Evaluating Immune-Inflammatory Indices for Risk Stratification in Cardiovascular Disease: An Umbrella Review of Systematic Reviews and Meta-Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Umbrella Review Methods
2.2. Search Strategy
2.3. Selection Criteria
2.4. Data Extraction
2.5. Quality Assessment of Methods and Evidence
2.6. Grading the Evidence
2.7. Statistical Analysis
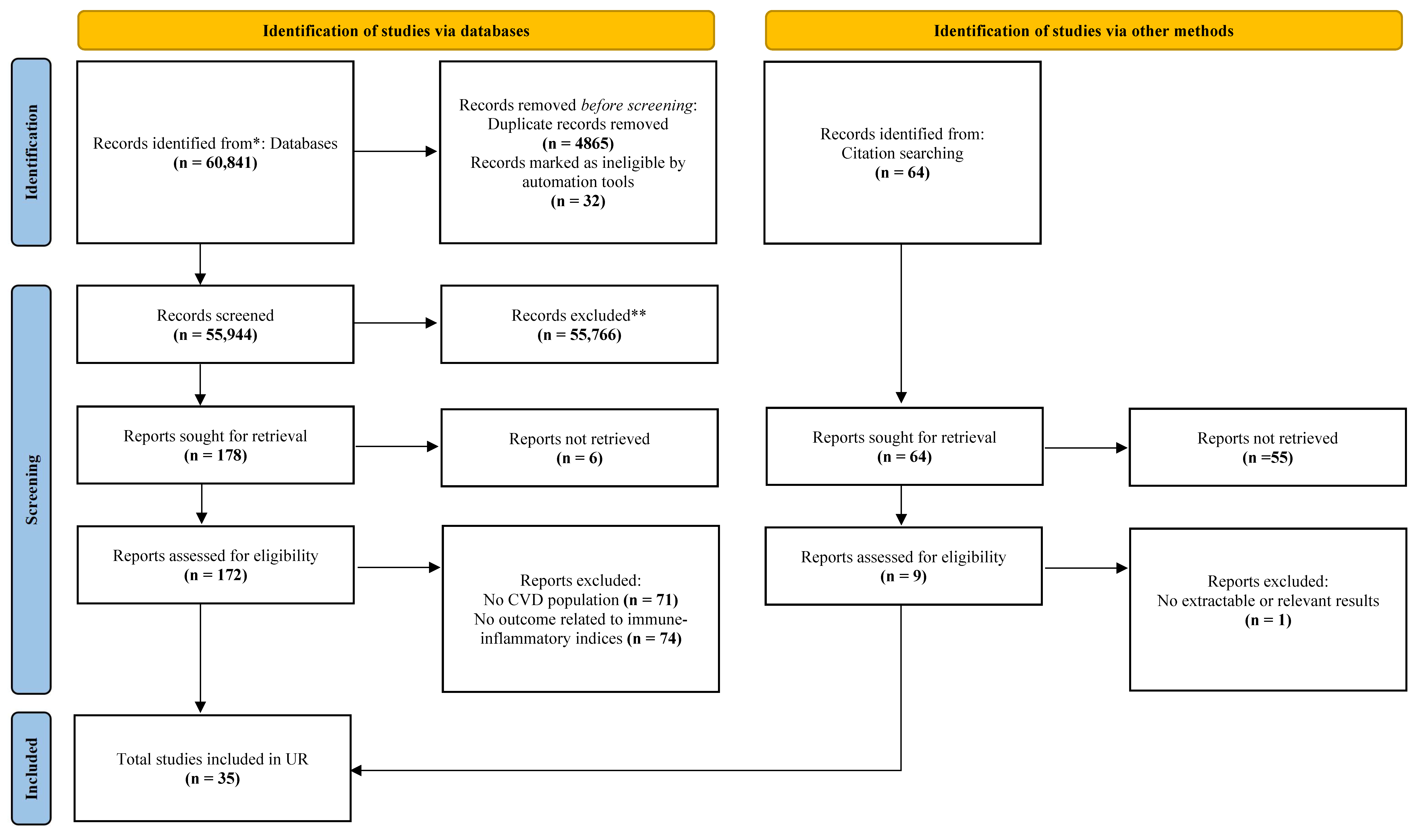
3. Results
3.1. Study Characteristics
3.2. Quality of Included Studies
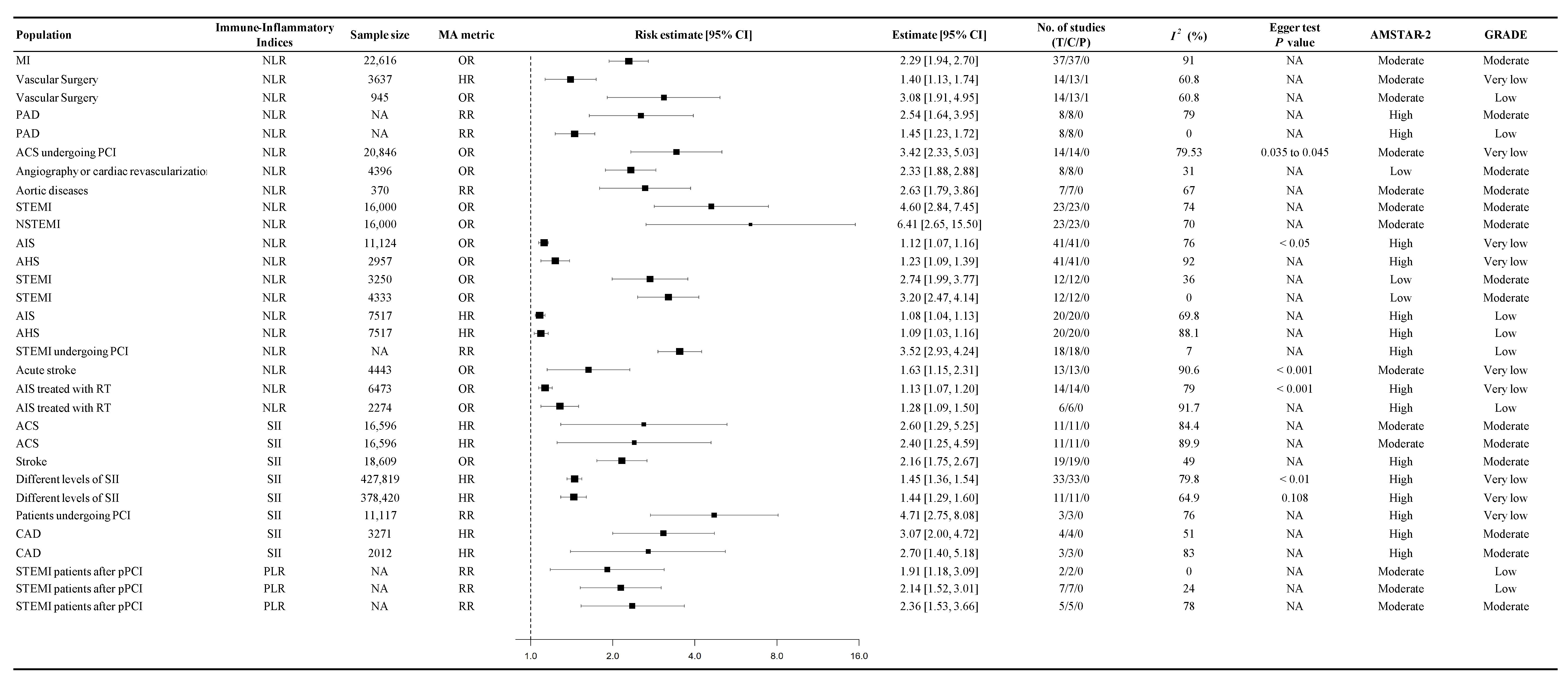
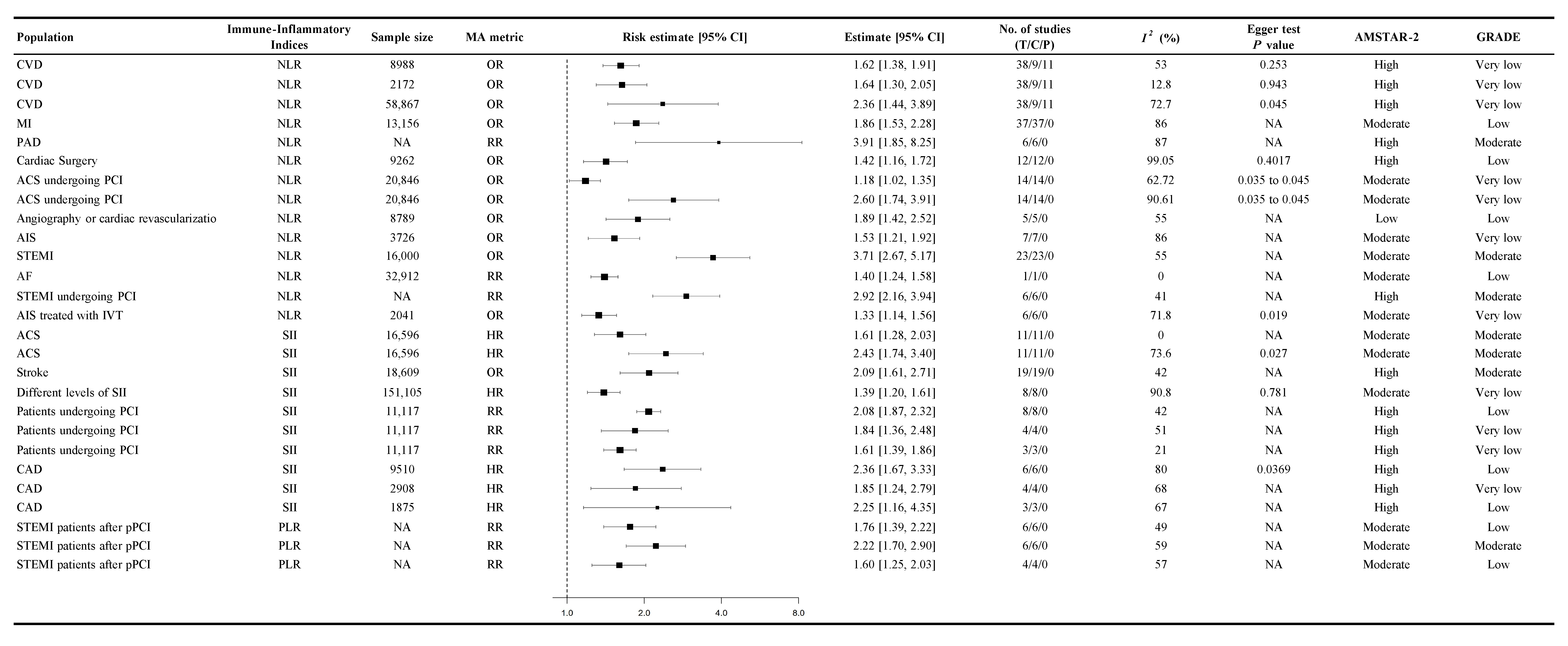
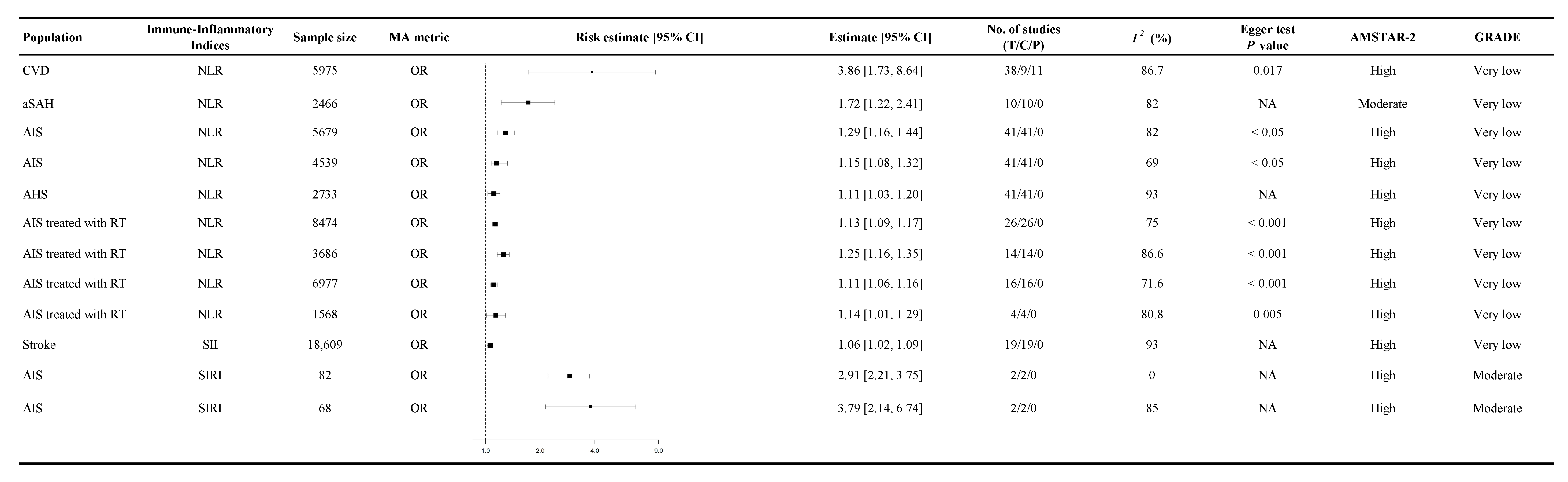
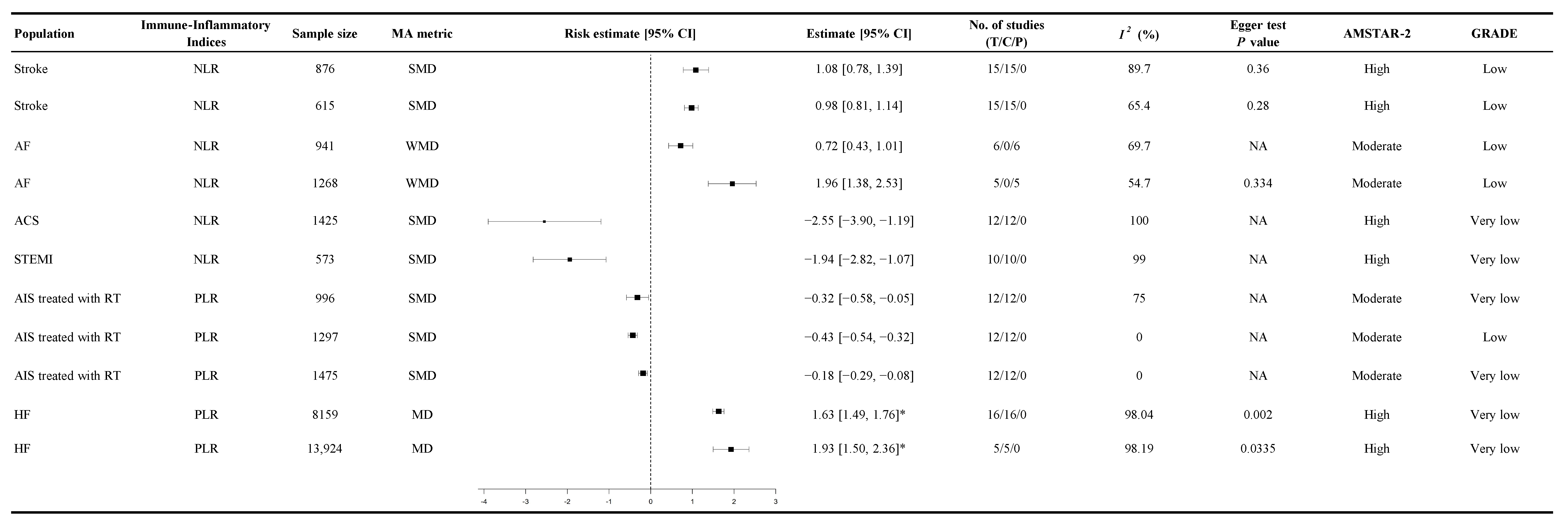
3.3. Association Between Immune-Inflammatory Indices and CVD Mortality
3.4. Association Between Immune-Inflammatory Indices and the Probability of Cardiovascular Events
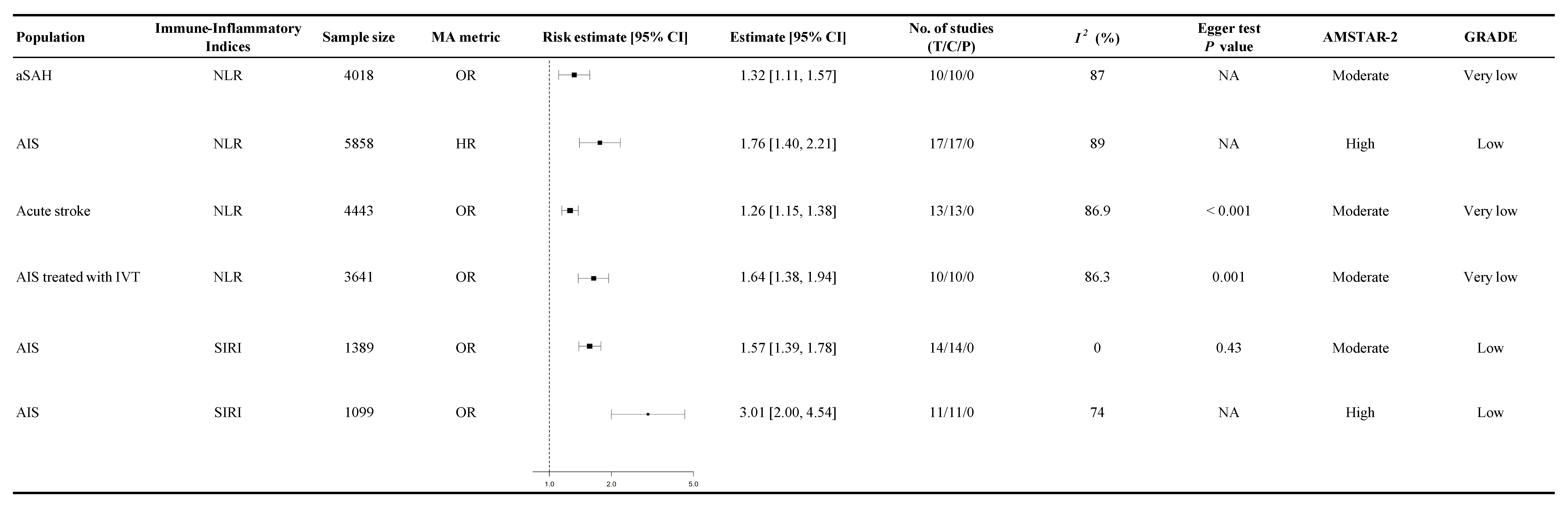
3.5. Association Between Immune-Inflammatory Indices and Functional Outcomes
3.6. Association Between Immune-Inflammatory Indices and Other Cardiovascular-Related Conditions
3.7. Inflammatory Burden
3.8. Heterogeneity
3.9. Assessment of Risk of Bias
4. Discussion
4.1. Neutrophil-to-Lymphocyte Ratio (NLR)
4.2. Systemic Immune-Inflammation Index (SII)
4.3. Platelet-to-Lymphocyte Ratio (PLR)
4.4. Systemic Inflammatory Response Index (SIRI)
4.5. Strengths
4.6. Limitations
4.7. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| ACM | all-cause mortality |
| AF | atrial fibrillation |
| AIS | acute ischemic stroke |
| AHS | acute hemorrhagic stroke |
| AMSTAR-2 | A Measurement Tool to Assess Systematic Reviews 2 |
| aSAH | aneurysmal subarachnoid hemorrhage |
| CAD | coronary artery disease |
| CDSS | clinical decision support system |
| CeVD | cerebrovascular disease |
| CRP | C-reactive protein |
| CI | confidence interval |
| CMPs | cardiomyopathies |
| CVD | cardiovascular disease |
| DALYs | disability-adjusted life years |
| DCI | delayed cerebral ischemia |
| EHR | electronic health record |
| END | early neurological deterioration |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| HF | heart failure |
| HR | hazard ratio |
| I2 | inconsistency index (statistical measure of heterogeneity) |
| IVT | intravenous thrombolysis |
| MACE | major adverse cardiovascular events |
| MD | mean difference |
| MI | myocardial infarction |
| MeSH | Medical Subject Headings |
| NA | not available or not applicable |
| NCD | non-communicable disease |
| NLR | neutrophil-to-lymphocyte ratio |
| NSTEMI | non-ST-elevation myocardial infarction |
| OR | odds ratio |
| PAD | peripheral artery disease |
| PCI | percutaneous coronary intervention |
| pPCI | primary percutaneous coronary intervention |
| PLR | platelet-to-lymphocyte ratio |
| RR | risk ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| PI[E]COS | Population, Intervention/Exposure, Comparison, Outcome, Study design |
| RT | reperfusion therapy |
| SAP | stroke-associated pneumonia |
| SII | systemic immune-inflammation index |
| SIRI | systemic inflammation response index |
| SIGN | Scottish Intercollegiate Guidelines Network |
| SMD | standardized mean difference |
| STEMI | ST-elevation myocardial infarction |
| SRMA | systematic review and meta-analysis |
| UR | umbrella review |
| vs. | versus |
| WHO | World Health Organization |
| WMD | weighted mean difference |
References
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222, Erratum in: Lancet 2020, 396, 1562. https://doi.org/10.1016/S0140-6736(20)32226-1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017, 6, 2048004016687211. [Google Scholar] [CrossRef]
