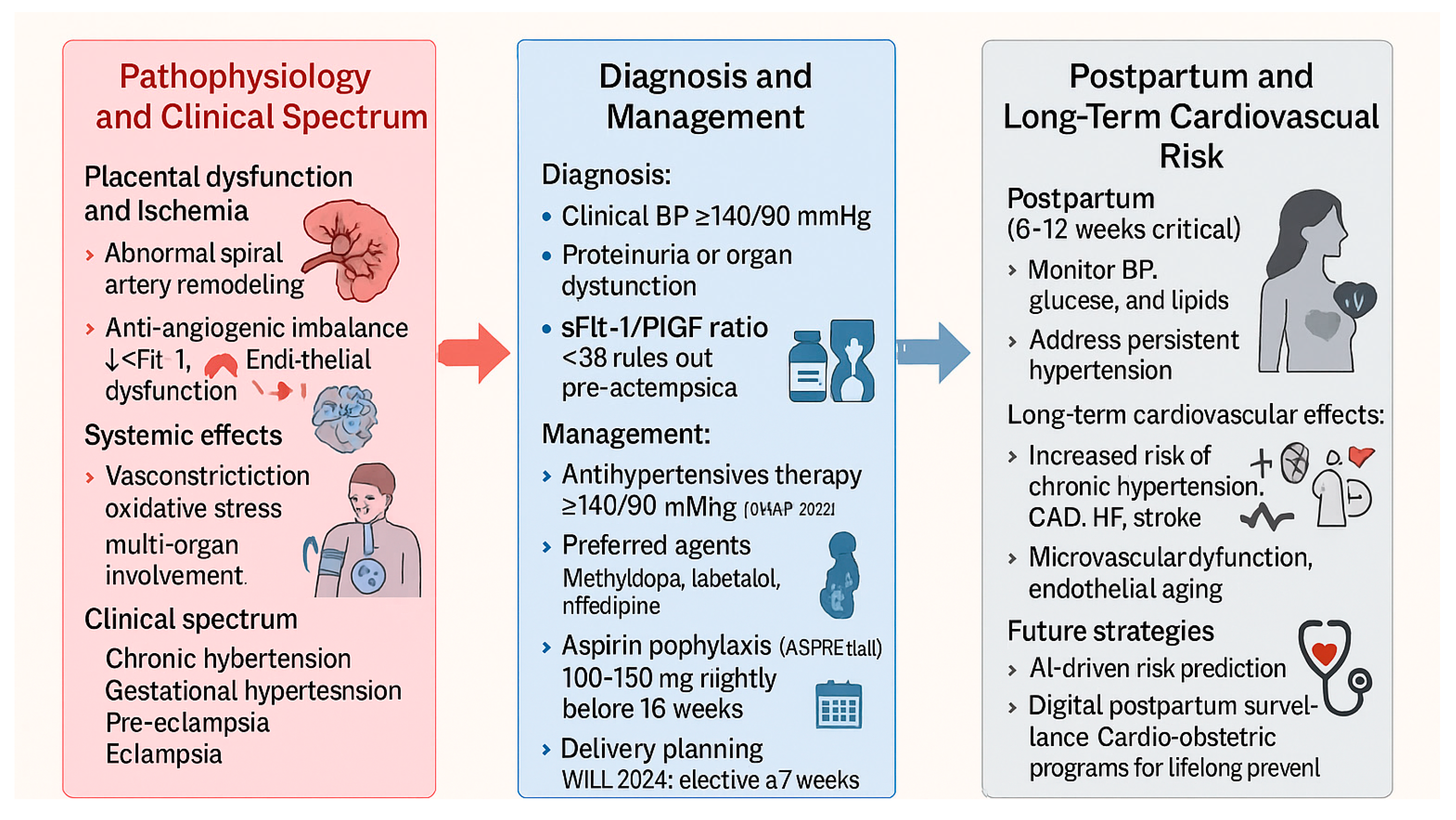
4. Hypertensive Disorders of Pregnancy and Long-Term Cardiovascular Risk
4.1. Pathophysiology and Clinical Spectrum
4.2. Diagnostic Advances and Biomarkers
4.3. Therapeutic Management and Comparative Guidelines
4.4. Long-Term Cardiovascular Risk
4.5. Future Directions
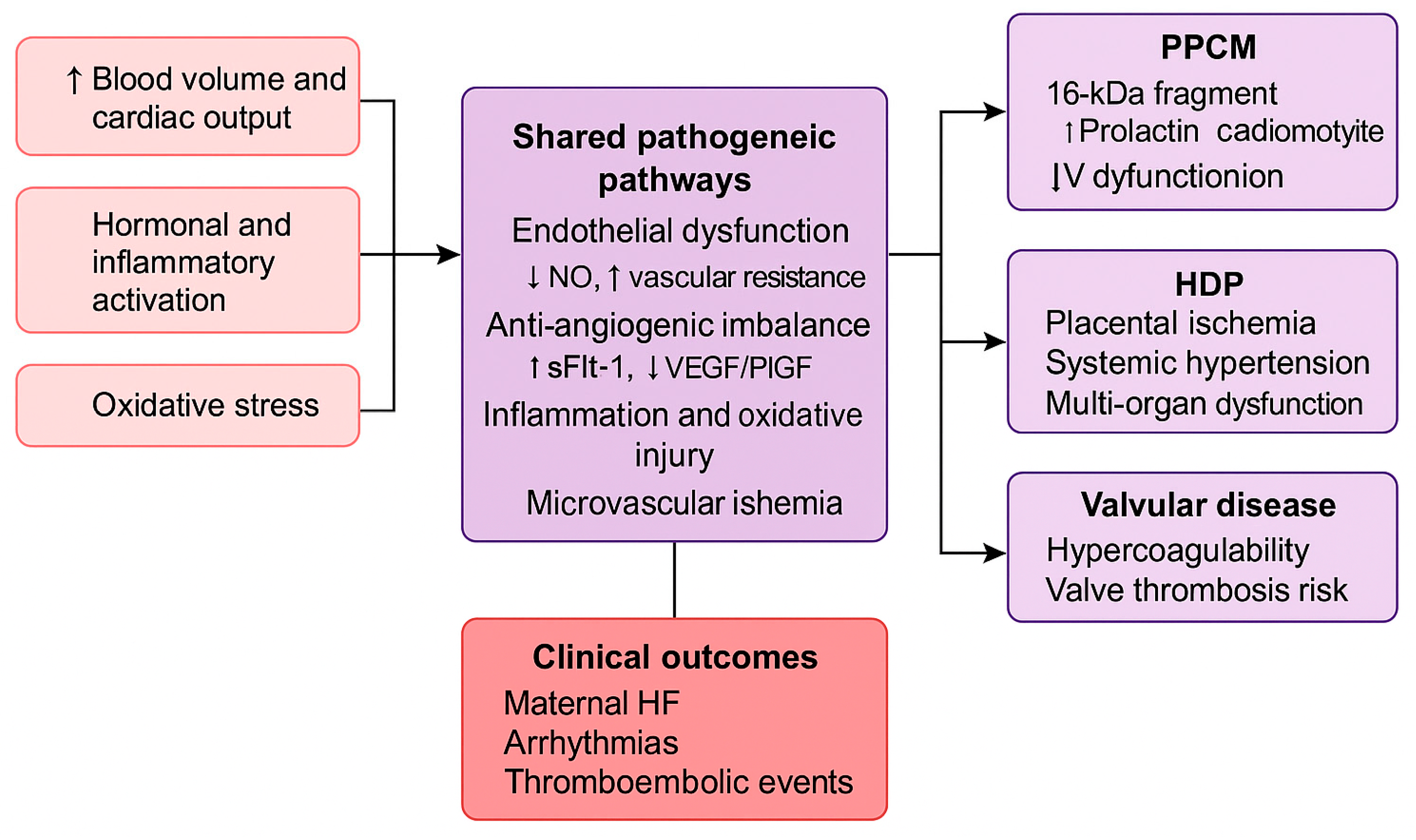
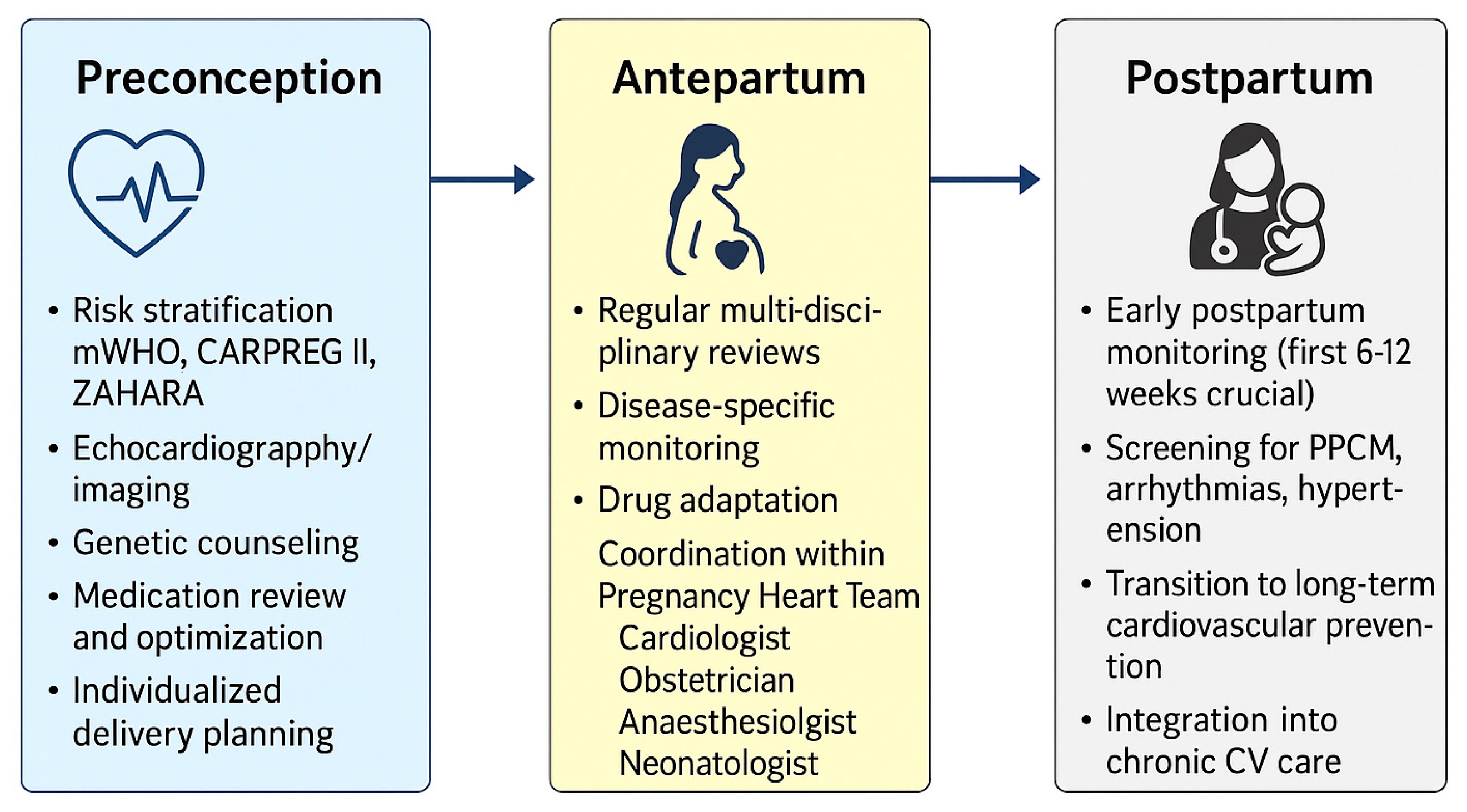
5. Discussion
5.1. Shared Mechanistic Pathways
5.2. Multidisciplinary Care and Lifelong Cardiovascular Health
5.3. Clinical Evidence and Knowledge Gaps
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Backer, J.; Haugaa, K.H.; Hasselberg, N.E.; de Hosson, M.; Brida, M.; Castelletti, S.; Cauldwell, M.; Cerbai, E.; Crotti, L.; de Groot, N.M.S.; et al. 2025 ESC Guidelines for the management of cardiovascular disease and pregnancy. Eur. Heart J. 2025, 46, 4462–4568. [Google Scholar] [CrossRef]
- Mehta, L.S.; Warnes, C.A.; Bradley, E.; Templin, C.; Singh, M.; Arora, G.; Wood, M.J.; Wilson, P.; Bairey Merz, C.N.; Michos, E.D.; et al. Cardiovascular considerations in caring for pregnant patients. Circulation 2020, 141, e884–e903. [Google Scholar] [CrossRef]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef]
- Petersen, E.E.; Davis, N.L.; Goodman, D.; Cox, S.; Mayes, N.; Johnston, E.; Syverson, C.; Seed, K.; Shapiro-Mendoza, C.K.; Callaghan, W.M.; et al. Pregnancy-related deaths in the United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 423–429. [Google Scholar]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef]
- Hauspurg, A.; Ying, W.; Hubel, C.A.; Michos, E.D.; Ouyang, P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin. Cardiol. 2018, 41, 239–246. [Google Scholar] [CrossRef]
- Boyd, H.A. Pregnancy complications as indicators of cardiovascular risk. J. Am. Heart Assoc. 2023, 12, e030452. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Silversides, C.K.; Grewal, J.; Mason, J.; Colman, J.; Siu, S.C.; Hornberger, L.K.; Kiess, M.; Canada, J.; Tam, K.W.; Wang, A.; et al. Pregnancy outcomes in women with heart disease (CARPREG II). J. Am. Coll. Cardiol. 2018, 71, 2419–2430. [Google Scholar] [PubMed]
