Visualization of the Persistent Avascular Retina with Ultra-Widefield Green Reflectance Imaging
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
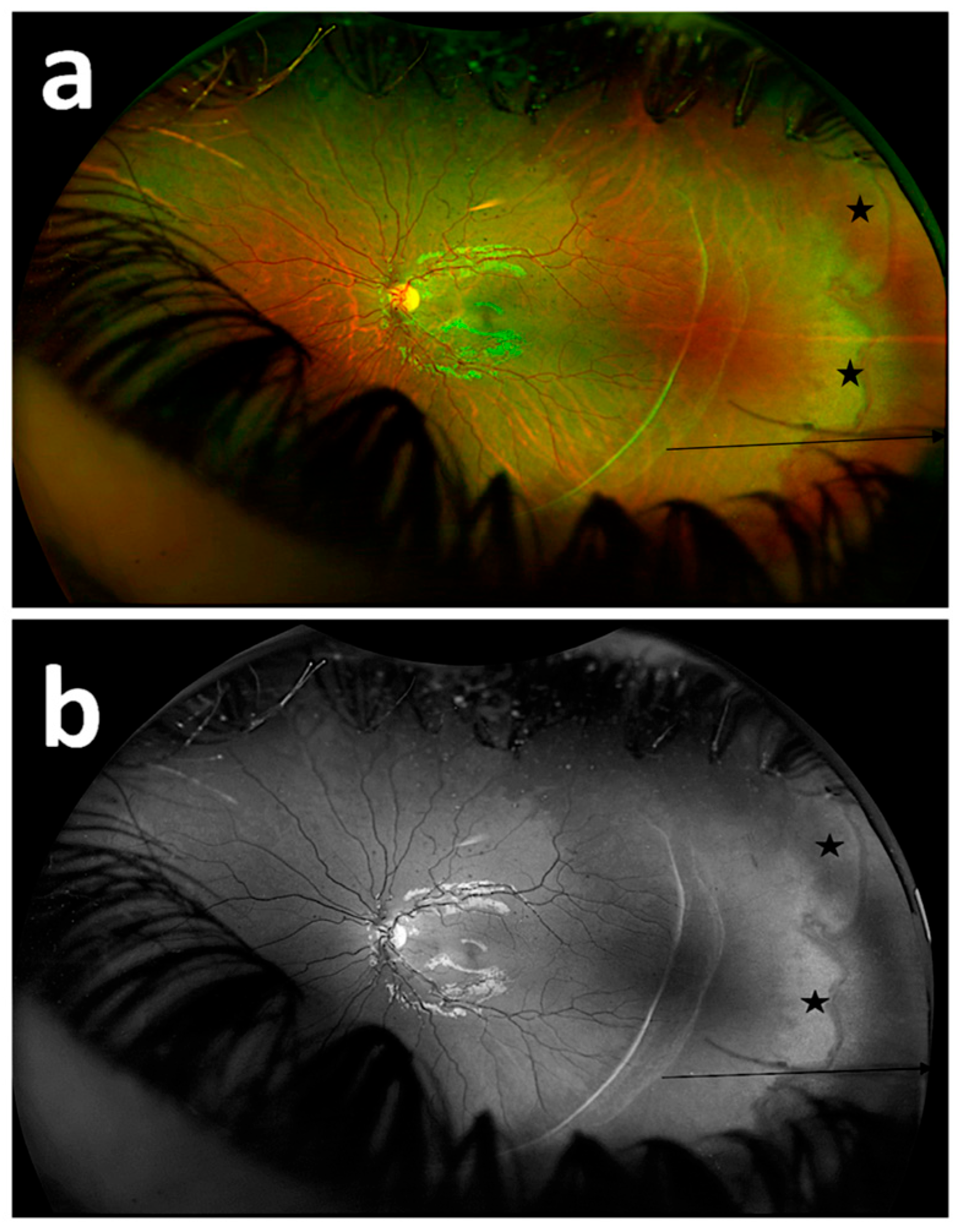
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, E.H.; Shin, Y.U.; Cho, H. Retinopathy of prematurity: A review of epidemiology and current treatment strategies. Clin. Exp. Pediatr. 2022, 65, 115–126. [Google Scholar] [CrossRef]
- Chung, E.J.; Kim, J.H.; Ahn, H.S.; Koh, H.J. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1727–1730. [Google Scholar] [CrossRef]
- Tan, T.F.; Tay, S.A.; Agarwal-Sinha, S.; Tan, G.S.W.; Wu, W.C.; Tsai, A.S.H. Persistent avascular retina in retinopathy of prematurity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2025, 263, 2177–2190. [Google Scholar] [CrossRef]
- Özdek, Ş.; Özdemir Zeydanlı, E.; Baumal, C.; Hoyek, S.; Patel, N.; Berrocal, A.; Lopez-Cañizares, A.; Al-Khersan, H.; Kusaka, S.; Mano, F.; et al. Avascular peripheral retina in infants. Turk. J. Ophthalmol. 2023, 53, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.E.; Moinuddin, O.; Blair, M.P.; Schechet, S.A.; Shapiro, M.J.; Quiram, P.A.; Mammo, D.A.; Berrocal, A.M.; Prakhunhungsit, S.; Cernichiaro-Espinosa, L.A.; et al. Late-onset retinal findings and complications in untreated retinopathy of prematurity. Ophthalmol. Retin. 2020, 4, 602–612. [Google Scholar] [CrossRef]