- Rehman, S.; Rehman, E.; Ikram, M.; Jianglin, Z. Cardiovascular disease (CVD): Assessment, prediction and policy implications. BMC Public. Health 2021, 21, 1299. [Google Scholar] [CrossRef]
- Biswas, T.; Townsend, N.; Gupta, R.D.; Ghosh, A.; Rawal, L.B.; Mørkrid, K.; Mamun, A. Clustering of metabolic and behavioural risk factors for cardiovascular diseases among the adult population in South and Southeast Asia: Findings from WHO STEPS data. Lancet Reg. Health-S. Asia 2023, 12, 100164. [Google Scholar] [CrossRef] [PubMed]
- Maimaris, W.; Paty, J.; Perel, P.; Legido-Quigley, H.; Balabanova, D.; Nieuwlaat, R.; McKee, M. The influence of health systems on hypertension awareness, treatment, and control: A systematic literature review. PLoS Med. 2013, 10, e1001490. [Google Scholar] [CrossRef]
- Nedkoff, L.; Briffa, T.; Murray, K.; Gaw, J.; Yates, A.; Sanfilippo, F.M.; Nicholls, S.J. Risk of early recurrence and mortality in high-risk myocardial infarction patients: A population-based linked data study. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 17, 200185. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids 2015, 2015, 971453. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Wu, Y.; Shi, Y.; Xu, G.; Wang, J.; Xiang, M.; Weng, S.; Jiang, J.; Ma, J. Predictive value of the relative lymphocyte count in coronary heart disease. Heart Vessel. 2010, 25, 469–473. [Google Scholar] [CrossRef]
- Ly, H.Q.; Kirtane, A.J.; Murphy, S.A.; Buros, J.; Cannon, C.P.; Braunwald, E.; Gibson, C.M.; Group, T.S. Association of Platelet Counts on Presentation and Clinical Outcomes in ST-Elevation Myocardial Infarction (from the TIMI Trials). Am. J. Cardiol. 2006, 98, 1–5. [Google Scholar] [CrossRef]
- Doring, Y.; Drechsler, M.; Soehnlein, O.; Weber, C. Neutrophils in atherosclerosis: From mice to man. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 288–295. [Google Scholar] [CrossRef]
- Ionita, M.G.; van den Borne, P.; Catanzariti, L.M.; Moll, F.L.; de Vries, J.P.; Pasterkamp, G.; Vink, A.; de Kleijn, D.P. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, J.; Koper, O.M.; Siedlecka-Czykier, E.; Matowicka-Karna, J.; Bychowski, J.; Kemona, H. The utility of inflammation and platelet biomarkers in patients with acute coronary syndromes. Saudi J. Biol. Sci. 2018, 25, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Perros, A.J.; Esguerra-Lallen, A.; Rooks, K.; Chong, F.; Engkilde-Pedersen, S.; Faddy, H.M.; Hewlett, E.; Naidoo, R.; Tung, J.P.; Fraser, J.F.; et al. Coronary artery bypass grafting is associated with immunoparalysis of monocytes and dendritic cells. J. Cell Mol. Med. 2020, 24, 4791–4803. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhao, Q.; Liu, Z.S.; Zhang, C.Y.; Yang, J.; Meng, K. Relationship between monocyte/lymphocyte ratio and non-culprit plaque vulnerability in patients with acute coronary syndrome: An optical coherence tomography study. Medicine 2020, 99, e21562. [Google Scholar] [CrossRef] [PubMed]
- Selek, U.; Pehlivan, B.; Sezen, D.; Besen, A.A.; Mertsoylu, H.; Ozdemir, Y.; Kucuk, A.; Topkan, E.; Bolukbasi, Y. Systemic Inflammation Response Index Predicts Survival Outcomes in Glioblastoma Multiforme Patients Treated with Standard Stupp Protocol. J. Immunol. Res. 2020, 2020, 8628540. [Google Scholar] [CrossRef]
- Firment, J.; Hulin, I. Zahorec index or Neutrophil-to-lymphocyte ratio, valid biomarker of inflammation and immune response to infection, cancer and surgery. Bratisl. Lek. Listy 2024, 125, 75–83. [Google Scholar] [CrossRef]