- Van Hagen, I.M.; Roos-Hesselink, J.W. Pregnancy in congenital heart disease. Heart 2020, 106, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Shotan, A.; Roos-Hesselink, J.; Baris, L.; Goland, S.; Yekel, Y.; Elkayam, U. Cardiomyopathy and pregnancy. Can. J. Cardiol. 2021, 37, 2067–2075. [Google Scholar] [CrossRef]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Groen, H.; Ferreira, E.; Presbitero, P.; Meizoso, J.; O’Hara, R.; McIntyre, H.D.; Elkayam, U.; et al. Peripartum cardiomyopathy position statement. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef]
- Fett, J.D.; Christie, L.G.; Carraway, R.D.; Murphy, J.G. Peripartum cardiomyopathy prognosis. Mayo Clin. Proc. 2005, 80, 1602–1606. [Google Scholar] [CrossRef]
- Hilfiker-Kleiner, D.; Sliwa, K. PPCM pathophysiology and epidemiology. Nat. Rev. Cardiol. 2014, 11, 364–370. [Google Scholar]
- Van der Meer, P.; Van Essen, B.J.; Viljoen, C.; Sliwa, K.; Hilfiker-Kleiner, D.; Brida, M.; Bracchi, M.; Boucek, M.; Groh, W.J.; Guenther, R.; et al. Bromocriptine treatment in PPCM. Eur. Heart J. 2025, 46, 1017–1027. [Google Scholar] [CrossRef]
- Sliwa, K.; Blauwet, L.; Tibazarwa, K.; Libhaber, E.; Smedema, J.P.; Becker, A.; McMurray, J.; Yamac, H.; Labidi, S.; Struman, I.; et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: A proof-of-concept pilot study. Circulation 2010, 121, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Petrie, M.C.; Van der Meer, P.; Hilfiker-Kleiner, D.; Mebazaa, A.; Mitchell, C.; Butler, J.; Stewart, S.; Hamshere, S.; Marelli, A.; et al. PPCM management and 6-month outcomes. Eur. Heart J. 2020, 41, 3787–3797. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.M.; Elkayam, U.; Alharethi, R.; Thompson, P.; Shah, P.J.; Toyoda, Y.; McCulloch, M.; Shah, M.; Mosceto, J.; Raitt, M.; et al. IPAC study. J. Am. Coll. Cardiol. 2015, 66, 905–914. [Google Scholar] [PubMed]
- Ilardi, F.; Manzo, R.; Peretto, G.; De Almeida, A.S.; Rosiello, E.; Marra, M.T.; Di Biase, L.; Calò, L.; Dello Iacono, R.; Camporeale, G.; et al. Italian registry PPCM. Int. J. Cardiol. 2026, 442, 133866. [Google Scholar] [CrossRef]