- Choudhry, N.; Duker, J.S.; Freund, K.B.; Kiss, S.; Querques, G.; Rosen, R.; Sarraf, D.; Souied, E.H.; Stanga, P.E.; Staurenghi, G.; et al. Classification and guidelines for widefield imaging. Ophthalmol. Retin. 2019, 3, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Surve, A.; Kumawat, D.; Takkar, B.; Azad, S.; Chawla, R.; Shroff, D.; Arora, A.; Singh, R.; Venkatesh, P. Ultra-wide field retinal imaging: A wider clinical perspective. Indian J. Ophthalmol. 2021, 69, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Imai, A.; Kasamatsu, H.; Kakihara, S.; Toriyama, Y.; Murata, T. Assessment of diabetic retinopathy using two ultra-wide-field fundus imaging systems, the Clarus® and Optos™ systems. BMC Ophthalmol. 2018, 18, 332. [Google Scholar] [CrossRef]
- Patel, S.N.; Shi, A.; Wibbelsman, T.D.; Klufas, M.A. Ultra-widefield retinal imaging: An update on recent advances. Ther. Adv. Ophthalmol. 2020, 20, 2515841419899495. [Google Scholar] [CrossRef]
- Erginay, A. True-to-life retinal imaging with the new ultrawidefield color RGB modality. Mod. Retin. Digit. Ed. 2024, 4, 2. Available online: https://www.modernretina.com/view/true-to-life-retinal-imaging-with-the-new-ultrawidefield-color-rgb-modality (accessed on 21 September 2025).
