Impact of Single- Versus Multiple-Type HPV Infections on Cervical Cytological and Histological Abnormalities: The Dominant Oncogenic Potential of HPV16 Single-Type Infections
Abstract
1. Introduction
2. Materials and Methods
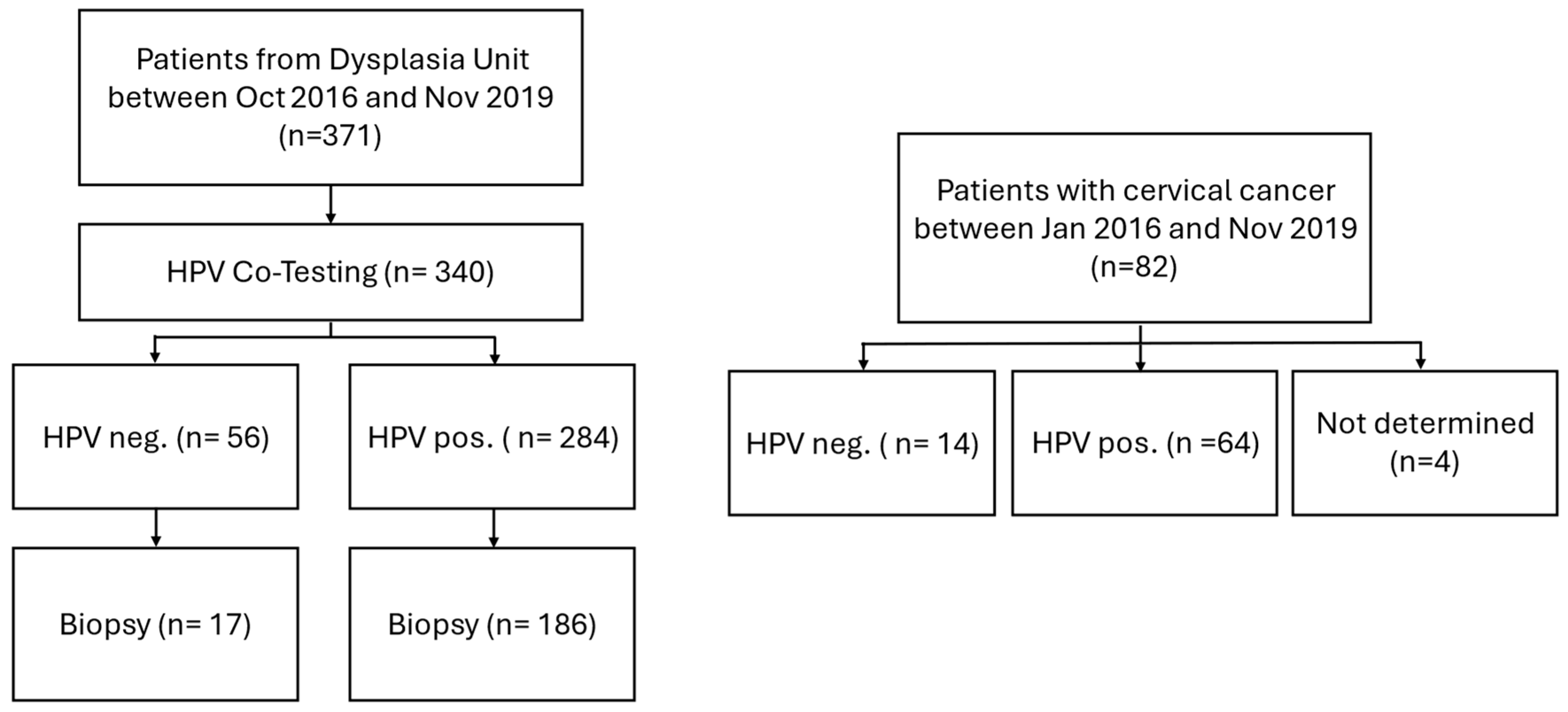
2.1. Study Population
2.2. Specimen Collection and HPV Genotyping
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Age (Years) | ≤19 | 20–29 | 30–39 | 40–49 | 50–59 | ≥60 | Total |
|---|---|---|---|---|---|---|---|
| ST | 2 | 43 | 69 | 37 | 11 | 3 | 165 |
| MT | 3 | 46 | 41 | 20 | 6 | 3 | 119 |
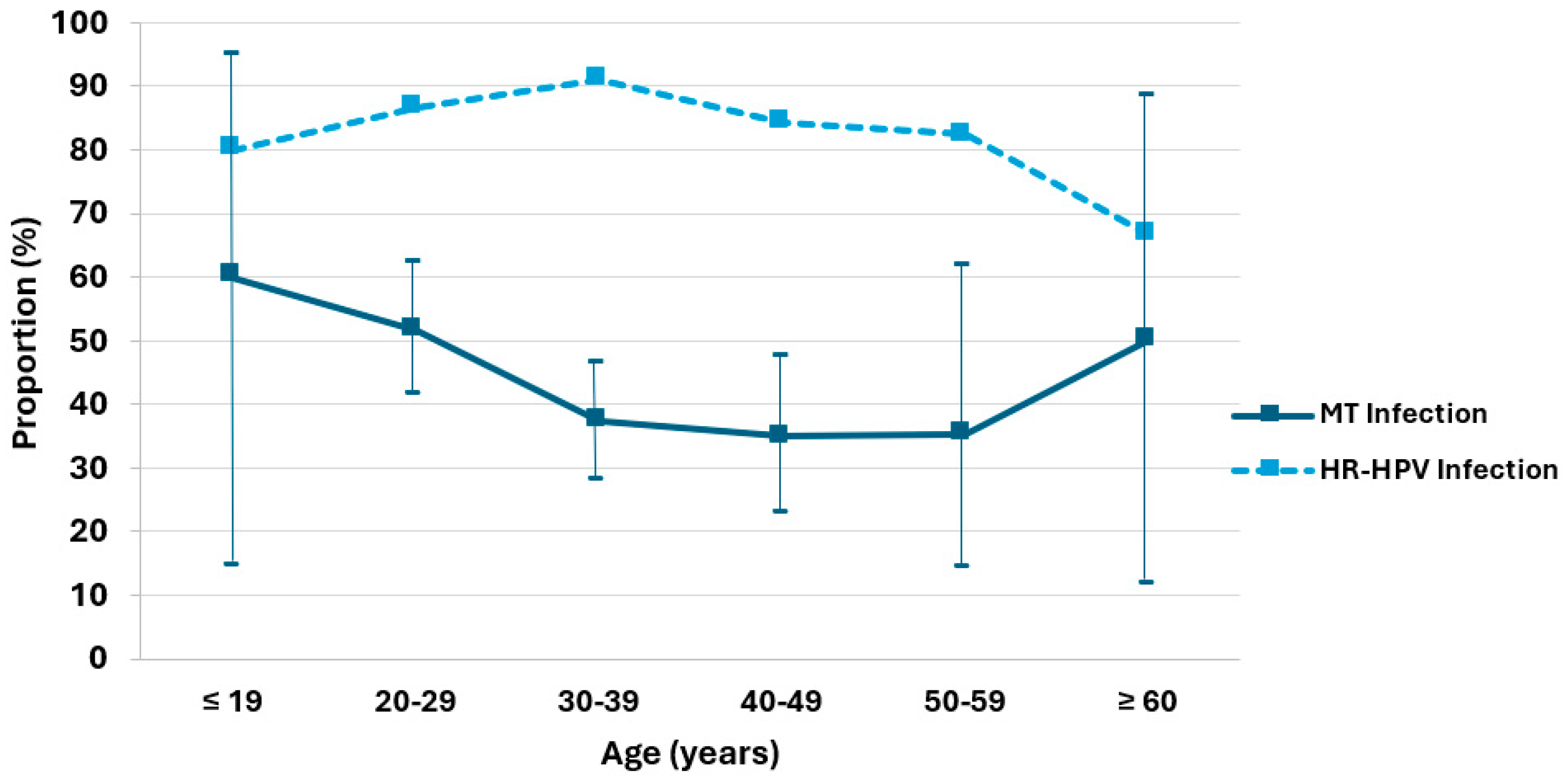
| MT/all HPV | 60 | 51.6 | 37.3 | 35.1 | 35.3 | 50 | |
| (% with 95% CI) | (14.7–94.7) | (41.3–62.1) | (28.2–46.3) | (22.7–47.5) | (14.2–61.7) | (11.8–88.2) | |
| HR HPV | 4 | 77 | 100 | 48 | 14 | 4 | 247 |
| HR HPV /all HPV (%) | 80 | 86.5 | 91 | 84.2 | 82.4 | 66.7 |
| HPV Type | Risk Group | CIN3+ ST (n) | CIN3+ MT (n) | CIN2− ST (n) | CIN2− MT (n) |
|---|---|---|---|---|---|
| 16 | High Risk | 74 | 19 | 15 | 7 |
| 18 | High Risk | 13 | 4 | 3 | 2 |
| 31 | High Risk | 2 | 12 | 3 | 1 |
| 33 | High Risk | 1 | 8 | 0 | 6 |
| 35 | High Risk | 1 | 3 | 0 | 2 |
| 39 | High Risk | 0 | 6 | 1 | 3 |
| 45 | High Risk | 4 | 7 | 0 | 3 |
| 51 | High Risk | 1 | 6 | 0 | 5 |
| 52 | High Risk | 2 | 12 | 1 | 6 |
| 56 | High Risk | 0 | 0 | 0 | 6 |
| 58 | High Risk | 2 | 5 | 1 | 8 |
| 59 | High Risk | 2 | 3 | 4 | 5 |
| 34 | Probably High Risk | 0 | 0 | 0 | 1 |
| 53 | Probably High Risk | 1 | 5 | 1 | 7 |
| 66 | Probably High Risk | 0 | 2 | 0 | 7 |
| 68 | Probably High Risk | 1 | 4 | 0 | 4 |
| 73 | Probably High Risk | 3 | 6 | 0 | 5 |
| 82 | Probably High Risk | 0 | 0 | 0 | 1 |
| 6 | Low Risk | 0 | 5 | 1 | 1 |
| 11 | Low Risk | 0 | 5 | 0 | 0 |
| 42 | Low Risk | 0 | 2 | 0 | 3 |
| 44 | Low Risk | 0 | 0 | 1 | 0 |
| 54 | Low Risk | 0 | 5 | 1 | 1 |
| 55 | Low Risk | 0 | 0 | 1 | 1 |
| 61 | Low Risk | 0 | 6 | 0 | 7 |
| 62 | Low Risk | 0 | 0 | 0 | 2 |
| 72 | Low Risk | 1 | 0 | 0 | 0 |
| 81 | Low Risk | 0 | 0 | 0 | 1 |