- Peretto, G.; Micaglio, E.; Ciconte, G.; Dello Russo, A.; Buzzatti, N.; Ziacchi, M.; Pulignano, G.; De Caterina, R.; Faggioni, M.; Sciarra, L.; et al. Arrhythmic presentation of PPCM. Front. Cardiovasc. Med. 2024, 11, 1362692. [Google Scholar] [CrossRef]
- James, A.H. Pregnancy-associated thrombosis. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 277–285. [Google Scholar] [CrossRef]
- Freedman, R.L.; Lucas, D.N. MBRRACE-UK maternal care. Int. J. Obstet. Anesth. 2015, 24, 161–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Adamo, M.; Zou, C.; Liu, L.; Liu, Y.; Xu, X.; Li, J.; Liu, C.; Mao, J.; Wang, J.; et al. Hypertrophic cardiomyopathy management. J. Cardiovasc. Med. 2024, 25, 399–419. [Google Scholar] [CrossRef]
- Joglar, J.A.; Kapa, S.; Saarel, E.V.; Viskin, S.; Ackerman, M.J.; Wilde, A.A.M.; Dorian, P.; Krahn, A.D.; Klein, G.J.; Conen, D.; et al. Arrhythmias during pregnancy. Heart Rhythm. 2023, 20, e175–e264. [Google Scholar] [CrossRef] [PubMed]
- Van der Zande, J.A.; Ramlakhan, K.P.; Sliwa, K.; Zeppenfeld, K.; Roos-Hesselink, J.W.; Thorne, S.A.; Niessen, H.W.M.; Kamp, O.; Berger, F.; Budts, W.; et al. Pregnancy with prosthetic valves. Eur. Heart J. 2025, ehaf265. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.; Ostro, J.; Shah, P.S.; Magee, L.A.; Kahn, S.R.; Lee, S.K.; Chan, W.S.; Hunt, D.; Pullenayegum, E.; Rodger, M.; et al. Anticoagulation in mechanical valves during pregnancy. Eur. Heart J. 2017, 38, 1509–1516. [Google Scholar] [CrossRef]
- Dempfle, C.E.; Koscielny, J.; Lindhoff-Last, E.; Greinacher, A.; Büller, H.R.; Rupprecht, H.J.; Göbel, A.; Junker, R.; Samama, M.M.; Büller, M.K.; et al. Fondaparinux in pregnancy. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211014575. [Google Scholar] [CrossRef] [PubMed]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Countouris, M.; Mahmoud, Z.; Cohen, J.B.; Friedman, A.M.; Hinkle, S.N.; Feingold, B.; Balachandren, N.; Parnell, B.; Wallace, K.; Froehlich, R.; et al. Hypertension in pregnancy. Circulation 2025, 151, 490–507. [Google Scholar] [CrossRef]
- Nie, X.; Xu, Z.; Ren, H. Risk factors for preeclampsia in chronic hypertension. BMC Pregnancy Childbirth 2024, 24, 307. [Google Scholar] [CrossRef]