- Gu, P.; Xu, P.; Chen, Y.; Li, J.; Sun, H.; Xu, H.; Lu, Q. The predictive value of pan-immune inflammatory index for early recurrence of atrial fibrillation after cryoablation. BMC Cardiovasc. Disord. 2024, 24, 669. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Q.; Wang, R.; Ji, H.; Chen, Y.; Quan, X.; Zhang, C. Systemic Immune-Inflammatory Index Predicts Clinical Outcomes for Elderly Patients with Acute Myocardial Infarction Receiving Percutaneous Coronary Intervention. Med. Sci. Monit. 2019, 25, 9690–9701. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhang, N.; Zhu, H. The relationship between the level of NMLR on admission and the prognosis of patients after cardiopulmonary resuscitation: A retrospective observational study. Eur. J. Med. Res. 2023, 28, 424. [Google Scholar] [CrossRef]
- Ninla-Aesong, P.; Kietdumrongwong, P.; Neupane, S.P.; Puangsri, P.; Jongkrijak, H.; Chotipong, P.; Kaewpijit, P. Relative value of novel systemic immune-inflammatory indices and classical hematological parameters in predicting depression, suicide attempts and treatment response. Sci. Rep. 2024, 14, 19018. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Wang, H.; Yuan, Y.; Chen, D.; Sun, Y.; Xu, Z. Systemic immune-inflammation index predicted short-term outcomes in ATAD patients undergoing surgery. J. Card. Surg. 2022, 37, 969–975. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Vakhshoori, M.; Bondariyan, N.; Sabouhi, S.; Kiani, K.; Alaei Faradonbeh, N.; Emami, S.A.; Shakarami, M.; Khanizadeh, F.; Sanaei, S.; Motamedi, N.; et al. The impact of platelet-to-lymphocyte ratio on clinical outcomes in heart failure: A systematic review and meta-analysis. Ther. Adv. Cardiovasc. Dis. 2024, 18, 17539447241227287. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Zhang, C.; Wang, J.; Liao, B.; Feng, J. The Systemic Immune Inflammation Index as a Novel Predictive Biomarker for Contrast-Induced Acute Kidney Injury Risk Following Percutaneous Coronary Intervention: A Meta-Analysis of Cohort Studies. Curr. Vasc. Pharmacol. 2024, 23, 136–145. [Google Scholar] [CrossRef]
- Shahsanaei, F.; Abbaszadeh, S.; Behrooj, S.; Rahimi Petrudi, N.; Ramezani, B. The value of neutrophil-to-lymphocyte ratio in predicting severity of coronary involvement and long-term outcome of percutaneous coronary intervention in patients with acute coronary syndrome: A systematic review and meta-analysis. Egypt. Heart J. 2024, 76, 39. [Google Scholar] [CrossRef]
- Huang, Y.W.; Zhang, Y.; Feng, C.; An, Y.H.; Li, Z.P.; Yin, X.S. Systemic inflammation response index as a clinical outcome evaluating tool and prognostic indicator for hospitalized stroke patients: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 474. [Google Scholar] [CrossRef]
- Amezcua-Castillo, E.; González-Pacheco, H.; Sáenz-San Martín, A.; Méndez-Ocampo, P.; Gutierrez-Moctezuma, I.; Massó, F.; Sierra-Lara, D.; Springall, R.; Rodríguez, E.; Arias-Mendoza, A.; et al. C-Reactive Protein: The Quintessential Marker of Systemic Inflammation in Coronary Artery Disease-Advancing toward Precision Medicine. Biomedicines 2023, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Alexios, S.A.; Angelopoulos, A.; Papanikolaou, P.; Simantiris, S.; Oikonomou Evangelos, K.; Vamvakaris, K.; Koumpoura, A.; Farmaki, M.; Trivella, M.; Vlachopoulos, C.; et al. Biomarkers of Vascular Inflammation for Cardiovascular Risk Prognostication. JACC Cardiovasc. Imaging 2022, 15, 460–471. [Google Scholar] [CrossRef]
