The Usefulness of Basic Laboratory Analyses in Diagnostics of Inherited Metabolic Diseases in Children
Abstract
1. Introduction
2. Usefulness of Basic Laboratory Analyses in Diagnosis of IMDs
3. Peripheral Blood Morphology
3.1. Megaloblastic Anemia
3.2. Microcytic Anemia
3.3. Hemolytic Anemia
3.4. Thrombocytopenia
3.5. Neutropenia
3.6. Pancytopenia
4. Laboratory (Biochemical) Markers of Liver Damage/Function
5. Serum Creatine Kinase
6. Serum Alkaline Phosphatase (ALP)
6.1. Elevated ALP Activity
6.2. Low ALP Activity
7. Serum Uric Acid
7.1. Hyperuricaemia
7.2. Hypouricaemia
7.3. Normouricaemia and Normouricosuria
8. Hypoglycaemia
8.1. Glycogen Storage Disorders
8.2. Gluconeogenesis Disorders
8.3. FAO Defects and Disorders of Ketone Body Metabolism
8.4. Idiopathic Ketotic Hypoglycemia
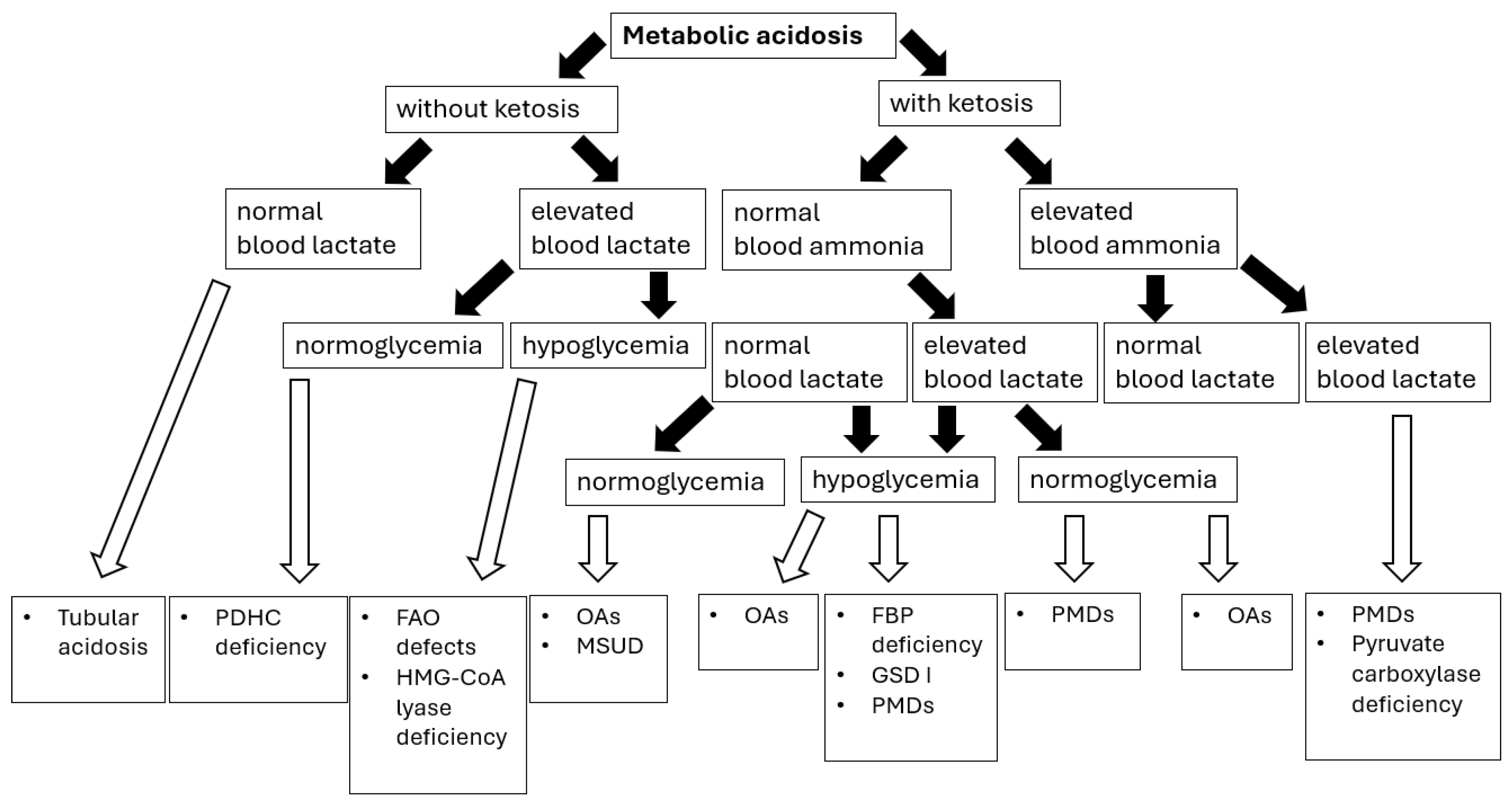
9. Hyperlactatemia with or Without Lactic Acidosis
10. Hyperammonemia
11. Lipid Profile
11.1. Hypercholesterolemia
11.2. Hypocholesterolemia
| Disease | Lipoprotein Abnormalities | Characteristic Features |
|---|---|---|
| Familial lipoprotein lipase or its activator (apoC-II) deficiency | ↑ chylomicrons. ↑ triacylglycerols. N/↑ cholesterol. | Creamy appearance of blood. Enlargement of the liver and spleen (triacylglycerol uptake by macrophages). Recurrent pancreatitis. Xanthelasma (triacylglycerols in skin macrophages). |
| Congenital deficiency of apoprotein A-I and apoprotein A-II (Tangier disease) | Abnormal chylomicrons and VLDL. Reduced plasma cholesterol concentration. | Enlarged palatine tonsils with an orange tint. Enlarged spleen, liver, and lymph nodes (accumulation of cholesterol esters). Neurological symptoms—sensory disturbances, muscle weakness. |
| Abetalipoproteinemia (lack of apoprotein B-48, apo-B48) | Chylomicrons, LDL, and VLDL are absent in plasma. Triacylglycerol and cholesterol concentrations are many times lower than physiological values. | Malabsorption syndrome (numerous fat droplets appear in the cytoplasm of intestinal epithelial cells)—steatorrhea, ADEK vitamin deficiency. Neurological disorders—spinocerebellar ataxia, peripheral neuropathy, myopathy, balance disorders, muscle weakness, and spastic muscle contractions. Visual disturbances—visual field defects, twilight vision, and symptoms of retinitis pigmentosa. |
| Lecithin–cholesterol acyltransferase deficiency | Very low HDL-C. Mild to severe hyperTG. Presence of lipoprotein X (Lp-X). Decreased apo A-I, A-II. | Corneal opacities. Hemolytic anemia. Proteinuria. Renal failure. |
12. Red Flags—Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, C.R.; van Karnebeek, C.D.M. Inborn Errors of Metabolism. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 449–481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saudubray, J.M.; Garcia-Cazorla, À. Inborn Errors of Metabolism Overview: Pathophysiology, Manifestations, Evaluation, and Management. Pediatr. Clin. N. Am. 2018, 65, 179–208. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H.J. Inborn Errors of Metabolism: Advances in Diagnosis and Therapy. JAMA Pediatr. 2015, 169, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; van Karnebeek, C.D.M.; Vockley, J.; Blau, N. A proposed nosology of inborn errors of metabolism. Genet. Med. 2019, 21, 102–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, C.R.; Rahman, S.; Keller, M.; Zschocke, J.; ICIMD Advisory Group. An international classification of inherited metabolic disorders (ICIMD). J. Inherit. Metab. Dis. 2021, 44, 164–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lalonde, E.; Rentas, S.; Lin, F.; Dulik, M.C.; Skraban, C.M.; Spinner, N.B. Genomic Diagnosis for Pediatric Disorders: Revolution and Evolution. Front. Pediatr. 2020, 8, 373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moutapam-Ngamby-Adriaansen, Y.; Maillot, F.; Labarthe, F.; Lioger, B. Blood cytopenias as manifestations of inherited metabolic diseases: A narrative review. Orphanet J. Rare Dis. 2024, 19, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cario, H.; Smith, D.E.; Blom, H.; Blau, N.; Bode, H.; Holzmann, K.; Pannicke, U.; Hopfner, K.P.; Rump, E.M.; Ayric, Z.; et al. Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease. Am. J. Hum. Genet. 2011, 88, 226–231. [Google Scholar] [CrossRef]
- Hilton, J.F.; Christensen, K.E.; Watkins, D.; Raby, B.A.; Renaud, Y.; de la Luna, S.; Estivill, X.; MacKenzie, R.E.; Hudson, T.J.; Rosenblatt, D.S. The molecular basis of glutamate formiminotransferase deficiency. Hum. Mutat. 2003, 22, 67–73. [Google Scholar] [CrossRef]
- Fujiwara, T.; Harigae, H. Pathophysiology and genetic mutations in congenital sideroblastic anemia. Pediatr. Int. 2013, 55, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Lecha, M.; Puy, H.; Deybach, J.C. Erythropoietic protoporphyria. Orphanet J. Rare Dis. 2009, 4, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balwani, M. Erythropoietic Protoporphyria and X-Linked Protoporphyria: Pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019, 128, 298–303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szlendak, U.; Bykowska, K.; Lipniacka, A. Clinical, Biochemical and Molecular Characteristics of the Main Types of Porphyria. Adv. Clin. Exp. Med. 2016, 25, 361–368. [Google Scholar] [CrossRef] [PubMed]