- An, H.; Jin, M.; Li, Z.; Wang, Y.; Zhang, Q.; Yang, J.; Yu, Y.; Xu, S.; Chen, L.; Zhou, X.; et al. Hypertension and preterm birth. BMJ Open 2022, 12, e058068. [Google Scholar] [CrossRef] [PubMed]
- Sławek-Szmyt, S.; Kawka-Paciorkowska, K.; Ciepłucha, A.; Lesiak, M.; Ropacka-Lesiak, M. Preeclampsia and future CVD. J. Clin. Med. 2022, 11, 6048. [Google Scholar] [CrossRef] [PubMed]
- Sá, C.P.N.; Jiménez, M.F.; Rosa, M.W.; Alves, A.J.; Vieira, M.; Vale, H.; Pinto-Silva, L.; Castro, M.; Rebelo, H.; Ribeiro, F.; et al. PlGF and sFlt-1 in preeclampsia diagnosis. Rev. Bras. Ginecol. Obs. 2020, 42, 697–704. [Google Scholar]
- Veisani, Y.; Jenabi, E.; Delpisheh, A.; Khazaei, S. Angiogenic factors and preeclampsia. Int. J. Reprod. Biomed. 2019, 17, 1–10. [Google Scholar] [PubMed]
- Magee, L.A.; Kirkham, K.; Tohill, S.; MacDonell, K.; Walker, M.; Zhang, W.; Broadhurst, C.; McDonald, S.; Wen, S.W.; Leduc, L.; et al. WILL trial. PLoS Med. 2024, 21, e1004481. [Google Scholar]
- Sharma, D.D.; Chandresh, N.R.; Javed, A.; Singh, M.; Kumar, D.; Patel, V.; Kumar, P.; Saini, D.; Mehra, D.; Garg, R.; et al. Management of preeclampsia. Cureus 2024, 16, e51512. [Google Scholar] [CrossRef]
- Mészáros, B.; Kukor, Z.; Valent, S. Prevention and screening of preeclampsia. J. Clin. Med. 2023, 12, 6020. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Syngelaki, A.; O’Gorman, N.; Wright, D.; Nicolaides, K.H.; Poon, L.C. Aspirin for evidence-based preeclampsia prevention trial: Effects of aspirin on maternal serum pregnancy-associated plasma protein A and placental growth factor trajectories in pregnancy. Am. J. Obstet. Gynecol. 2024, 231, 342.e1–342.e9. [Google Scholar] [CrossRef]
- Kamp, J.C.; von Kaisenberg, C.; Greve, S.; Jankowich, M.; Barst, R.; Humbert, M.; Ghofrani, A.; Sitbon, O.; Apitz, C.; Englert, M.; et al. Pregnancy in pulmonary arterial hypertension. J. Heart Lung Transplant. 2021, 40, 229–233. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Simonneau, G.; Torbicki, A.; Jais, X.; Galiè, N.; Sitbon, O.; Rott, H.; Grünig, E.; et al. 2022 ESC/ERS PH Guidelines. Eur Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- El Iskandarani, M.; Golamari, R.; Bettinotti, B.G.M.; Alhabib, K.; Alsaileek, A.; Wahbi, B.; Ramadan, M.; Alraies, M.C.; Manla, Y.; Alhaddad, I.A.; et al. Pregnancy in pulmonary hypertension: Meta-analysis. J. Thorac. Dis. 2025, 17, 5108–5121. [Google Scholar] [CrossRef]