- García-Escobar, A.; Vera-Vera, S.; Tébar-Márquez, D.; Rivero-Santana, B.; Jurado-Román, A.; Jiménez-Valero, S.; Galeote, G.; Cabrera, J.-Á.; Moreno, R. Neutrophil-to-lymphocyte ratio an inflammatory biomarker, and prognostic marker in heart failure, cardiovascular disease and chronic inflammatory diseases: New insights for a potential predictor of anti-cytokine therapy responsiveness. Microvasc. Res. 2023, 150, 104598. [Google Scholar] [CrossRef]
- Ye, Z.; Hu, T.; Wang, J.; Xiao, R.; Liao, X.; Liu, M.; Sun, Z. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 933913. [Google Scholar] [CrossRef]
- Kurtul, A.; Ornek, E. Platelet to Lymphocyte Ratio in Cardiovascular Diseases: A Systematic Review. Angiology 2019, 70, 802–818. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, B.; Peng, C.; Liu, Y.; Lai, W. Prognostic implications of system inflammation response index in atrial fibrillation patients with type 2 diabetes mellitus. Sci. Rep. 2025, 15, 1025. [Google Scholar] [CrossRef]
- Agyeman, K.B.; Shafi, N.; Contreras, R.; Parackal, V.; Shah, D.N.; Gurram, A.; Keetha, N.R.; Ameen, D. The association between systemic immune-inflammation index and cardiovascular diseases: An in-depth umbrella review of meta-analyses with GRADE assessment. Heliyon 2025, 11, e42736. [Google Scholar] [CrossRef]
- Choi, G.J.; Kang, H. Introduction to Umbrella Reviews as a Useful Evidence-Based Practice. J. Lipid Atheroscler. 2023, 12, 3–11. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. Evid. Based Ment. Health 2018, 21, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Yuan, X.; Li, J.; Wang, W.; Dai, T.; Chen, H.; Wang, Y.; et al. Dietary sugar consumption and health: Umbrella review. BMJ 2023, 381, e071609. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Ioannidis, J.P.A.; Tzoulaki, I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015, 14, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, X.; Zheng, Y.; Li, J.; Wang, Y.; Lin, Y.; He, Z.; Zhao, W.; Chen, C.; Qiu, K.; et al. Association of glucose-lowering medications with cardiovascular outcomes: An umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020, 8, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Angkananard, T.; Anothaisintawee, T.; McEvoy, M.; Attia, J.; Thakkinstian, A. Neutrophil Lymphocyte Ratio and Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2018, 2018, 2703518. [Google Scholar] [CrossRef] [PubMed]
- Banahene, N.O.; Sinha, T.; Shaikh, S.; Zin, A.K.; Khreis, K.; Chaudhari, S.S.; Wei, C.R.; Palleti, S.K. Effect of Elevated Neutrophil-to-Lymphocyte Ratio on Adverse Outcomes in Patients With Myocardial Infarction: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e61647. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.M.; Perry, L.A.; Borg, C.; Ramson, D.M.; Campbell, R.; Liu, Z.; Nguyen, J.; Douglas, N.; Kok, J.; Penny-Dimri, J. Prognostic Significance of Preoperative Neutrophil-Lymphocyte Ratio in Vascular Surgery: Systematic Review and Meta-Analysis. Vasc. Endovasc. Surg. 2020, 54, 697–706. [Google Scholar] [CrossRef]
- Khanzadeh, S.; Lucke-Wold, B.; Eshghyar, F.; Rezaei, K.; Clark, A. The Neutrophil to Lymphocyte Ratio in Poststroke Infection: A Systematic Review and Meta-Analysis. Dis. Markers 2022, 2022, 1983455. [Google Scholar] [CrossRef]
- Kurniawan, R.B.; Siahaan, P.P.; Saputra, P.B.; Arnindita, J.N.; Savitri, C.G.; Faizah, N.N.; Andira, L.H.; D’Oria, M.; Eko Putranto, J.N.; Alkaff, F.F. Neutrophil-to-lymphocyte ratio as a prognostic biomarker in patients with peripheral artery disease: A systematic review and meta-analysis. Vasc. Med. 2024, 29, 687–699. [Google Scholar] [CrossRef]