| 83 | Low Risk | 0 | 2 | 0 | 1 |
| 84 | Low Risk | 0 | 3 | 0 | 2 |
| 90 | Low Risk | 0 | 1 | 0 | 3 |
| CIN2− | CIN3+ | p | |
|---|---|---|---|
| a9 + non-a7/9 | 13 | 15 | 0.608 |
| a7 + non-a7/9 | 3 | 7 | |
| non-a7/9 + non-a7/9 | 6 | 4 | |
| a9 + a9 | 5 | 8 | |
| a9 + a7 | 8 | 15 |
References
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F. WHO International Agency for Research on Cancer Carcinogenicity of Human Papillomaviruses. Lancet Oncol. 2005, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Smith, J.S.; Plummer, M.; Muñoz, N.; Franceschi, S. Human Papillomavirus Types in Invasive Cervical Cancer Worldwide: A Meta-Analysis. Br. J. Cancer 2003, 88, 63–73. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Li, Z.; Xirasagar, S.; Maes, P.; Kaminsky, D.; Liou, D.; Sun, Q.; Kaur, R.; Huyen, Y.; McBride, A.A. The Papillomavirus Episteme: A Major Update to the Papillomavirus Sequence Database. Nucleic Acids Res. 2017, 45, D499–D506. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological Agents. Volume 100B. A Review of Human Carcinogens; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100B; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- de Villiers, E.-M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Aitken, C.A.A.; van Agt, H.M.E.; Siebers, A.G.; van Kemenade, F.J.; Niesters, H.G.M.; Melchers, W.J.G.; Vedder, J.E.M.; Schuurman, R.; van den Brule, A.J.C.; van der Linden, H.C.; et al. Introduction of primary screening using high-risk HPV DNA detection in the Dutch cervical cancer screening programme: A population-based cohort study. BMC Med. 2019, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, L.; Zhang, K.; Zhang, Y.; Zheng, T.; Dai, M. Multiple Human Papillomavirus Infections among Chinese Women with and without Cervical Abnormalities: A Population-Based Multi-Center Cross-Sectional Study. Front. Oncol. 2011, 1, 38. [Google Scholar] [CrossRef]