- To-Figueras, J.; Erwin, A.L.; Aguilera, P.; Millet, O.; Desnick, R.J. Congenital erythropoietic porphyria. Liver Int. 2024, 44, 1842–1855. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, A.; Ishikawa, K.; Niemeyer, C.; Grünert, S.C. Pearson syndrome: A multisystem mitochondrial disease with bone marrow failure. Orphanet J. Rare Dis. 2022, 17, 379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riley, L.G.; Heeney, M.M.; Rudinger-Thirion, J.; Frugier, M.; Campagna, D.R.; Zhou, R.; Hale, G.A.; Hilliard, L.M.; Kaplan, J.A.; Kwiatkowski, J.L.; et al. The phenotypic spectrum of germline YARS2 variants: From isolated sideroblastic anemia to mitochondrial myopathy, lactic acidosis and sideroblastic anemia 2. Haematologica 2018, 103, 2008–2015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipiński, P.; Tylki-Szymańska, A. The Liver and Lysosomal Storage Diseases: From Pathophysiology to Clinical Presentation, Diagnostics, and Treatment. Diagnostics 2024, 14, 1299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steward, C.G.; Groves, S.J.; Taylor, C.T.; Maisenbacher, M.K.; Versluys, B.; Newbury-Ecob, R.A.; Ozsahin, H.; Damin, M.K.; Bowen, V.M.; McCurdy, K.R.; et al. Neutropenia in Barth syndrome: Characteristics, risks, and management. Curr. Opin. Hematol. 2019, 26, 6–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grünert, S.C.; Derks, T.G.J.; Mundy, H.; Dalton, R.N.; Donadieu, J.; Hofbauer, P.; Jones, N.; Uçar, S.K.; LaFreniere, J.; Contreras, E.L.; et al. Treatment recommendations for glycogen storage disease type IB- associated neutropenia and neutrophil dysfunction with empagliflozin: Consensus from an international workshop. Mol. Genet. Metab. 2024, 141, 108144. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Austin, S.L.; Abdenur, J.E.; Arn, P.; Bali, D.S.; Boney, A.; Chung, W.K.; Dagli, A.I.; Dale, D.; Koeberl, D.; et al. Diagnosis and management of glycogen storage disease type I: A practice guideline of the American College of Medical Genetics and Genomics. Genet. Med. 2014, 16, e1. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, C.; Francisco, R.; Mexia, P.; Luis Pereira, B.; Granjo, P.; Coelho, H.; Barbosa, M.; Dos Reis Ferreira, V.; Videira, P.A. Revisiting the immunopathology of congenital disorders of glycosylation: An updated review. Front. Immunol. 2024, 15, 1350101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, X.; Ichikawa, M.; Lyons, J.J.; Datta, S.; Lamborn, I.T.; Jing, H.; Kim, E.S.; Biancalana, M.; Wolfe, L.A.; et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J. Allergy Clin. Immunol. 2014, 133, 1400–1409.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stray-Pedersen, A.; Backe, P.H.; Sorte, H.S.; Mørkrid, L.; Chokshi, N.Y.; Erichsen, H.C.; Gambin, T.; Elgstøen, K.B.; Bjørås, M.; Wlodarski, M.W.; et al. PGM3 mutations cause a congenital disorder of glycosylation with severe immunodeficiency and skeletal dysplasia. Am. J. Hum. Genet. 2014, 95, 96–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forny, P.; Hörster, F.; Ballhausen, D.; Chakrapani, A.; Chapman, K.A.; Dionisi-Vici, C.; Dixon, M.; Grünert, S.C.; Grunewald, S.; Haliloglu, G.; et al. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: First revision. J. Inherit. Metab. Dis. 2021, 44, 566–592, Erratum in J. Inherit. Metab. Dis. 2022, 45, 862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, C.R.; Cassiman, D.; Blau, N. Clinical and biochemical footprints of inherited metabolic diseases. II. Metabolic liver diseases. Mol. Genet. Metab. 2019, 127, 117–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seker Yilmaz, B.; Baruteau, J.; Rahim, A.A.; Gissen, P. Clinical and Molecular Features of Early Infantile Niemann Pick Type C Disease. Int. J. Mol. Sci. 2020, 21, 5059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klouwer, F.C.; Berendse, K.; Ferdinandusse, S.; Wanders, R.J.; Engelen, M.; Poll-The, B.T. Zellweger spectrum disorders: Clinical overview and management approach. Orphanet J. Rare Dis. 2015, 10, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heubi, J.E.; Setchell, K.D.; Bove, K.E. Inborn errors of bile acid metabolism. Semin. Liver Dis. 2007, 27, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Spiekerkoetter, U. Mitochondrial fatty acid oxidation disorders: Clinical presentation of long-chain fatty acid oxidation defects before and after newborn screening. J. Inherit. Metab. Dis. 2010, 33, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Guerra, I.M.S.; Ferreira, H.B.; Melo, T.; Rocha, H.; Moreira, S.; Diogo, L.; Domingues, M.R.; Moreira, A.S.P. Mitochondrial Fatty Acid β-Oxidation Disorders: From Disease to Lipidomic Studies-A Critical Review. Int. J. Mol. Sci. 2022, 23, 13933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; IJlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, W.S.; Sokol, R.J. Mitochondrial hepatopathies: Advances in genetics and pathogenesis. Hepatology 2007, 45, 1555–1565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopan, A.; Sarma, M.S. Mitochondrial hepatopathy: Respiratory chain disorders-‘breathing in and out of the liver’. World J. Hepatol. 