- Li, Z.; Lian, D.; Lin, R.; Li, J.; Wang, W. Meta-analysis of the relationship between neutrophil to lymphocyte ratio and short-term prognosis of patients with acute aortic dissection. Arch. Clin. Psychiatry 2023, 50, 143. [Google Scholar]
- Liu, Z.; Nguyen Khuong, J.; Borg Caruana, C.; Jackson, S.M.; Campbell, R.; Ramson, D.M.; Penny-Dimri, J.C.; Kluger, M.; Segal, R.; Perry, L.A. The Prognostic Value of Elevated Perioperative Neutrophil-Lymphocyte Ratio in Predicting Postoperative Atrial Fibrillation After Cardiac Surgery: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2020, 29, 1015–1024. [Google Scholar] [CrossRef]
- Shi, M.; Yang, C.; Tang, Q.W.; Xiao, L.F.; Chen, Z.H.; Zhao, W.Y. The Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Patients With Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis of Observational Studies. Front. Neurol. 2021, 12, 745560. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Jiang, X.; Zhu, H.; Lu, Z.; Xu, L. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: A meta-analysis of observational studies. Atherosclerosis 2014, 234, 206–213. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, H.; Qiu, Z.; Cheng, X. Prognostic role of neutrophil-to-lymphocyte ratio in aortic disease: A meta-analysis of observational studies. J. Cardiothorac. Surg. 2020, 15, 215. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, X.; Hu, W.; Zhao, L.; Zhao, S.; Zhang, J.; Chu, Z.; Xu, Y. Neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation in ischemic stroke: A meta-analysis. Brain Behav. 2019, 9, e01382. [Google Scholar] [CrossRef]
- Dentali, F.; Nigro, O.; Squizzato, A.; Gianni, M.; Zuretti, F.; Grandi, A.M.; Guasti, L. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: A systematic review and meta-analysis of the literature. Int. J. Cardiol. 2018, 266, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lekkala, S.P.; Mellacheruvu, S.P.; Gill, K.S.; Khela, P.S.; Singh, G.; Jitta, S.R.; Patel, M.; Hingora, M.J.; Desai, R. Association between preablation and postablation neutrophil-lymphocyte ratio and atrial fibrillation recurrence: A meta-analysis. J. Arrhythm. 2024, 40, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hou, M.; Ding, Z.; Liu, X.; Shao, Y.; Li, X. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 686983. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Liu, R.; He, X.; Hou, B. Predictive value of neutrophil to lymphocyte ratio for ischemic stroke in patients with atrial fibrillation: A meta-analysis. Front. Neurol. 2022, 13, 1029010. [Google Scholar] [CrossRef]
- Mikhael, R.; Hindoro, E.; Taner, S.; Lukito, A.A. Neutrophil-to-lymphocyte ratio for predictor of in-hospital mortality in ST-segment elevation myocardial infarction: A meta-analysis. Med. J. Indones. 2020, 29, 172–182. [Google Scholar] [CrossRef]
- Pruc, M.; Kubica, J.; Banach, M.; Swieczkowski, D.; Rafique, Z.; Peacock, W.F.; Siudak, Z.; Kurek, K.; Nanayakkara, P.; Szarpak, L. Diagnostic and prognostic performance of the neutrophil-to-lymphocyte ratio in acute coronary syndromes: A meta-analysis of 90 studies including 45 990 patients. Kardiol. Pol. 2024, 82, 276–284. [Google Scholar] [CrossRef]
- Song, S.Y.; Zhao, X.X.; Rajah, G.; Hua, C.; Kang, R.J.; Han, Y.P.; Ding, Y.C.; Meng, R. Clinical Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Patients With Ischemic Stroke or Hemorrhagic Stroke: An Updated Meta-Analysis. Front. Neurol. 2019, 10, 1032. [Google Scholar] [CrossRef]