- Seth, R.; Moss, A.J.; McNitt, S.; Zareba, W.; Robinson, J.L.; Locati, E.; Schwartz, P.J.; Goldenberg, I.; Priori, S.G.; Wilde, A.A.M. Long QT syndrome and pregnancy. J. Am. Coll. Cardiol. 2007, 49, 1092–1098. [Google Scholar] [CrossRef]
- Marcinkeviciene, A.; Rinkuniene, D.; Puodziukynas, A. Long QT management during pregnancy. Medicina 2022, 58, 1694. [Google Scholar] [CrossRef]
- Smith, K.; Gros, B. Aortic dissection in Marfan pregnancy. Congenit. Heart Dis. 2017, 12, 251–260. [Google Scholar] [CrossRef]
- Roman, M.J.; Pugh, N.L.; Hendershot, T.P.; Cooper, K.; Davis, J.T.; Jefferies, J.L.; McGaughran, J.; Ueda, T.; Mitchell, D.; Chan, D.; et al. Aortic complications in Marfan syndrome. J. Am. Heart Assoc. 2016, 5, e004052. [Google Scholar] [CrossRef]
- Russo, M.; Boehler-Tatman, M.; Albright, C.; Gornik, I.; Mansukhani, R.; Barsness, K.; Young, M.; Kearon, C.; Azarbal, B.; Litt, H.; et al. Aortic dissection in pregnancy. Semin. Vasc. Surg. 2022, 35, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, J.; McLaughlin, M.A.; Dolan, S.M.; Govindarajulu, U.; Sieh, W.; Sundquist, K. Adverse pregnancy outcomes and long term risk of ischemic heart disease in mothers: National cohort and co-sibling study. BMJ 2023, 380, e072112. [Google Scholar] [CrossRef] [PubMed]
- Tita, A.T.; Szychowski, J.M.; Boggess, K.; Dugoff, L.; Sibai, B.; Lawrence, K.; Hughes, B.L.; Bell, J.; Aagaard, K.; Edwards, R.K.; et al. Treatment for mild chronic hypertension. N. Engl. J. Med. 2022, 386, 1781–1792. [Google Scholar] [CrossRef] [PubMed]

| Clinical Domain | ESC 2025 | AHA 2020 | Key Differences |
|---|---|---|---|
| PPCM | Defines PPCM as HF with LVEF < 45% developing in late pregnancy or ≤6 months postpartum. Bromocriptine recommended (Class IIa) in severe PPCM with anticoagulation. | Similar diagnostic definition. Bromocriptine may be considered in research or specialized centers. | ESC provides stronger support for bromocriptine based on registry evidence, while AHA remains cautious due to lack of RCTs. |