2021, 13, 1707–1726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Hussaini, A.; Faqeih, E.; El-Hattab, A.W.; Alfadhel, M.; Asery, A.; Alsaleem, B.; Bakhsh, E.; Ali, A.; Alasmari, A.; Lone, K.; et al. Clinical and molecular characteristics of mitochondrial DNA depletion syndrome associated with neonatal cholestasis and liver failure. J. Pediatr. 2014, 164, 553–559.e2. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Kuranobu, N.; Ogawa-Tominaga, M.; Akiyama, N.; Sugiyama, Y.; Ebihara, T.; Fushimi, T.; Ichimoto, K.; Matsunaga, A.; Tsuruoka, T.; et al. Clinical and molecular basis of hepatocerebral mitochondrial DNA depletion syndrome in Japan: Evaluation of outcomes after liver transplantation. Orphanet J. Rare Dis. 2020, 15, 169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aleksovska, K.; Kyriakides, T.; Angelini, C.; Argov, Z.; Claeys, K.G.; de Visser, M.; Filosto, M.; Jovanovic, I.; Kostera-Pruszczyk, A.; Molnar, M.J.; et al. What Are the Normal Serum Creatine Kinase Values for Skeletal Muscle? A Worldwide Systematic Review. Eur. J. Neurol. 2025, 32, e70240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urtizberea, J.A.; Severa, G.; Malfatti, E. Metabolic Myopathies in the Era of Next-Generation Sequencing. Genes 2023, 14, 954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lilleker, J.B.; Keh, Y.S.; Roncaroli, F.; Sharma, R.; Roberts, M. Metabolic myopathies: A practical approach. Pract. Neurol. 2018, 18, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.A.; Kariyawasam, D.; Grattan, S.; Bayley, K.; Davis, M.; Holland, S.; Waddel, L.B.; Jones, K.; Lorentzos, M.; Ravine, A.; et al. Newborn Screening for the Diagnosis and Treatment of Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2023, 10, 15–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarnopolsky, M.A. Myopathies Related to Glycogen Metabolism Disorders. Neurotherapeutics 2018, 15, 915–927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gümüş, E.; Özen, H. Glycogen storage diseases: An update. World J. Gastroenterol. 2023, 29, 3932–3963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kishnani, P.S.; Steiner, R.D.; Bali, D.; Berger, K.; Byrne, B.J.; Case, L.E.; Crowley, J.F.; Downs, S.; Howell, R.R.; Kravitz, R.M.; et al. Pompe disease diagnosis and management guideline. Genet. Med. 2006, 8, 267–288, Erratum in Genet. Med. 2006, 8, 382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olimpio, C.; Tiet, M.Y.; Horvath, R. Primary mitochondrial myopathies in childhood. Neuromuscul. Disord. 2021, 31, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Bangeas, A.; Poulidou, V.; Liampas, I.; Marogianni, C.; Aloizou, A.M.; Tsouris, Z.; Sgantzos, M.; Arnaoutoglou, M.; Bogdanos, D.P.; Dardiotis, E.; et al. Advances in Management of Mitochondrial Myopathies. Int. J. Mol. Sci. 2025, 26, 5411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, S.T.; Craven, L.; Russell, O.M.; Turnbull, D.M.; Vincent, A.E. Diagnosis and Treatment of Mitochondrial Myopathies. Neurotherapeutics 2018, 15, 943–953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cannalire, G.; Pilloni, S.; Esposito, S.; Biasucci, G.; Di Franco, A.; Street, M.E. Alkaline phosphatase in clinical practice in childhood: Focus on rickets. Front. Endocrinol. 2023, 14, 1111445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strauch, J.M.; Vogel, M.; Meigen, C.; Ceglarek, U.; Kratzsch, J.; Willenberg, A.; Kiess, W. Pediatric reference values of alkaline phosphatase: Analysis from a German population-based cohort and influence of anthropometric and blood parameters. Bone 2023, 174, 116809. [Google Scholar] [CrossRef] [PubMed]
- Colantonio, D.A.; Kyriakopoulou, L.; Chan, M.K.; Daly, C.H.; Brinc, D.; Venner, A.A.; Pasic, M.D.; Armbruster, D.; Adeli, K. Closing the gaps in pediatric laboratory reference intervals: A CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin. Chem. 2012, 58, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Ridefelt, P.; Gustafsson, J.; Aldrimer, M.; Hellberg, D. Alkaline phosphatase in healthy children: Reference intervals and prevalence of elevated levels. Horm. Res. Pediatr. 2014, 82, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Schottmann, G.; Meinecke, P. Hyperphosphatasia with mental retardation, brachytelephalangy, and a distinct facial gestalt: Delineation of a recognizable syndrome. Eur. J. Med. Genet. 2010, 53, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Carmody, L.C.; Blau, H.; Danis, D.; Zhang, X.A.; Gourdine, J.P.; Vasilevsky, N.; Krawitz, P.; Thompson, M.D.; Robinson, P.N. Significantly different clinical phenotypes associated with mutations in synthesis and transamidase+remodeling glycosylphosphatidylinositol (GPI)-anchor biosynthesis genes. Orphanet J. Rare Dis. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hutny, M.; Lipinski, P.; Jezela-Stanek, A. Characteristics of Neuroimaging and Behavioural Phenotype in Polish Patients with PIGV-CDG-An Observational Study and Literature Review. Genes 2023, 14, 1208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messina, M.; Manea, E.; Cullup, T.; Tuschl, K.; Batzios, S. Hyperphosphatasia with mental retardation syndrome 3: Cerebrospinal fluid abnormalities and correction with pyridoxine and Folinic acid. JIMD Rep. 2022, 64, 42–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shkalim Zemer, V.; Hoshen, M.; Levinsky, Y.; Richenberg, Y.; Yosef, N.; Oberman, B.; Cohen, M.; Cohen, A.H. Benign transient hyperphosphatasemia in infants and children: A retrospective database study. Eur. J. Pediatr. 2023, 182, 3211–3216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huh, S.Y.; Feldman, H.A.; Cox, J.E.; Gordon, C.M. Prevalence of transient hyperphosphatasemia among healthy infants and toddlers. Pediatrics 2009, 124, 703–709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whyte, M.P. Hypophosphatasia—Aetiology, nosology, pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2016, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Mornet, E. Hypophosphatasia. Metabolism 2018, 82, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.L. Hypophosphatasia: An overview of the disease and its treatment. Osteoporos. Int. 2015, 26, 2743–2757. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Lecca, D.; Abbracchio, M.P.; Ceruti, S. Pathophysiological Role of Purines and Pyrimidines in Neurodevelopment: Unveiling New Pharmacological Approaches to Congenital Brain Diseases. Front. Pharmacol. 2017, 8, 941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fathallah-Shaykh, S.A.; Cramer, M.T. Uric acid and the kidney. Pediatr. Nephrol. 2014, 29, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Simoni, R.E.; Gomes, L.N.; Scalco, F.B.; Oliveira, C.P.; Aquino Neto, F.R.; de Oliveira, M.L. Uric acid changes in urine and plasma: An effective tool in screening for purine inborn errors of metabolism and other pathological conditions. J. Inherit. Metab. Dis. 2007, 30, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.J.; Puig, J.G. Hypoxanthine-guanine phosophoribosyltransferase (HPRT) deficiency: Lesch-Nyhan syndrome. Orphanet J. Rare Dis. 2007, 2, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torun, B.; Bilgin, A.; Orhan, D.; Gocmen, R.; Kılıc, S.S.; Kuskonmaz, B.; Cetinkaya, D.; Tezcan, I.; Cagdas, D. Combined immunodeficiency due to purine nucleoside phosphorylase deficiency: Outcome of three patients. Eur. J. Med. Genet. 2022, 65, 104428. [Google Scholar] [CrossRef] [PubMed]
- Peretz, H.; Lagziel, A.; Bittner, F.; Kabha, M.; Shtauber-Naamati, M.; Zhuravel, V.; Usher, S.; Rump, S.; Wollers, S.; Bork, B.; et al. Classical Xanthinuria in Nine Israeli Families and Two Isolated Cases from Germany: Molecular, Biochemical and Population Genetics Aspects. Biomedicines 2021, 9, 788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, S.K.; Schwarz, G.; Tasic, V.; Křížková, M.; Krijt, J.; Roeper, J.; Honzík, T.; Šebesta, I.; Kožich, V.; Šaligová, J.; et al. A prevalent MOCS2 variant in the Roma population is associated with a novel mild form of molybdenum cofactor deficiency. Eur. J. Pediatr. 2025, 184, 499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dąbrowska-Leonik, N.; Piątosa, B.; Słomińska, E.; Bohynikova, N.; Bernat-Sitarz, K.; Bernatowska, E.; Wolska-Kuśnierz, B.; Kałwak, K.; Kołtan, S.; Dąbrowska, A.; et al. National experience with adenosine deaminase deficiency related SCID in Polish children. Front. Immunol. 2023, 13, 1058623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jurecka, A.; Zikanova, M.; Kmoch, S.; Tylki-Szymańska, A. Adenylosuccinate lyase deficiency. J. Inherit. Metab. Dis. 2015, 38, 231–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quarta, A.; Iannucci, D.; Guarino, M.; Blasetti, A.; Chiarelli, F. Hypoglycemia in Children: Major Endocrine-Metabolic Causes and Novel Therapeutic Perspectives. Nutrients 2023, 15, 3544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casertano, A.; Rossi, A.; Fecarotta, S.; Rosanio, F.M.; Moracas, C.; Di Candia, F.; Parenti, G.; Franzese, A.; Mozzillo, E. An Overview of Hypoglycemia in Children Including a Comprehensive Practical Diagnostic Flowchart for Clinical Use. Front. Endocrinol. 2021, 12, 684011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hannah, W.B.; Derks, T.G.J.; Drumm, M.L.; Grünert, S.C.; Kishnani, P.S.; Vissing, J. Glycogen storage diseases. Nat. Rev. Dis. Primers 2023, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Goldstein, J.; Austin, S.L.; Arn, P.; Bachrach, B.; Bali, D.S.; Chung, W.K.; El-Gharbawy, A.; Brown, L.M.; Kahler, S.; et al. Diagnosis and management of glycogen storage diseases type VI and IX: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2019, 21, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.L.; Soler-Alfonso, C.; Kiely, B.T.; Asai, A.; Smith, A.L.; Bali, D.S.; Kang, P.B.; Landstrom, A.P.; Akman, H.O.; Burrow, T.A.; et al. Diagnosis and management of glycogen storage disease type IV, including adult polyglucosan body disease: A clinical practice resource. Mol. Genet. Metab. 2023, 138, 107525. [Google Scholar] [CrossRef] [PubMed]