- Ul Hussain, H.; Kumar, K.A.; Zahid, M.; Husban Burney, M.; Khan, Z.; Asif, M.; Rehan, S.T.; Ahmad Cheema, H.; Swed, S.; Yasmin, F.; et al. Neutrophil to lymphocyte ratio as a prognostic marker for cardiovascular outcomes in patients with ST-segment elevation myocardial infarction after percutaneous coronary intervention: A systematic review and meta-analysis. Medicine 2024, 103, e38692. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, X.; Zhen, Y.; Chen, X.; Yao, P.; Liu, W.; Lu, E.; Du, Y.; Liu, H.; Zhao, S. The predictive role of the neutrophil-lymphocyte ratio in the prognosis of adult patients with stroke. Chin. Neurosurg. J. 2020, 6, 22. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Ji, M.; Mang, J.; Xu, Z. Prognostic value of the neutrophil-to-lymphocyte ratio in acute ischemic stroke patients treated with intravenous thrombolysis: A systematic review and meta-analysis. BMC Neurol. 2021, 21, 191. [Google Scholar] [CrossRef]
- Wu, B.; Liu, F.; Sun, G.; Wang, S. Prognostic role of dynamic neutrophil-to-lymphocyte ratio in acute ischemic stroke after reperfusion therapy: A meta-analysis. Front. Neurol. 2023, 14, 1118563. [Google Scholar] [CrossRef]
- Huang, Y.W.; Yin, X.S.; Li, Z.P. Association of the systemic immune-inflammation index (SII) and clinical outcomes in patients with stroke: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 1090305. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, X.; Diao, H.; Yang, Y.; Ding, L.; Huan, W.; Chen, Y.; Cui, W. Systemic immune inflammation index with all-cause and cause-specific mortality: A meta-analysis. Inflamm. Res. 2024, 73, 2199–2216. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, G. Association Between Systemic Immune-Inflammation Index and Adverse Outcomes in Patients With Acute Coronary Syndrome: A Meta-Analysis. Angiology 2024, 76, 33197241263399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, M.; Liu, L.; Deng, L.; Yulei, X.; Zhong, Y.; Liao, B.; Yu, L.; Feng, J. Systemic immune-inflammation index as a novel predictor of major adverse cardiovascular events in patients undergoing percutaneous coronary intervention: A meta-analysis of cohort studies. BMC Cardiovasc. Disord. 2024, 24, 189. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Sun, T.; Huang, X.; Ma, M.; Yang, S.; Zhou, Y. Prognostic value of systemic immune-inflammation index in CAD patients: Systematic review and meta-analyses. Eur. J. Clin. Investig. 2024, 54, e14100. [Google Scholar] [CrossRef]
- Dong, G.; Huang, A.; Liu, L. Platelet-to-lymphocyte ratio and prognosis in STEMI: A meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13386. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lin, N. Systemic Inflammatory Response Index and the Short-Term Functional Outcome of Patients with Acute Ischemic Stroke: A Meta-analysis. Neurol. Ther. 2024, 13, 1431–1451. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Bhaskar, S.M.M. Prognostic Role of the Platelet-Lymphocyte Ratio in Acute Ischemic Stroke Patients Undergoing Reperfusion Therapy: A Meta-Analysis. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221110373. [Google Scholar] [CrossRef]