- Moon, J.Y.; Wai, K.M.; Patel, N.S.; Katz, R.; Dahrouj, M.; Miller, J.B. Visualization of retinal breaks on ultra-widefield fundus imaging using a digital green filter. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 935–940. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Ducrey, N.M.; Delori, F.C.; Gragoudas, E.S. Monochromatic ophthalmoscopy and fundus photography. II. The pathological fundus. Arch. Ophthalmol. 1979, 97, 288–293. [Google Scholar] [CrossRef]
- Shakha; Chandra, P. Red-free visualization enhances ease of laser therapy for retinopathy of prematurity. Indian J. Ophthalmol. 2025, 73, S178–S179. [Google Scholar] [CrossRef]
- Iqbal, M.I. Red-free (green) filter-enhanced gonioscopy with smartphone: A pilot study. Cureus 2024, 16, e51559. [Google Scholar] [CrossRef]
- Sharma, P.; Dhami, A.; Dhami, N.B.; Dhami, G.S. Comparison of patient satisfaction with red-free (green) versus yellow light using binocular indirect ophthalmoscope for retinal examination. Indian J. Ophthalmol. 2022, 70, 2038–2040. [Google Scholar] [CrossRef]
- Ahn, S.E.; Kim, S.W.; Oh, J.; Huh, K. Ultra-wide-field green (532 nm) and red (633 nm) reflectance imaging of the “sunset glow” fundus in chronic Vogt-Koyanagi-Harada disease. Indian J. Ophthalmol. 