- Berling, É.; Laforêt, P.; Wahbi, K.; Labrune, P.; Petit, F.; Ronzitti, G.; O’Brien, A. Narrative review of glycogen storage disorder type III with a focus on neuromuscular, cardiac and therapeutic aspects. J. Inherit. Metab. Dis. 2021, 44, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Belu, A.; Filip, N.; Trandafir, L.M.; Spoială, E.L.; Țarcă, E.; Zamosteanu, D.; Ghiga, G.; Bernic, J.; Jehac, A.; Cojocaru, E. Lactate, an Essential Metabolic Marker in the Diagnosis and Management of Pediatric Conditions. Diagnostics 2025, 15, 816. [Google Scholar] [CrossRef]
- Schumann, A.; Schultheiss, U.T.; Ferreira, C.R.; Blau, N. Clinical and biochemical footprints of inherited metabolic diseases. XIV. Metabolic kidney diseases. Mol. Genet. Metab. 2023, 140, 107683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, J.; Radej, J.; Horak, J.; Karvunidis, T.; Valesova, L.; Kriz, M.; Matejovic, M. Lactate: The Fallacy of Oversimplification. Biomedicines 2023, 11, 3192. [Google Scholar] [CrossRef]
- Shayota Brian, J. Biomarkers of mitochondrial disorders. Neurotherapeutics 2024, 21, e00325. [Google Scholar] [CrossRef]
- Ganetzky, R.; McCormick, E.M.; Falk, M.J. Primary Pyruvate Dehydrogenase Complex Deficiency Overview. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2021. [Google Scholar] [PubMed]
- Savvidou, A.; Ivarsson, L.; Naess, K.; Eklund, E.A.; Lundgren, J.; Dahlin, M.; Frithiof, D.; Sofou, K.; Darin, N. Novel imaging findings in pyruvate dehydrogenase complex (PDHc) deficiency-Results from a nationwide population-based study. J. Inherit. Metab. Dis. 2022, 45, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, D.A.; Correia, C.E.; Saunders, A.C.; Wolfsdorf, J.I. Hepatic glycogen synthase deficiency: An infrequently recognized cause of ketotic hypoglycemia. Mol. Genet. Metab. 2006, 87, 284–288. [Google Scholar] [CrossRef]
- Häberle, J. Clinical practice: The management of hyperammonemia. Eur. J. Pediatr. 2011, 170, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Häberle, J.; Burlina, A.; Chakrapani, A.; Dixon, M.; Karall, D.; Lindner, M.; Mandel, H.; Martinelli, D.; Pintos-Morell, G.; Santer, R.; et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J. Inherit. Metab. Dis. 2019, 42, 1192–1230. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients. Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5 (Suppl. S3), S1–S8. [Google Scholar] [CrossRef]
- Nielsen, S.T.; Lytsen, R.M.; Strandkjaer, N.; Rasmussen, I.J.; Sillesen, A.-S.; Vøgg, R.O.B.; Raja, A.A.; Nordestgaard, B.G.; Kamstrup, P.R.; Iversen, K.; et al. Significance of lipids, lipoproteins, and apolipoproteins during the first 14–16 months of life. Eur. Heart J. 2023, 44, 4408–4418. [Google Scholar] [CrossRef]
- Myśliwiec, M.; Bandura, M.; Wołoszyn-Durkiewicz, A.; Hennig, M.; Walczak, M.; Peregud-Pogorzelski, J.; Sykut-Cegielska, J.; Miszczak-Knecht, M.; Chlebus, K.; Wasąg, B.; et al. 2024 Polish recommendations for the management of familial hypercholesterolemia in children and adolescents. Arch. Med. Sci. 2024, 20, 1741–1753. [Google Scholar] [CrossRef]
- Shah, A.S.; Wilson, D.P. Genetic Disorders Causing Hypertriglycerydemia in Children and Adolescents; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. [Google Scholar]
- Moutzouri, E.; Elisaf, M.; Liberpoulos, E.N. Hypocholesterolemia. Curr. Vasc. Pharmacol. 2011, 9, 200–212. [Google Scholar] [CrossRef]
- Groselj, U.; Kafol, J.; Molk, N.; Sedej, K.; Mlinaric, M.; Sikonja, J.; Sustar, U.; Kern, B.C.; Kovac, J.; Battelino, T.; et al. Prevalence, genetic variants, and clinical implications of hypocholesterolemia in children. Atherosclerosis 2025, 400, 119065. [Google Scholar] [CrossRef]

| Urea Cycle Disorders | Organic Acidurias | Aminoacidopathies | Inborn Errors of Carbohydrate Metabolism | Fatty Acid Oxidation Defects | Congenital Lactic Acidoses | |
|---|---|---|---|---|---|---|
| Metabolic acidosis | Respiratory alkalosis | ++ | ± | ± | ± | ± |
| Hyperammonemia | +++ | ++ | ± | − | ± | − |
| Hypoglycemia | − | ± | ± | ++ | ++ | ± |
| Ketonuria/ketonaemia | − | ++ | + | + | − | + |
| Elevated serum lactate | − | ± | ± | + | ± | +++ |
| Macrocytosis or Macrocytic Anemia | Total Vitamin B12 | Folate | MMA | Hcy | |
|---|---|---|---|---|---|
| Neonatal cbl deficiency | + | ↓ | N | ↑ | ↑ |
| Nutritional cbl deficiency | + | ↓ | N | ↑ | ↑ |
| cblC | +/− | N | N | ↑ | ↑ |
| cblD | + | N | N | N/↑ | ↑ |
| cblF/cblJ | + | N | N | ↑ | ↑ |
| cblE/G | + | N | N | N | N |
| Transcobalamin II deficiency | + | N | N | ↑ | ↑ |
| Folate deficiency or malabsorption | + | N | ↓ | N | ↑ |
| DHFR deficiency | + | N | ↓ | N | N |
| MTHFD1 deficiency | + | N | ↓ | N | ↑ |
| Hepatomegaly or hepatosplenomegaly with cholestasis | α-1-antitrypsin deficiency, progressive familial intrahepatic cholestasis, bile acid synthesis disorders, Niemann–Pick type C disease, citrin deficiency, Zellweger syndrome spectrum disorders, adenosine kinase deficiency; less frequently—galactosemia, fructosemia, tyrosinemia type I, congenital disorders of glycosylation |