| Anticoagulation in Mechanical Valves | Individualized strategy. VKA preferred throughout pregnancy if daily dose ≤5 mg; LMWH acceptable with strict anti-Xa monitoring (peak 0.8–1.2 U/mL). Transition to heparin at 36 weeks. | Similar strategy; emphasizes shared decision-making. LMWH targets: peak 0.7–1.2 U/mL, trough monitoring optional. | General agreement, but ESC provides more specific anti-Xa targets; need for standardization persists. |
| Antihypertensive Therapy | Initiate at ≥140/90 mmHg (based on CHAP). Labetalol, nifedipine, and methyldopa as first-line agents. | Same BP threshold and agents. | Full alignment after CHAP; both discourage ACE inhibitors, ARBs, MRAs. |
| Aspirin Prophylaxis for Pre-eclampsia | 100–150 mg nightly starting <16 weeks in high-risk women. | 81–162 mg daily before 16 weeks in high-risk women. | Concordant strategy with minor dosing differences. |
| Delivery Timing in Hypertensive Pregnancy | Planned delivery at 37 weeks for stable women with chronic or gestational hypertension (WILL 2024). | Delivery between 37 and 39 weeks for controlled hypertension. | Harmonized recommendations based on recent RCTs. |
| Postpartum Cardiovascular Follow-up | Evaluation of BP, glucose, and lipids within 6–12 weeks; transition to cardiovascular prevention programs. | Similar recommendations emphasizing coordination with primary care. | Consensus achieved but implementation uneven across systems. |
| Section | Study/Registry | Population/Design | Main Findings/Clinical Message | Ref. |
|---|---|---|---|---|
| Peripartum and Preexisting Cardiomyopathies | EORP PPCM Registry | International prospective registry | Bromocriptine + standard HF therapy associated with higher LVEF recovery and lower 6-month mortality | [15,17] |
| IPAC Study | North American multicenter cohort | ~50% LV recovery at 6 months; baseline LVEF <30% predicts poor outcome | [18] | |
| Bromocriptine Pilot (Sliwa et al.) | Prospective interventional pilot | Prolactin blockade improves LV recovery; mechanistic support for targeted therapy | [16] | |