2013, 61, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Koto, T.; Hirota, K.; Hirakata, A. Ultra-widefield fundus imaging in gas-filled eyes after vitrectomy. BMC Ophthalmol. 2017, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Wai, K.M.; Moon, J.; Dahrouj, M.; Miller, J. Application of a digital green filter for improved visualization of retinal breaks on ultra-widefield fundus imaging. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1885. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2773487 (accessed on 21 September 2025).
- Ciardella, A.; Brown, D. Fundus Fluorescein and Indocyanine Green Angiography: A Textbook and Atlas. New York, Slack Incorporated. In Wide Field Imaging; Agarwal, A., Ed.; SLACK incorporated: San Francisco, CA, USA, 2007; pp. 79–83. [Google Scholar]
- Choudhry, N.; Golding, J.; Manry, M.W.; Rao, R.C. Ultra-widefield steering-based spectral-domain optical coherence tomography imaging of the retinal periphery. Ophthalmology 2016, 123, 1368–1374. [Google Scholar] [CrossRef]
- Witmer, M.T.; Parlitsis, G.; Patel, S.; Kiss, S. Comparison of ultra-widefield fluorescein angiography with the Heidelberg Spectralis® noncontact ultra-widefield module versus the Optos® Optomap®. Clin. Ophthalmol. 2013, 7, 389–394. [Google Scholar] [CrossRef]
- Optos. The Benefits of Optomap. 2021. Available online: https://www.optos.com/products/the-benefits-of-optomap/ (accessed on 21 September 2025).