- Kops, N.L.; Caierão, J.; Bessel, M.; Horvath, J.D.C.; Domingues, C.M.; Benzaken, A.S.; Villa, L.L.; de Souza, F.M.A.; Pereira, G.F.M.; Wendland, E.M. Behavioral Factors Associated with Multiple-Type HPV Genital Infections: Data from a Cross-Sectional Study in Young Women in Brazil. Reprod. Health 2021, 18, 201. [Google Scholar] [CrossRef]
- Finan, R.R.; Chemaitelly, H.; Racoubian, E.; Aimagambetova, G.; Almawi, W.Y. Genetic Diversity of Human Papillomavirus (HPV) as Specified by the Detection Method, Gender, and Year of Sampling: A Retrospective Cross-Sectional Study. Arch. Gynecol. Obstet. 2023, 307, 1469–1479. [Google Scholar] [CrossRef]
- Mejlhede, N.; Bonde, J.; Fomsgaard, A. High Frequency of Multiple HPV Types in Cervical Specimens from Danish Women. APMIS 2009, 117, 108–114. [Google Scholar] [CrossRef]
- Salazar, K.L.; Zhou, H.S.; Xu, J.; Peterson, L.E.; Schwartz, M.R.; Mody, D.R.; Ge, Y. Multiple Human Papilloma Virus Infections and Their Impact on the Development of High-Risk Cervical Lesions. Acta Cytol. 2015, 59, 391–398. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Katki, H.A.; Hildesheim, A.; Rodríguez, A.C.; Quint, W.; Schiffman, M.; Van Doorn, L.-J.; Porras, C.; Wacholder, S.; Gonzalez, P.; et al. Human Papillomavirus Infection with Multiple Types: Pattern of Coinfection and Risk of Cervical Disease. J. Infect. Dis. 2011, 203, 910–920. [Google Scholar] [CrossRef]
- Argyri, E.; Papaspyridakos, S.; Tsimplaki, E.; Michala, L.; Myriokefalitaki, E.; Papassideri, I.; Daskalopoulou, D.; Tsiaoussi, I.; Magiakos, G.; Panotopoulou, E. A Cross Sectional Study of HPV Type Prevalence According to Age and Cytology. BMC Infect. Dis. 2013, 13, 53. [Google Scholar] [CrossRef]
- Song, F.; Yan, P.; Huang, X.; Wang, C.; Du, H.; Qu, X.; Wu, R. Roles of Extended Human Papillomavirus Genotyping and Multiple Infections in Early Detection of Cervical Precancer and Cancer and HPV Vaccination. BMC Cancer 2022, 22, 42. [Google Scholar] [CrossRef]