| CARPREG II | Risk prediction model | Identifies predictors of maternal cardiac events; cornerstone for pre-pregnancy counseling | [6] | |
| Italian Multicentre Registry (Ilardi et al.) | Multicentre national cohort | Heterogeneous clinical and echocardiographic features; LV recovery predictors require further investigation | [19] | |
| Arrhythmic PPCM Presentation (Peretto et al.) | Case series + critical review | PPCM may present with malignant ventricular arrhythmias; arrhythmic phenotype may precede LV dysfunction | [20] | |
| Anticoagulation and Mechanical Valve Management | ROPAC III Registry | 613 pregnancies (411 mechanical valves, 202 bioprosthetic) | Valve thrombosis 4–17%; higher risk with LMWH when anti-Xa targets unmet | [25] |
| D’Souza Meta-analysis | Systematic review (VKA vs. LMWH) | VKAs provide best valve protection; LMWH safer for fetus but requires strict anti-Xa monitoring | [26] | |
| Hypertensive Disorders of Pregnancy (HDPs) | CHAP Trial | 2408 women with mild chronic hypertension | Antihypertensive treatment at ≥140/90 mmHg reduces adverse pregnancy outcomes; adopted by ESC 2025/AHA 2020 | [48] |
| ASPRE Trial | High-risk women, aspirin prophylaxis | Aspirin 150 mg nightly <16 weeks reduces preterm pre-eclampsia by ~60% | [41] | |
| WILL Trial | Chronic or gestational hypertension | Planned delivery at 37 weeks reduces maternal complications without increasing neonatal risk | [43] | |
| PROGNOSIS Study | Suspected pre-eclampsia | sFlt-1/PlGF ≤38 rules out pre-eclampsia for 7 days; elevated ratio predicts imminent disease | [37] | |
| Other Pregnancy-Related CV Conditions | PPCM Registries (European cohorts) | Multicenter European cohorts | ~50% LV recovery at 6 months; poor outcome associated with LVEF < 30% or LVEDD > 60 mm | [14] |
| Preeclampsia and Future CVD Risk | Systematic review and meta-analysis | 2–4× increased lifetime risk of chronic hypertension, IHD, and stroke after pre-eclampsia | [9] |
| Condition | Established Evidence/Current Consensus | Unresolved or Controversial Issues |
|---|---|---|