| Enlarged liver without evidence of hepatocellular damage with enlarged spleen | Lysosomal storage diseases (e.g., Gaucher disease, mucopolysaccharidoses) |
| Enlarged liver with signs of hepatocellular damage and possible enlargement of the spleen | Acid sphingomyelinase deficiency (formerly Niemann–Pick disease type A, B, A/B), lysosomal lipase deficiency—early-onset form (Wolman disease) and late-onset form (cholesteryl ester storage disease) |
| Hepatomegaly with tubulopathy | Galactosemia associated with GALT deficiency, hereditary fructose intolerance, tyrosinemia type I, transaldolase deficiency (TALDO), GSD type XI, congenital disorders of glycosylation (e.g., PMM2-CDG) |
| Hepatomegaly with hypoglycemia | Hepatic glycogenoses (GSD types I, III, VI, IX, XI), gluconeogenesis disorders (e.g., fructose-1,6-bisphosphatase deficiency), galactosemia associated with GALT deficiency *, hereditary fructose intolerance *, mitochondrial hepatopathies, congenital disorders of glycosylation (e.g., PMM2-CDG, MPI-CDG) |
| Hepatomegaly with hyperlipidemia | Visceral form of acid sphingomyelinase deficiency (formerly Niemann–Pick disease type B), late-onset form of lysosomal lipase deficiency (cholesteryl ester storage disease), hepatic glycogenoses (GSD I, III, VI, IX), primary defects of lipoprotein metabolism (apo-CII deficiency, lipoprotein lipase deficiency) |
| Acute liver failure < 3 months of age | Gestational alloimmune liver disease (GALD), galactosemia associated with GALT deficiency, tyrosinemia type I, mitochondrial hepatopathies, urea cycle disorders, fatty acid β-oxidation disorders, Wolman disease, transaldolase deficiency (TALDO), congenital disorders of glycosylation |
| Acute liver failure at age 3 months–2 years | Galactosemia associated with GALT deficiency, hereditary fructose intolerance, tyrosinemia type I, mitochondrial hepatopathies, urea cycle disorders, fatty acid β-oxidation disorders, transaldolase deficiency (TALDO), congenital disorders of glycosylation |
| Acute liver failure > 2 years of age | Wilson’s disease, mitochondrial hepatopathies, urea cycle disorders, fatty acid β-oxidation disorders, congenital glycosylation disorders |
| Carnitine transporter (OCTN2) deficiency—primary carnitine deficiency | Early (first 2 years of life) hepato-muscular manifestation with hepatomegaly, elevated transaminases, hypoketotic hypoglycemia, and hepatic encephalopathy; in older children, cardiomyopathy, skeletal muscle weakness, and slightly elevated CK levels. |
| Muscular form in an early childhood with dilated cardiomyopathy, hypotonia, muscle weakness, and elevated CK levels. | |
| Adult form with cardiac arrhythmia in pregnant women, muscle fatigue. | |
| Carnitine-palmitoyl transferase type 1A (CPT1A) deficiency—hepatic form | Isolated liver involvement with hepatomegaly, elevated transaminases, hepatic encephalopathy. |
| Carnitine-palmitoyl transferase type 2 (CPT2) deficiency | Neonatal/infantile form with severe hepato-muscular manifestation—liver failure, cardiomyopathy, respiratory failure, and/or cardiac arrhythmias. |
| Severe childhood hepato-muscular form with liver failure, cardiomyopathy, cardiac arrhythmias, and myopathy. | |
| Classic muscular form—from infancy to adulthood (onset is generally observed in childhood or early adulthood); recurrent episodes of muscle pain and weakness and rhabdomyolysis; no signs/symptoms of myopathy are observed between attacks. | |
| Very long chain acyl-CoA dehydrogenase deficiency (VLCADD) | Severe infantile form with hypertrophic cardiomyopathy and acute liver failure. |
| Hepato-muscular form with a milder course in childhood. | |
| Adult form with recurrent episodes of rhabdomyolysis. | |
| Long-chain fatty acid 3-OH-acyl-CoA dehydrogenase deficiency (LCHADD) | Newborns with the severe phenotype present with hypoglycemia, hepatomegaly, encephalopathy, and often cardiomyopathy within a few days of birth. |
| Intermediate phenotype—hypoketotic hypoglycemia caused by infection or starvation in infancy. | |
| Mild (late-onset) phenotype—myopathy and/or neuropathy. | |
| Peripheral neuropathy in adolescence or adulthood (in approximately 80% of patients with MTP deficiency and in approximately 5–10% of patients with LCHAD deficiency)—a slow, progressive sensorimotor polyneuropathy, along with limb–girdle myopathy with recurrent episodes of myoglobinuria. Retinitis pigmentosa—affects approximately 30–50% of patients with LCHAD deficiency and approximately 5–13% of patients with MTP deficiency; deterioration of color vision, vision in low light, and vision in the center of the visual field, up to complete vision loss. | |
| Medium-chain acyl-CoA dehydrogenase deficiency (MCADD) | Before the era of screening, the presentation was similar to CPT1A deficiency: liver failure with hepatic encephalopathy. |
| Currently, MCAD deficiency is usually diagnosed before decompensation occurs, treatment is initiated early, and acute metabolic decompensation is rare. |