- Trottier, H.; Mahmud, S.; Costa, M.C.; Sobrinho, J.P.; Duarte-Franco, E.; Rohan, T.E.; Ferenczy, A.; Villa, L.L.; Franco, E.L. Human Papillomavirus Infections with Multiple Types and Risk of Cervical Neoplasia. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1274–1280. [Google Scholar] [CrossRef]
- De Brot, L.; Pellegrini, B.; Moretti, S.T.; Carraro, D.M.; Soares, F.A.; Rocha, R.M.; Baiocchi, G.; da Cunha, I.W.; de Andrade, V.P. Infections with Multiple High-Risk HPV Types Are Associated with High-Grade and Persistent Low-Grade Intraepithelial Lesions of the Cervix. Cancer Cytopathol. 2017, 125, 138–143. [Google Scholar] [CrossRef]
- Pista, A.; Oliveira, A.; Verdasca, N.; Ribeiro, F. Single and Multiple Human Papillomavirus Infections in Cervical Abnormalities in Portuguese Women. Clin. Microbiol. Infect. 2011, 17, 941–946. [Google Scholar] [CrossRef]
- Spinillo, A.; Dal Bello, B.; Gardella, B.; Roccio, M.; Dacco’, M.D.; Silini, E.M. Multiple Human Papillomavirus Infection and High Grade Cervical Intraepithelial Neoplasia among Women with Cytological Diagnosis of Atypical Squamous Cells of Undetermined Significance or Low Grade Squamous Intraepithelial Lesions. Gynecol. Oncol. 2009, 113, 115–119. [Google Scholar] [CrossRef]
- Andersson, E.; Kärrberg, C.; Rådberg, T.; Blomqvist, L.; Zetterqvist, B.-M.; Ryd, W.; Lindh, M.; Horal, P. Type-Dependent E6/E7 mRNA Expression of Single and Multiple High-Risk Human Papillomavirus Infections in Cervical Neoplasia. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2012, 54, 61–65. [Google Scholar] [CrossRef]
- Wentzensen, N.; Nason, M.; Schiffman, M.; Dodd, L.; Hunt, W.C.; Wheeler, C.M. No Evidence for Synergy Between Human Papillomavirus Genotypes for the Risk of High-Grade Squamous Intraepithelial Lesions in a Large Population-Based Study. J. Infect. Dis. 2014, 209, 855–864. [Google Scholar] [CrossRef]