- Stanga, P.E.; Bravo, F.J.V.; Reinstein, U.I.; Stanga, S.F.E. New 200° single-capture color red-green-blue ultra-widefield retinal imaging technology: First clinical experience. Ophthalmic Surg. Lasers Imaging Retin. 2023, 54, 714–718. [Google Scholar] [CrossRef]
- Toslak, D.; Son, T.; Erol, M.K.; Kim, H.; Kim, T.H.; Chan, R.V.P.; Yao, X. Portable ultra-widefield fundus camera for multispectral imaging of the retina and choroid. Biomed. Opt. Express 2020, 11, 6281–6292. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Khan, M.I.A.; Hossain, M.T.; Biswas, A.; Nakai, T.; Rohdin, J. Which color channel is better for diagnosing retinal diseases automatically in color fundus photographs? Life 2022, 12, 973. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Shi, C.; Wu, B.; Pazo, E.E.; Shen, L. Vision degrading myodesopsia assessed with optos ultra-widefield scanning laser ophthalmoscope. BMC Ophthalmol. 2023, 23, 425. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Paul Chan, R.V.; Berrocal, A.; Binenbaum, G.; Blair, M.; Peter Campbell, J.; Capone, A., Jr.; et al. International classification of retinopathy of prematurity, third edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.J.; Chang, Y.S.; Park, W.S. Combined intravitreal bevacizumab injection and zone I sparing laser photocoagulation in patients with zone I retinopathy of prematurity. Retina 2014, 34, 77–82. [Google Scholar] [CrossRef]