- Ye, B.; Gan, J.; Han, Y.; Yu, L.; Huang, Y.; Ye, B. Relationship between Prediction of Platelet-Lymphocyte Ratio and Atrial Fibrillation in Perioperative Patients: A Systematic Review and Meta-Analysis. Heart Surg. Forum 2024, 27, E180–E187. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Teperman, J.; Carruthers, D.; Guo, Y.; Barnett, M.P.; Harris, A.A.; Sedlis, S.P.; Pillinger, M.; Babaev, A.; Staniloae, C.; Attubato, M.; et al. Relationship between neutrophil-lymphocyte ratio and severity of lower extremity peripheral artery disease. Int. J. Cardiol. 2017, 228, 201–204. [Google Scholar] [CrossRef]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef]
- Liu, J.; Ao, W.; Zhou, J.; Luo, P.; Wang, Q.; Xiang, D. The correlation between PLR-NLR and prognosis in acute myocardial infarction. Am. J. Transl. Res. 2021, 13, 4892–4899. [Google Scholar]
- Verdoia, M.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G.; Novara Atherosclerosis Study, G. Neutrophil to Lymphocyte Ratio and the Extent of Coronary Artery Disease: Results From a Large Cohort Study. Angiology 2016, 67, 75–82. [Google Scholar] [CrossRef]
- Stef, A.; Bodolea, C.; Bocsan, I.C.; Cainap, S.S.; Achim, A.; Serban, A.; Solomonean, A.G.; Tintiuc, N.; Buzoianu, A.D. The Value of Biomarkers in Major Cardiovascular Surgery Necessitating Cardiopulmonary Bypass. Rev. Cardiovasc. Med. 2024, 25, 355. [Google Scholar] [CrossRef] [PubMed]
- Ivascu, R.; Torsin, L.I.; Hostiuc, L.; Nitipir, C.; Corneci, D.; Dutu, M. The Surgical Stress Response and Anesthesia: A Narrative Review. J. Clin. Med. 2024, 13, 3017. [Google Scholar] [CrossRef] [PubMed]
- Cil, C.; Biteker, F.S.; Celik, O.; Ozlek, B.; Ozlek, E.; Gokcek, A.; Dogan, V. Biomarkers in Cardiac Surgery. Blood Purif. 2019, 48, 191. [Google Scholar] [CrossRef]
- Ramkumar, N.; Jacobs, J.P.; Berman, R.B.; Parker, D.M.; MacKenzie, T.A.; Likosky, D.S.; DiScipio, A.; Malenka, D.J.; Brown, J.R. Cardiac Biomarkers Predict Long-term Survival After Cardiac Surgery. Ann. Thorac. Surg. 2019, 108, 1776–1782. [Google Scholar] [CrossRef]
- Xu, M.; Chen, R.; Liu, L.; Liu, X.; Hou, J.; Liao, J.; Zhang, P.; Huang, J.; Lu, L.; Chen, L.; et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: The Dongfeng-Tongji cohort study. Atherosclerosis 2021, 323, 20–29. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, C.; Wu, L.; Li, Z.; Li, H.; Zhang, J. Systemic Immune Inflammation Index (SII), System Inflammation Response Index (SIRI) and Risk of All-Cause Mortality and Cardiovascular Mortality: A 20-Year Follow-Up Cohort Study of 42,875 US Adults. J. Clin. Med. 2023, 12, 1128. [Google Scholar] [CrossRef]
- Bakogiannis, C.; Sachse, M.; Stamatelopoulos, K.; Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019, 122, 154157. [Google Scholar] [CrossRef]
- Pasalic, L.; Wang, S.S.; Chen, V.M. Platelets as biomarkers of coronary artery disease. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers: New York, NY, USA, 2016; pp. 223–233. [Google Scholar]
- Willim, H.A.; Harianto, J.C.; Cipta, H. Platelet-to-Lymphocyte Ratio at Admission as a Predictor of In-Hospital and Long-Term Outcomes in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Cardiol. Res. 2021, 12, 109–116. [Google Scholar] [CrossRef]
- Han, J.-B.; Shu, Q.-H.; Zhang, Y.-F.; Yi, Y.-X. Predictive Value of Inflammation Biomarkers in Patients with Portal Vein Thrombosis. J. Clin. Transl. Hepatol. 2021, 9, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Slichter, S.J.; Davis, K.; Enright, H.; Braine, H.; Gernsheimer, T.; Kao, K.J.; Kickler, T.; Lee, E.; McFarland, J.; McCullough, J.; et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood 2005, 105, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Arnoldussen, I.A.C.; Witkamp, R.F. Effects of Nutrients on Platelet Function: A Modifiable Link between Metabolic Syndrome and Neurodegeneration? Biomolecules 2021, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.E. Determinants of platelet count in humans. Haematologica 2011, 96, 10–13. [Google Scholar] [CrossRef]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.