- Zhong, F.; Yu, T.; Ma, X.; Wang, S.; Cong, Q.; Tao, X. Extensive HPV Genotyping Reveals High Association between Multiple Infections and Cervical Lesions in Chinese Women. Dis. Markers 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhang, Y.; Jing, X.; Wu, N.; Hou, Y.; Hao, C. Correlation between Multi—Type Human Papillomavirus Infections and Viral Loads and the Cervical Pathological Grade. Int. J. Gynecol. Obstet. 2021, 152, 96–102. [Google Scholar] [CrossRef]
- Senapati, R.; Nayak, B.; Kar, S.K.; Dwibedi, B. HPV Genotypes Co-Infections Associated with Cervical Carcinoma: Special Focus on Phylogenetically Related and Non-Vaccine Targeted Genotypes. PLoS ONE 2017, 12, e0187844. [Google Scholar] [CrossRef]
- Wentzensen, N.; Sun, C.; Ghosh, A.; Kinney, W.; Mirabello, L.; Wacholder, S.; Shaber, R.; LaMere, B.; Clarke, M.; Lorincz, A.T.; et al. Methylation of HPV18, HPV31, and HPV45 Genomes and Cervical Intraepithelial Neoplasia Grade 3. J. Natl. Cancer Inst. 2012, 104, 1738–1749. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Myers, L.; Hammons, A.F.; Clark, R.A.; Dunlap, K.; Kissinger, P.J.; Hagensee, M.E. Prevalence and Clustering Patterns of Human Papillomavirus Genotypes in Multiple Infections. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2005, 14, 2439–2445. [Google Scholar] [CrossRef]
- Stuebs, F.A.; Koch, M.C.; Dietl, A.K.; Adler, W.; Geppert, C.; Hartmann, A.; Knöll, A.; Beckmann, M.W.; Mehlhorn, G.; Schulmeyer, C.E.; et al. Cytology and High-Risk Human Papillomavirus Test for Cervical Cancer Screening Assessment. Diagnostics 2022, 12, 1748. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs; International Agency for Research on Cancer (IARC): Lyon, France, 2014; ISBN 978-92-832-2435-8. [Google Scholar]
- Channir, H.I.; Bendtsen, S.K.; Melchior, L.C.; Sandholm, P.R.; Mordhorst, C.; Carlander, A.-L.F.; von Buchwald, C.; Kiss, K. Validation of the VisionArray® Chip Assay for HPV DNA Testing in Histology Specimens of Oropharyngeal Squamous Cell Carcinoma. Head Neck Pathol. 2024, 18, 27. [Google Scholar] [CrossRef]
- VisionArray HPV—ZytoVision GmbH. Available online: https://www.zytovision.com/products/visionarray/hpv/ (accessed on 6 June 2025).