- Hu, J.; Blair, M.P.; Shapiro, M.J.; Lichtenstein, S.J.; Galasso, J.M.; Kapur, R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch. Ophthalmol. 2012, 130, 1000–1006. [Google Scholar] [CrossRef]
- Robles-Holmes, H.; Coyner, A.S.; Campbell, J.P.; Nudleman, E. Imaging features associated with persistent avascular retina in retinopathy of prematurity. Ophthalmology 2024, 131, 122–124. [Google Scholar] [CrossRef]
- Hanif, A.M.; Gensure, R.H.; Scruggs, B.A.; Anderson, J.; Chiang, M.F.; Campbell, J.P. Prevalence of persistent avascular retina in untreated children with a history of retinopathy of prematurity screening. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2022, 26, 29–31. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Chen, F.; Chen, W.; Jiang, N.; Xiang, D. Study protocol for prognosis and treatment strategy of peripheral persistent avascular retina after intravitreal anti-VEGF therapy in retinopathy of prematurity. Trials 2020, 21, 493. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, P.J.; Russell, M.; Ma, P.E.; Isbister, C.M.; Maberley, D.A. Sensitivity and specificity of the optos optomap for detecting peripheral retinal lesions. Retina 2007, 27, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Nagel, I.D.; Heinke, A.; Agnihotri, A.P.; Yassin, S.; Cheng, L.; Camp, A.S.; Scott, N.L.; Kalaw, F.G.P.; Borooah, S.; Bartsch, D.G.; et al. Comparison of a Novel Ultra-Widefield Three-Color Scanning Laser Ophthalmoscope to Other Retinal Imaging Modalities in Chorioretinal Lesion Imaging. Transl. Vis. Sci. Technol. 2025, 14, 11. [Google Scholar] [CrossRef]
- Karlin, D.B.; Curtin, B.J. Peripheral chorioretinal lesions and axial length of the myopic eye. Am. J. Ophthalmol. 1976, 81, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.; Ly, A.; Katalinic, P.; Coroneo, M.T.; Chang, A.; Kalloniatis, M.; Madigan, M.C.; Nivison-Smith, L. Visualisation of peripheral retinal degenerations and anomalies with ocular imaging. Semin. Ophthalmol. 2022, 37, 554–582. [Google Scholar] [CrossRef]
- Orlin, A.; Fatoo, A.; Ehrlich, J.; D’Amico, D.J.; Chan, R.P.; Kiss, S. Ultra-widefield fluorescein angiography of white without pressure. Clin. Ophthalmol. 2013, 7, 959–964. [Google Scholar] [CrossRef]
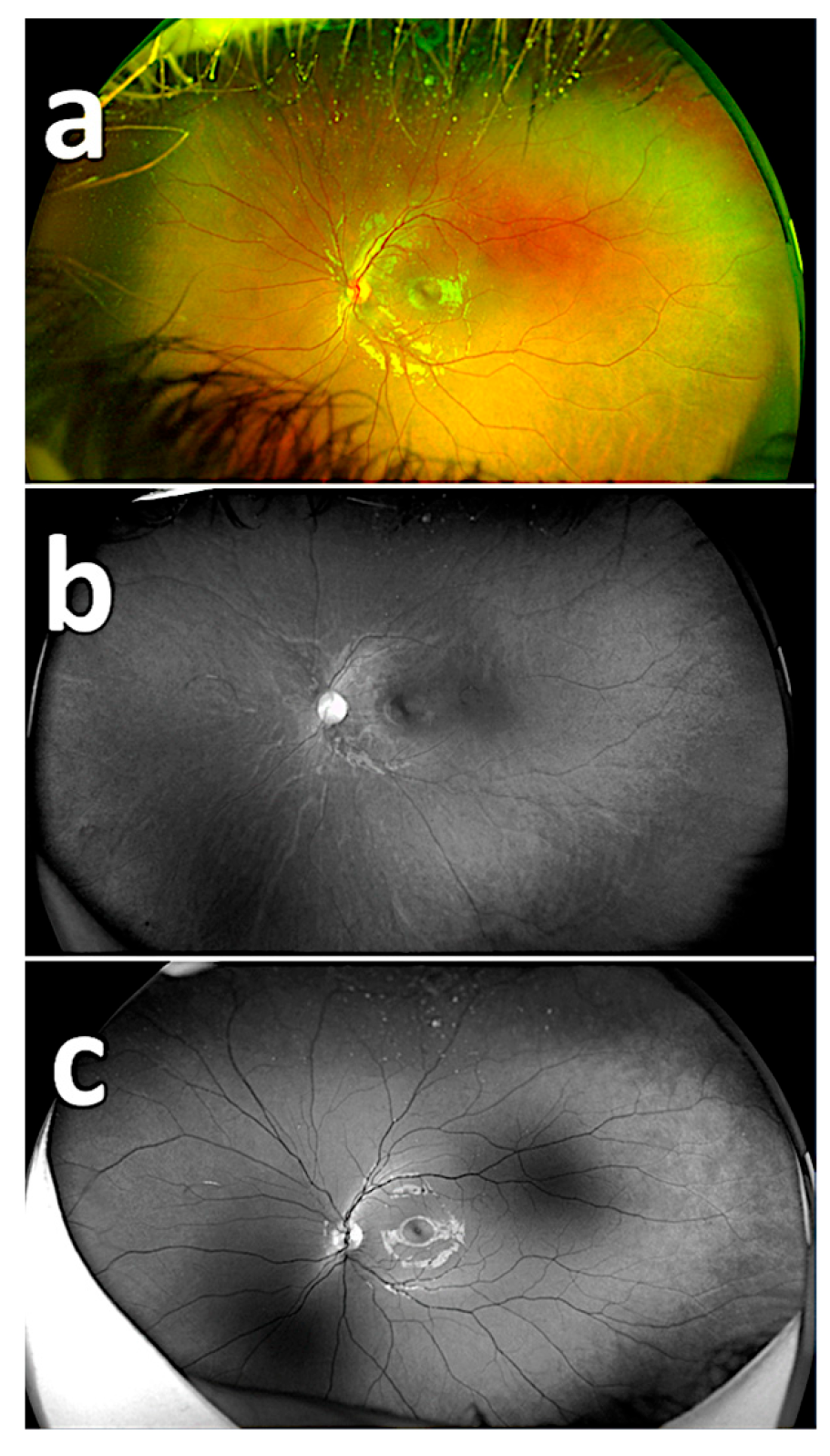
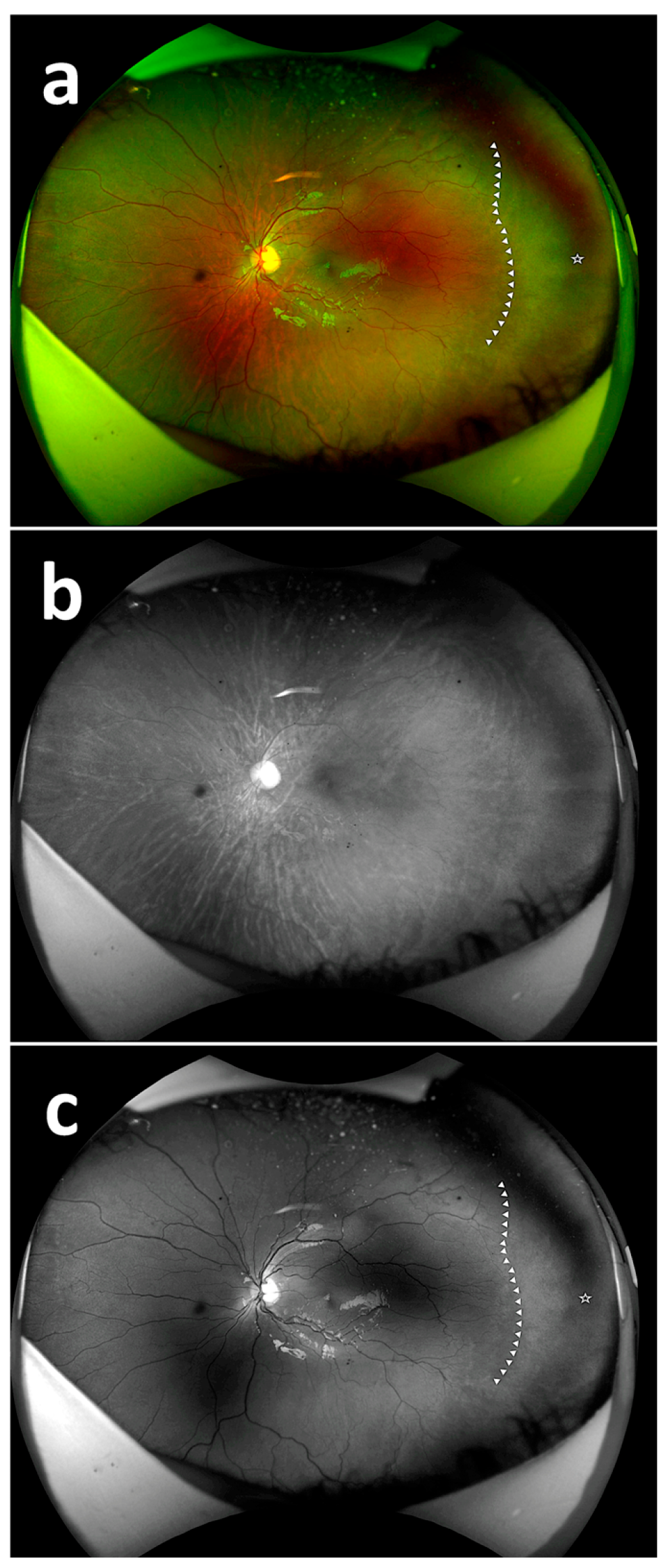

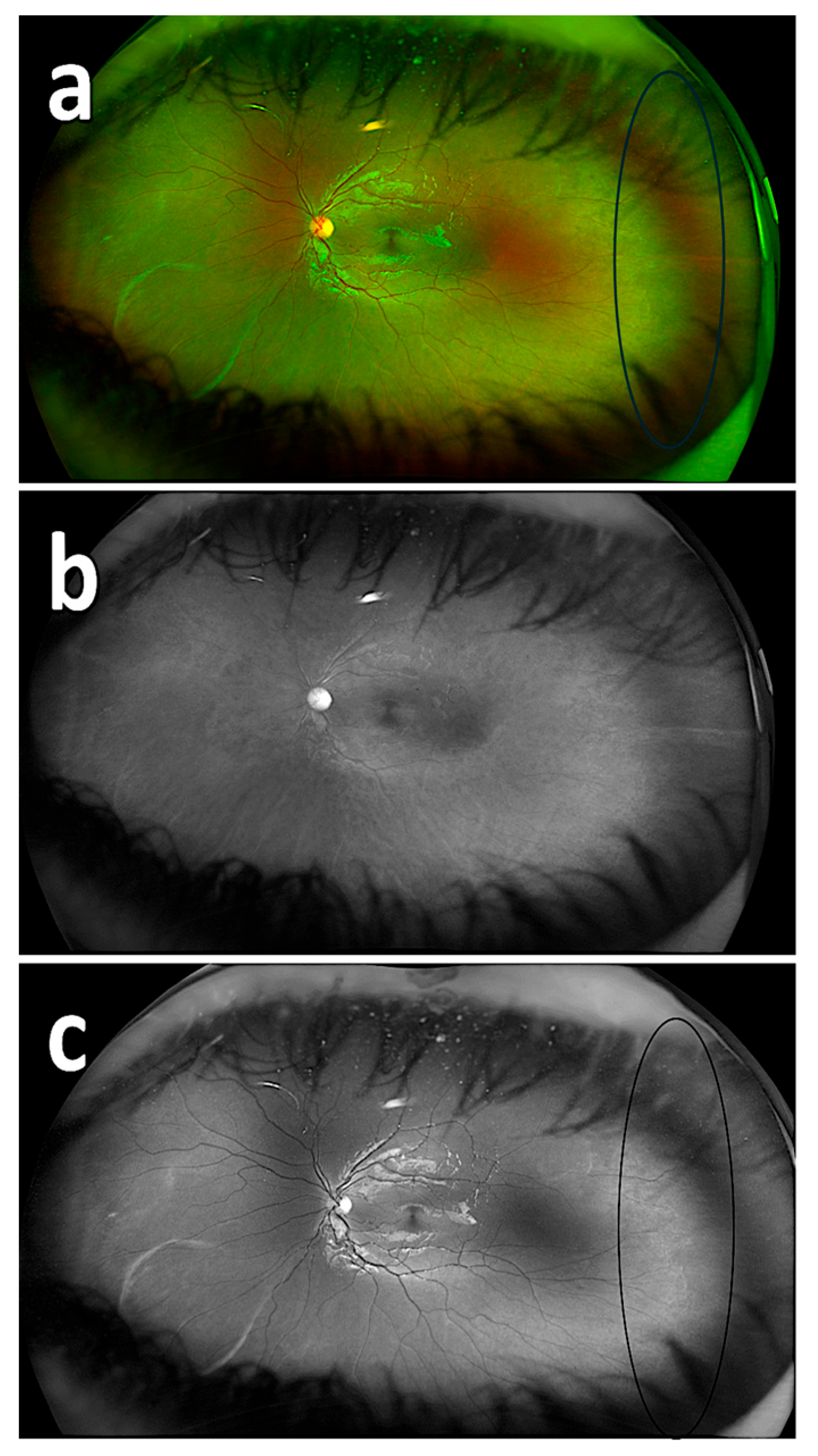
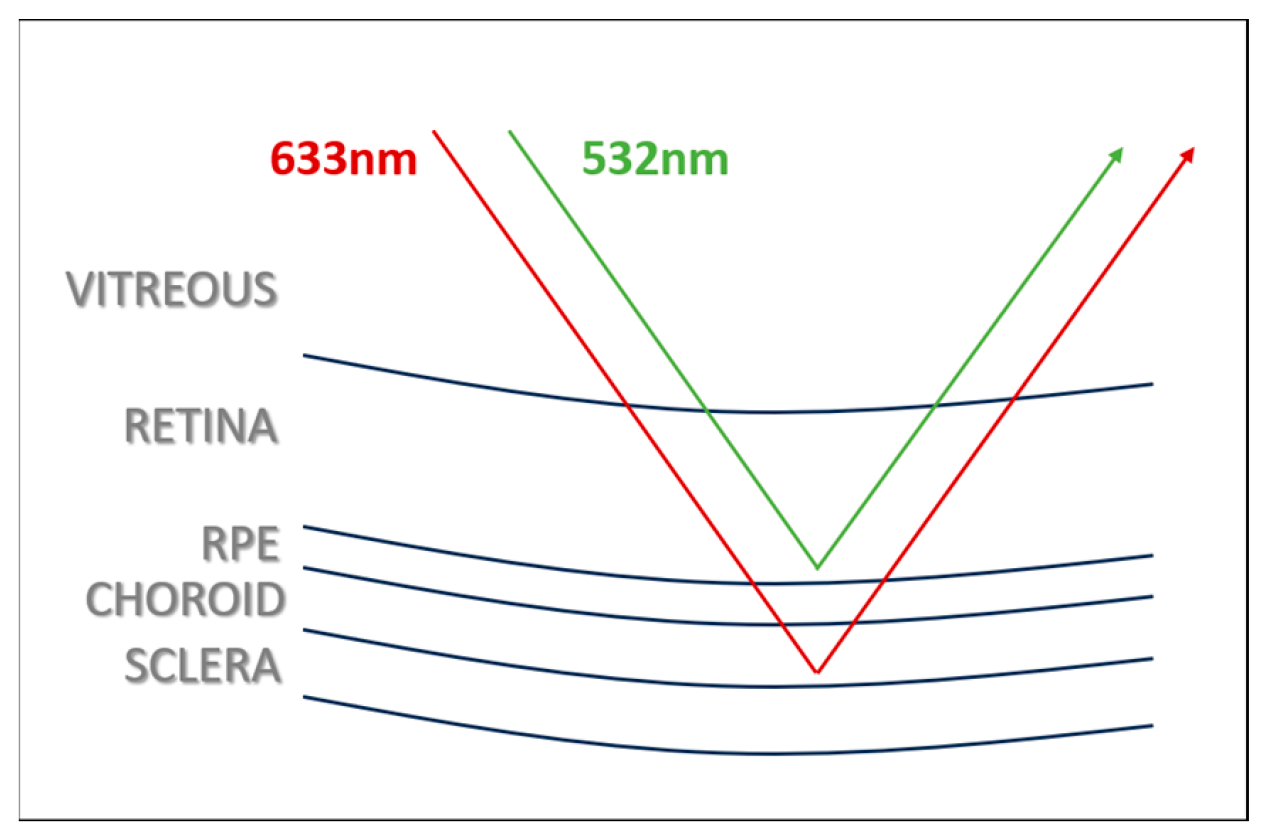
| No. of Respondents | Composite Imaging (20) | Red Reflectance Imaging (20) | Green Reflectance Imaging (20) |
|---|---|---|---|
| 1. | 15 | 3 | 18 |
| 2. | 16 | 2 | 19 |
| 3. | 12 | 1 | 17 |
| 4. | 13 | 4 | 20 |