| Disease | Pompe Dis-ease | GSD IIIa | GSD IV | GSD V | GSD VII | GSD X | GSD XI | GSD XIII | GSD XIV | GSD XV | PCD | CPT II | MELAS | MERRF | TK2 Deficiency | Kearn–Sayre Syndrome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myalgia | + | + | + | + | + | + | + | |||||||||
| Proximal muscle weakness | + | + | + | + | + | + | + | + | + | |||||||
| Distal muscles weakness | + | |||||||||||||||
| Rhabdomyolysis | +/− | +/− | + | + | + | + | + | + | + | +/− | +/− | + | ||||
| Second-wind phenomenon | + | |||||||||||||||
| Exercise intolerance | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Hepatomegaly | +/− | + | + | + | + | + | ||||||||||
| Cardiomyopathy | +/− | + | +/− | + | + | +/− |
| MELAS Mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes | Onset of symptoms 2–10 years of age: re-current headaches, recurrent vomiting, migraines, seizures Encephalomyopathy with stroke-like episodes (most often with amblyopia) in a location inconsistent with the anatomical course of the cerebral vessels Lactic acidosis Exercise intolerance Short stature Hearing loss Diabetes Most often (80%) associated with the m.3243A > G variant in the MTTL1 gene encoding tRNALeu |
| MERRF Myoclonic epilepsy with ragged red fibers | Myoclonic epilepsy, including other types of seizures Symptoms in childhood after a period of normal development Cerebellar ataxia, myopathy, hearing loss, optic atrophy, cardiac arrhythmias, dementia Muscle biopsy (Gomori trichrome stain) Shows pathognomonic, ragged muscle fibers with red granules under the sarco-lemma—ragged red fibers Most often (80%) associated with the m.8344A > G mutation in the MTTK gene encoding tRNALeu |
| Kearns–Sayre syndrome | Age of onset < 20 years Retinitis pigmentosa (twilight vision disturbances, narrowing of the visual field to telescope vision, photophobia) Progressive external ophthalmoplegia (ptosis, limited eye movement) Cardiac conduction disorders (AV blocks) Hearing loss, diabetes, hypoparathyroidism, growth hormone deficiency, elevated CSF protein levels, cerebral folate deficiency (leukoencephalopathy) mtDNA deletions (most often m.8470_13446del4977) |
| MNGIE Mitochondrial neurogastrointestinal encephalomyopathy | Symptoms onset: childhood, adolescence/young adulthood (typical), adult-hood (late onset, >40 years) GI symptoms/signs: sub-occlusive episodes, nausea, vomiting, early satiety, severe abdominal pain, abdominal distension, dysphagia, constipation and diarrhea, acute peritonitis due to small bowel perforation Unexplained weight loss, thinness, cachexia Radiological GI signs: small bowel diverticulosis, GI dilation (e.g., gastric or intestinal dilation) Neurological symptoms/signs: chronic progressive external ophthalmoplegia (CPEO), ptosis, peripheral neuropathy, hearing loss Neuroradiological signs: leukoencephalopathy without other neuroradiological abnormalities Metabolic alterations: liver steatosis evolving in cirrhosis, pancreatitis, early-onset diabetes mellitus, increased triglyceride levels, elevated plasma lactate |
| Type of HPP | Clinical Features |
|---|---|
| Lethal perinatal HPP | polyhydramnios, bowed and short long bones, low or absent skeletal mineralization, caput membranaceum, hypoplastic thoracic cage; respiratory distress due to pulmonary hypoplasia, tracheomalacia, chest deformity and profound muscular weakness; pyridoxine-dependent seizures; increased intracranial pressure (papilledema, vomiting); hypercalcemia and hypercalciuria and sometimes nephrocalcinosis |
| Benign perinatal HPP | limb shortening with bowing of the long bones showing spontaneous improvement in the last gestational trimester or at birth |
| Infantile HPP | newborns appear healthy at the time of birth; failure to thrive, poor feeding, muscular weakness, developmental delay, signs resembling rickets, i.e., wide fontanelles and rachitic deformities; respiratory failure due to pulmonary hypoplasia, small thorax, gracile bones, recurrent fractures, and tracheomalacia; hypercalcemia and hypercalciuria and sometimes nephrocalcinosis; craniosynostosis and intracranial hypertension; pyridoxine-dependent seizures; untreated patients with infantile HPP have 50% mortality in the first year of life |
| Childhood HPP Mild form | minor or no symptoms; early tooth loss (premature painless exfoliation of one or more deciduous teeth with intact roots before age 5 years); radiographic skeletal changes are very subtle, e.g., low bone mass |
| Childhood HPP Severe form | premature tooth loss; skeletal pain; muscle weakness (delayed walking, waddling gait, difficulty in climbing stairs); skeletal deformities—pectus excavatum, craniosynostosis, scoliosis and deformed long bones (slow-healing recurrent fractures; genu varum or genu valgum, swollen wrists (metaphyseal flaring)) |
| OdontoHPP | early loss of deciduous (before 3–5 years of age) and permanent teeth without signs of periodontal inflammation; defects in the shape, structure, and color of teeth, hypoplasia of enamel and dentine, thin dentinal walls, wide pulp chambers, thin and short roots, and dental caries |