- Luo, Q.; Zeng, X.; Luo, H.; Pan, L.; Huang, Y.; Zhang, H.; Han, N. Epidemiologic Characteristics of High-Risk HPV and the Correlation between Multiple Infections and Cervical Lesions. BMC Infect. Dis. 2023, 23, 667. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Zheng, J.; Xue, H.; Chen, J.; Zheng, X. Prevalence of Multiple Human Papillomavirus Infections and Association with Cervical Lesions among Outpatients in Fujian, China: A Cross-Sectional Study. J. Med. Virol. 2022, 94, 6028–6036. [Google Scholar] [CrossRef]
- Rousseau, M.C.; Pereira, J.S.; Prado, J.C.; Villa, L.L.; Rohan, T.E.; Franco, E.L. Cervical Coinfection with Human Papillomavirus (HPV) Types as a Predictor of Acquisition and Persistence of HPV Infection. J. Infect. Dis. 2001, 184, 1508–1517. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schiffman, M.; Dunn, S.T.; Zuna, R.E.; Gold, M.A.; Allen, R.A.; Zhang, R.; Sherman, M.E.; Wacholder, S.; Walker, J.; et al. Multiple HPV Genotype Infections in Cervical Cancer Progression in the Study to Understand Cervical Cancer Early Endpoints and Determinants (SUCCEED). Int. J. Cancer J. Int. Cancer 2009, 125, 2151–2158. [Google Scholar] [CrossRef]
- Rositch, A.F.; Burke, A.E.; Viscidi, R.P.; Silver, M.I.; Chang, K.; Gravitt, P.E. Contributions of Recent and Past Sexual Partnerships on Incident Human Papillomavirus Detection: Acquisition and Reactivation in Older Women. Cancer Res. 2012, 72, 6183–6190. [Google Scholar] [CrossRef]
- Paul, P.; Hammer, A.; Rositch, A.F.; Burke, A.E.; Viscidi, R.P.; Silver, M.I.; Campos, N.; Youk, A.O.; Gravitt, P.E. Rates of New Human Papillomavirus Detection and Loss of Detection in Middle-Aged Women by Recent and Past Sexual Behavior. J. Infect. Dis. 2020, 223, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, C.M.; Romão, F.; Castelo-Branco, C. Menopause and Aging: Changes in the Immune System--a Review. Maturitas 2010, 67, 316–320. [Google Scholar] [CrossRef]
- Wira, C.R.; Patel, M.V.; Ghosh, M.; Mukura, L.; Fahey, J.V. Innate Immunity in the Human Female Reproductive Tract: Endocrine Regulation of Endogenous Antimicrobial Protection Against HIV and Other Sexually Transmitted Infections. Am. J. Reprod. Immunol. 2011, 65, 196–211. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, L.; Yu, M.; Zhou, X.; Zhang, X.; Zheng, W.; Niu, S.; Zhou, F. Prevalence of Cervical Precancers or Cancers in Women with ASC-H/HSIL Cytology According to Aptima HPV (AHPV) Assay-Detected HPV Genotypes and Age. J. Cancer 2024, 15, 140–148. [Google Scholar] [CrossRef]
- Li, W.; Meng, Y.; Wang, Y.; Cheng, X.; Wang, C.; Xiao, S.; Zhang, X.; Deng, Z.; Hu, M.; Shen, P.; et al. Association of Age and Viral Factors with High-Risk HPV Persistence: A Retrospective Follow-up Study. Gynecol. Oncol. 2019, 154, 345–353. [Google Scholar] [CrossRef]
- Feng, T.; Cheng, B.; Sun, W.; Yang, Y. Outcome and Associated Factors of High-Risk Human Papillomavirus Infection without Cervical Lesions. BMC Womens Health 2023, 23, 599. [Google Scholar] [CrossRef]