| PPCM | Bromocriptine improves LV recovery when combined with standard HF therapy; anticoagulation indicated if LVEF < 35%. | Lack of randomized trials on bromocriptine; unclear recurrence risk modifiers in subsequent pregnancies. |
| Mechanical Valves and Anticoagulation | Low-dose VKA (<5 mg/day) remains safest for valve protection; LMWH feasible with rigorous monitoring; warfarin safe during lactation. | Optimal anti-Xa targets not standardized; limited RCT data comparing regimens; LMWH underdosing remains common. |
| Hypertensive Disorders of Pregnancy | CHAP and WILL trials define modern BP thresholds and delivery timing; aspirin prophylaxis reduces preterm pre-eclampsia risk by ~60%. | Need for real-world data on aspirin adherence and dosing; mechanisms linking HDP and long-term CVD still incompletely understood. |
| Postpartum Follow-up | HDP and PPCM recognized as markers of long-term cardiovascular risk; structured follow-up recommended at 6–12 weeks. | Implementation gaps in postpartum care; unclear optimal duration and modality of cardiovascular surveillance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tognola, C.; Brucato, F.; Maloberti, A.; Varrenti, M.; Preda, A.; Mazzone, P.; Giannattasio, C.; Guarracini, F. Cardiovascular Disease in Pregnancy: When Two Hearts Beat as One. Diagnostics 2025, 15, 2921. https://doi.org/10.3390/diagnostics15222921
Tognola C, Brucato F, Maloberti A, Varrenti M, Preda A, Mazzone P, Giannattasio C, Guarracini F. Cardiovascular Disease in Pregnancy: When Two Hearts Beat as One. Diagnostics. 2025; 15(22):2921. https://doi.org/10.3390/diagnostics15222921
Chicago/Turabian StyleTognola, Chiara, Filippo Brucato, Alessandro Maloberti, Marisa Varrenti, Alberto Preda, Patrizio Mazzone, Cristina Giannattasio, and Fabrizio Guarracini. 2025. "Cardiovascular Disease in Pregnancy: When Two Hearts Beat as One" Diagnostics 15, no. 22: 2921. https://doi.org/10.3390/diagnostics15222921
APA StyleTognola, C., Brucato, F., Maloberti, A., Varrenti, M., Preda, A., Mazzone, P., Giannattasio, C., & Guarracini, F. (2025). Cardiovascular Disease in Pregnancy: When Two Hearts Beat as One. Diagnostics, 15(22), 2921. https://doi.org/10.3390/diagnostics15222921