-H.; Zhang, H.; Pang, X.-W.; Chen, L.; Zhou, L.-Q.; Chen, M.; Tian, D.-S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Taha, A.; Bobi, J.; Dammers, R.; Dijkhuizen, R.M.; Dreyer, A.Y.; van Es, A.; Ferrara, F.; Gounis, M.J.; Nitzsche, B.; Platt, S.; et al. Comparison of Large Animal Models for Acute Ischemic Stroke: Which Model to Use? Stroke 2022, 53, 1411–1422. [Google Scholar] [CrossRef]
- Becker, K.J. Inflammation and the Silent Sequelae of Stroke. Neurotherapeutics 2016, 13, 801–810. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Michalak, M.; Olasinska-Wisniewska, A.; Rodzki, M.; Witkowska, A.; Gasecka, A.; Buczkowski, P.; Perek, B.; Jemielity, M. Neutrophil Counts, Neutrophil-to-Lymphocyte Ratio, and Systemic Inflammatory Response Index (SIRI) Predict Mortality after Off-Pump Coronary Artery Bypass Surgery. Cells 2022, 11, 1124. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Liang, C.; Ling, Y. The association between systemic inflammatory response index and new-onset atrial fibrillation in patients with ST-elevated myocardial infarction treated with percutaneous coronary intervention. BMC Cardiovasc. Disord. 2022, 22, 525. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

| Category | Inclusion Criteria |
|---|---|
| Population | Patients with a broad range of CVD, including but not limited to CAD, CeVD (e.g., stroke), PAD, HF, CMPs, and other cardiac conditions |
| Exposure | At least one immune-inflammatory index (NLR, PLR, SII, SIRI) |
| Comparison | Low vs. high levels of immune-inflammatory indices |
| Outcome | Clinical outcomes of CVD patients, such as mortality, MACE, hospitalization, revascularization, recurrence rates, and other related outcomes |
| Study design | Systematic reviews with meta-analyses containing quantitative data |
| Language | English |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, P.; Wu, L.H.; Wu, F.; Zhou, X.; Li, Y.; Su, H.; Zang, J.; Ji, X.; Xiao, X.; et al. Evaluating Immune-Inflammatory Indices for Risk Stratification in Cardiovascular Disease: An Umbrella Review of Systematic Reviews and Meta-Analyses. Diagnostics 2025, 15, 2862. https://doi.org/10.3390/diagnostics15222862
Liu H, Wang P, Wu LH, Wu F, Zhou X, Li Y, Su H, Zang J, Ji X, Xiao X, et al. Evaluating Immune-Inflammatory Indices for Risk Stratification in Cardiovascular Disease: An Umbrella Review of Systematic Reviews and Meta-Analyses. Diagnostics. 2025; 15(22):2862. https://doi.org/10.3390/diagnostics15222862
Chicago/Turabian StyleLiu, Hanxin, Pingwu Wang, Lik Hang Wu, Fan Wu, Xinya Zhou, Yuhan Li, Hui Su, Jiayi Zang, Xinchen Ji, Xueling Xiao, and et al. 2025. "Evaluating Immune-Inflammatory Indices for Risk Stratification in Cardiovascular Disease: An Umbrella Review of Systematic Reviews and Meta-Analyses" Diagnostics 15, no. 22: 2862. https://doi.org/10.3390/diagnostics15222862
APA StyleLiu, H., Wang, P., Wu, L. H., Wu, F., Zhou, X., Li, Y., Su, H., Zang, J., Ji, X., Xiao, X., Wu, Y.-K., Pakkiri, L. S., & Drum, C. L. (2025). Evaluating Immune-Inflammatory Indices for Risk Stratification in Cardiovascular Disease: An Umbrella Review of Systematic Reviews and Meta-Analyses. Diagnostics, 15(22), 2862. https://doi.org/10.3390/diagnostics15222862