- Ronco, G.; Giorgi-Rossi, P.; Carozzi, F.; Confortini, M.; Dalla Palma, P.; Del Mistro, A.; Ghiringhello, B.; Girlando, S.; Gillio-Tos, A.; De Marco, L.; et al. Efficacy of Human Papillomavirus Testing for the Detection of Invasive Cervical Cancers and Cervical Intraepithelial Neoplasia: A Randomised Controlled Trial. Lancet Oncol. 2010, 11, 249–257. [Google Scholar] [CrossRef]
- Rijkaart, D.C.; Berkhof, J.; Rozendaal, L.; van Kemenade, F.J.; Bulkmans, N.W.J.; Heideman, D.A.M.; Kenter, G.G.; Cuzick, J.; Snijders, P.J.F.; Meijer, C.J.L.M. Human Papillomavirus Testing for the Detection of High-Grade Cervical Intraepithelial Neoplasia and Cancer: Final Results of the POBASCAM Randomised Controlled Trial. Lancet Oncol. 2012, 13, 78–88. [Google Scholar] [CrossRef] [PubMed]
- van der Marel, J.; Quint, W.G.V.; Schiffman, M.; van de Sandt, M.M.; Zuna, R.E.; Dunn, S.T.; Smith, K.; Mathews, C.A.; Gold, M.A.; Walker, J.; et al. Molecular Mapping of High-Grade Cervical Intraepithelial Neoplasia Shows Etiological Dominance of HPV16. Int. J. Cancer 2012, 131, E946–E953. [Google Scholar] [CrossRef] [PubMed]
- Dickson, E.L.; Vogel, R.I.; Bliss, R.L.; Downs, L.S. Multiple-Type Human Papillomavirus (HPV) Infections: A Cross-Sectional Analysis of the Prevalence of Specific Types in 309,000 Women Referred for HPV Testing at the Time of Cervical Cytology. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2013, 23, 1295–1302. [Google Scholar] [CrossRef]
- Vaccarella, S.; Franceschi, S.; Snijders, P.J.F.; Herrero, R.; Meijer, C.J.L.M.; Plummer, M. The IARC HPV Prevalence Surveys Study Group Concurrent Infection with Multiple Human Papillomavirus Types: Pooled Analysis of the IARC HPV Prevalence Surveys. Cancer Epidemiol. Biomark. Prev. 2010, 19, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.L.; Hildesheim, A.; Burk, R.D.; Gravitt, P.; Wacholder, S.; Manos, M.M.; Scott, D.R.; Sherman, M.E.; Kurman, R.J.; Glass, A.G.; et al. A Prospective Study of Human Papillomavirus (HPV) Type 16 DNA Detection by Polymerase Chain Reaction and Its Association with Acquisition and Persistence of Other HPV Types. J. Infect. Dis. 2001, 183, 8–15. [Google Scholar] [CrossRef]
- Thomas, K.K.; Hughes, J.P.; Kuypers, J.M.; Kiviat, N.B.; Lee, S.K.; Adam, D.E.; Koutsky, L.A. Concurrent and Sequential Acquisition of Different Genital Human Papillomavirus Types. J. Infect. Dis. 2000, 182, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Hoes, J.; Pasmans, H.; Schurink-van ’t Klooster, T.M.; van der Klis, F.R.M.; Donken, R.; Berkhof, J.; de Melker, H.E. Review of Long-Term Immunogenicity Following HPV Vaccination: Gaps in Current Knowledge. Hum. Vaccines Immunother. 2022, 18, 1908059. [Google Scholar] [CrossRef]
- Xi, L.F.; Edelstein, Z.R.; Meyers, C.; Ho, J.; Cherne, S.L.; Schiffman, M. Human Papillomavirus Types 16 and 18 DNA Load in Relation to Coexistence of Other Types, Particularly Those in the Same Species. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2507–2512. [Google Scholar] [CrossRef]


| Histology | ||||||
|---|---|---|---|---|---|---|