| 1. Defects of pyruvate metabolism Pyruvate dehydrogenase deficiency Pyruvate carboxylase deficiency |
| 2. Defects of NADH oxidation Defects of the mitochondrial electron transfer chain |
| 3. Gluconeogenesis disorders/glycogen storage disorders Glucose-6-phosphatase deficiency (GSD I) Fructose-1,6-bisphosphatase deficiency Phosphoenolpyruvate carboxykinase deficiency Glycogen debrancher deficiency (GSD III) Glycogen synthase deficiency (GSD 0) |
| 4. Defects of fatty acid oxidation |
| 5. Defects of biotin metabolism Biotinidase deficiency Holocarboxylase synthase deficiency |
| 6. Defects of organic acid metabolism Propionic acidosis Methylmalonic acidosis |
| 7. Other Hereditary fructose intolerance |
| IMDs |
| Urea cycle defects Organic acidurias Fatty acid oxidation disorders Hypoglycemia–hyperammonemia syndrome |
| Secondary |
| Liver failure Portosystemic shunt Medications: valproate, L-asparaginase Physical exertion (e.g., seizures, respiratory failure) |
| False-positive results |
| Blood squeezing Sample hemolysis Long-term blood sample storage |
| Acute symptoms/clinical features |
| Consciousness disturbances (ranging from drowsiness to coma) Seizures Vomiting Encephalopathy Acute liver failure, coagulopathy (especially in OTCD and HHH) Circulatory failure, multi-organ failure Psychiatric symptoms (hallucinations, mania, psychosis, emotional. or personality disorders) In newborns: sepsis-like appearance, body temperature instability, hyperventilation |
| Chronic symptoms/clinical features |
| Recurrent symptoms Exacerbation following infections, excessive protein intake, or fasting Protein aversion Consciousness disturbances Cerebellar symptoms (tremor, ataxia, dysarthria) Headaches (migraine-like) Learning difficulties, cognitive impairment Epilepsy Progressive spastic diplegia or tetraplegia (described as ARG1D, HHH syndrome) Recurrent abdominal pain and vomiting Poor physical development (underweight and height) Elevated aminotransferase levels Psychiatric symptoms: hyperactivity, mood swings, behavioral changes, aggression Autistic features Trichorrhesis nodosa (ASLD) |
| Factors that may cause decompensation |
| Infection, especially with fever Excessive protein intake or fasting Gastrointestinal bleeding Prolonged, intense physical exertion Surgery under general anesthesia Medications: valproate, L-asparaginase, high-dose glucocorticosteroids, topiramate, carbamazepine, phenobarbital, phenytoin, primidone, furosemide, hydrochlorothiazide, salicylates |
| Correlations of Clinical and Biochemical Features Suggestive for IMD |
| Megaloblastic anemia + feeding difficulties/failure to thrive + developmental delay + seizures Megaloblastic anemia + renal manifestations (haemolytic uraemic syndrome) Hepatosplenomegaly (suggestive for storage) + thrombocytopenia Acute (hyperammonemic) encephalopathy Neurological deterioration ± lactic acidosis ± hyperammonaemia ± hypoglycemia Hepatopathy (elevated serum transaminases, prolonged INR) + myopathy (elevated CK activity, cardiomyopathy) + hypoketotic hypoglycemia ± lactic acidosis ± hyperammonaemia Early-onset encephalopathy + lactic acidosis ± brain malformations Acute/chronic encephalopathy + neutropenia/pancytopenia ± cardiomyopathy Acute/chronic encephalopathy + neutropenia/pancytopenia ± chronic renal failure Hepatomegaly + elevated serum transaminases + hypercholesterolemia ± hypoglycemia Recurrent hypoglycemia + hepatomegaly Hepatomegaly + elevated serum transaminases + tubulopathy Fasting hypoglycemia + hepatomegaly + elevated serum transaminases ± neutropenia Acute liver failure (especially in early childhood) Recurrent myalgia (exercise-induced) + rhabdomyolisis Permanently elevated CK activity + hepatomegaly + elevated serum transaminases + hypoglycemia Hyperuricemia + developmental delay/intellectual disability + movement disorders Hypouricemia + neonatal epileptic encephalopathy Hypouricemia + urolithiasis Neurological manifestation (ataxia, peripheral neuropathy) + hypocholesterolemia Recurrent pancreatitis + hypertriglyceridemia Elevated ALP activity + intellectual disability ± epilepsy Decreased ALP activity + rickets-like features |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipiński, P.; Doroba, A. The Usefulness of Basic Laboratory Analyses in Diagnostics of Inherited Metabolic Diseases in Children. Diagnostics 2025, 15, 2806. https://doi.org/10.3390/diagnostics15212806
Lipiński P, Doroba A. The Usefulness of Basic Laboratory Analyses in Diagnostics of Inherited Metabolic Diseases in Children. Diagnostics. 2025; 15(21):2806. https://doi.org/10.3390/diagnostics15212806
Chicago/Turabian StyleLipiński, Patryk, and Anna Doroba. 2025. "The Usefulness of Basic Laboratory Analyses in Diagnostics of Inherited Metabolic Diseases in Children" Diagnostics 15, no. 21: 2806. https://doi.org/10.3390/diagnostics15212806
APA StyleLipiński, P., & Doroba, A. (2025). The Usefulness of Basic Laboratory Analyses in Diagnostics of Inherited Metabolic Diseases in Children. Diagnostics, 15(21), 2806. https://doi.org/10.3390/diagnostics15212806