| 5. | 13 | 3 | 18 |
| 6. | 11 | 2 | 17 |
| 7. | 14 | 1 | 19 |
| 8. | 10 | 4 | 20 |
| 9. | 12 | 3 | 19 |
| 10. | 11 | 2 | 20 |
| Color Imaging | n | Mean ± SD (Min–Max) | Median (Q1–Q3) | p Value |
|---|---|---|---|---|
| Composite | 10 | 0.63 ± 0.09 (0.5–0.8) A | 0.63 (0.55–0.7) | |
| Red | 10 | 0.12 ± 0.05 (0.05–0.2) B | 0.13 (0.1–0.15) | |
| Green | 10 | 0.94 ± 0.06 (0.85–1) C | 0.95 (0.9–1) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cengiz Ünal, A.; Akıdan, M.; Erol, M.K. Visualization of the Persistent Avascular Retina with Ultra-Widefield Green Reflectance Imaging. Diagnostics 2025, 15, 2873. https://doi.org/10.3390/diagnostics15222873
Cengiz Ünal A, Akıdan M, Erol MK. Visualization of the Persistent Avascular Retina with Ultra-Widefield Green Reflectance Imaging. Diagnostics. 2025; 15(22):2873. https://doi.org/10.3390/diagnostics15222873
Chicago/Turabian StyleCengiz Ünal, Ayşe, Melih Akıdan, and Muhammet Kazım Erol. 2025. "Visualization of the Persistent Avascular Retina with Ultra-Widefield Green Reflectance Imaging" Diagnostics 15, no. 22: 2873. https://doi.org/10.3390/diagnostics15222873
APA StyleCengiz Ünal, A., Akıdan, M., & Erol, M. K. (2025). Visualization of the Persistent Avascular Retina with Ultra-Widefield Green Reflectance Imaging. Diagnostics, 15(22), 2873. https://doi.org/10.3390/diagnostics15222873