| Age (Years) | Total (%) | Benign (%) | CIN1 (%) | CIN2 (%) | CIN3 (%) | CC (%) |
| ≤19 | 5 (2) | 2 (40) | 2 (40) | 1 (20) | ||
| 20–29 | 56 (22.4) | 7 (12.5) | 1 (1.8) | 14 (25) | 30 (53.6) | 4 (7.1) |
| 30–39 | 108 (43.2) | 11 (10.2) | 10 (9.3) | 16 (14.8) | 46 (42.6) | 25 (23.1) |
| 40–49 | 51 (20.4) | 8 (15.7) | 5 (9.8) | 4 (7.8) | 17 (33.3) | 17 (33.3) |
| 50–59 | 21 (8.4) | 3 (14.3) | 1 (4.8) | 1 (4.8) | 5 (23.8) | 11 (52.4) |
| ≥60 | 9 (3.6) | 1 (11.1) | 8 (88.9) | |||
| Total | 250 (100) | 29 (11.6) | 19 (7.6) | 35 (14) | 101 (40.4) | 66 (26.4) |
| Histology | ||||||
|---|---|---|---|---|---|---|
| Cytology | Total (%) | Benign (%) | CIN1 (%) | CIN2 (%) | CIN3 (%) | CC (%) |
| NILM | 12 (6.5) | 6 (50) | 2 (16.7) | 1 (8.3) | 3 (25) | |
| ASC-US/LSIL | 20 (10.8) | 7 (35) | 2 (10) | 7 (35) | 4 (20) | |
| ASC-H/HSIL | 154 (82.8) | 16 (10.4) | 15 (9.7) | 27 (17.5) | 94 (61.0) | 2 (1.3) |
| Cytology | Histology | |||||||
|---|---|---|---|---|---|---|---|---|
| χ2 | p | χ2 | p | |||||
| N/ACS-US/LSIL | ACS-H/HSIL | CIN2− | CIN3+ | |||||
| ST | 38 | 126 | 0.0549 | 0.815 | 33 | 107 | 8.1311 | 0.004 |
| MT | 29 | 90 | 35 | 49 | ||||
| HR HPV ST | 27 | 128 | 1.0919 | 0.296 | 28 | 101 | 7.118 | 0.008 |
| HR HPV MT | 24 | 82 | 29 | 45 | ||||
| HPV16 ST | 12 | 73 | 0.3024 | 0.582 | 15 | 74 | 1.3187 | 0.251 |
| HPV16 MT | 7 | 32 | 7 | 19 | ||||
| HPV16 ST | 12 | 73 | 1.4263 | 0.232 | 15 | 74 | 3.9753 | 0.046 |
| HR HPV (HPV16-) ST | 15 | 55 | 13 | 27 | ||||
| HPV16 MT | 7 | 32 | 0.7758 | 0.378 | 7 | 19 | 2.3687 | 0.124 |
| HR HPV (HPV16-) MT | 17 | 50 | 20 | 24 | ||||
| HR HPV (HPV16-) ST | 15 | 55 | 0.2975 | 0.585 | 13 | 27 | 1.9474 | 0.163 |
| HRHPV (HPV16-) MT | 17 | 50 | 20 | 24 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.; Ludwig, S.; Sievers, S.L.; Einzmann, T.; Zhao, Y.; Pilch, H. Impact of Single- Versus Multiple-Type HPV Infections on Cervical Cytological and Histological Abnormalities: The Dominant Oncogenic Potential of HPV16 Single-Type Infections. Diagnostics 2025, 15, 2880. https://doi.org/10.3390/diagnostics15222880
Baek S, Ludwig S, Sievers SL, Einzmann T, Zhao Y, Pilch H. Impact of Single- Versus Multiple-Type HPV Infections on Cervical Cytological and Histological Abnormalities: The Dominant Oncogenic Potential of HPV16 Single-Type Infections. Diagnostics. 2025; 15(22):2880. https://doi.org/10.3390/diagnostics15222880
Chicago/Turabian StyleBaek, Sunhwa, Sebastian Ludwig, Sophie Lee Sievers, Thomas Einzmann, Yue Zhao, and Henryk Pilch. 2025. "Impact of Single- Versus Multiple-Type HPV Infections on Cervical Cytological and Histological Abnormalities: The Dominant Oncogenic Potential of HPV16 Single-Type Infections" Diagnostics 15, no. 22: 2880. https://doi.org/10.3390/diagnostics15222880
APA StyleBaek, S., Ludwig, S., Sievers, S. L., Einzmann, T., Zhao, Y., & Pilch, H. (2025). Impact of Single- Versus Multiple-Type HPV Infections on Cervical Cytological and Histological Abnormalities: The Dominant Oncogenic Potential of HPV16 Single-Type Infections. Diagnostics, 15(22), 2880. https://doi.org/10.3390/diagnostics15222880






