Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism—Radiological and Clinical Pitfalls and Dilemmas
Abstract
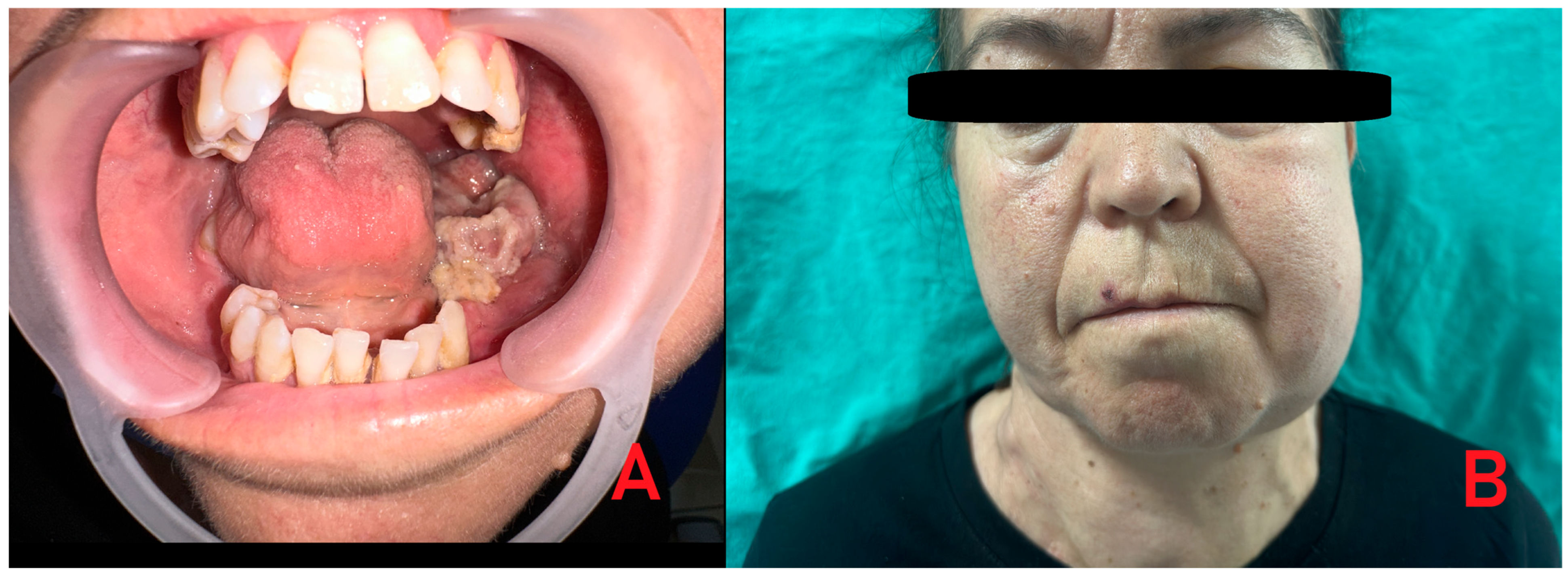
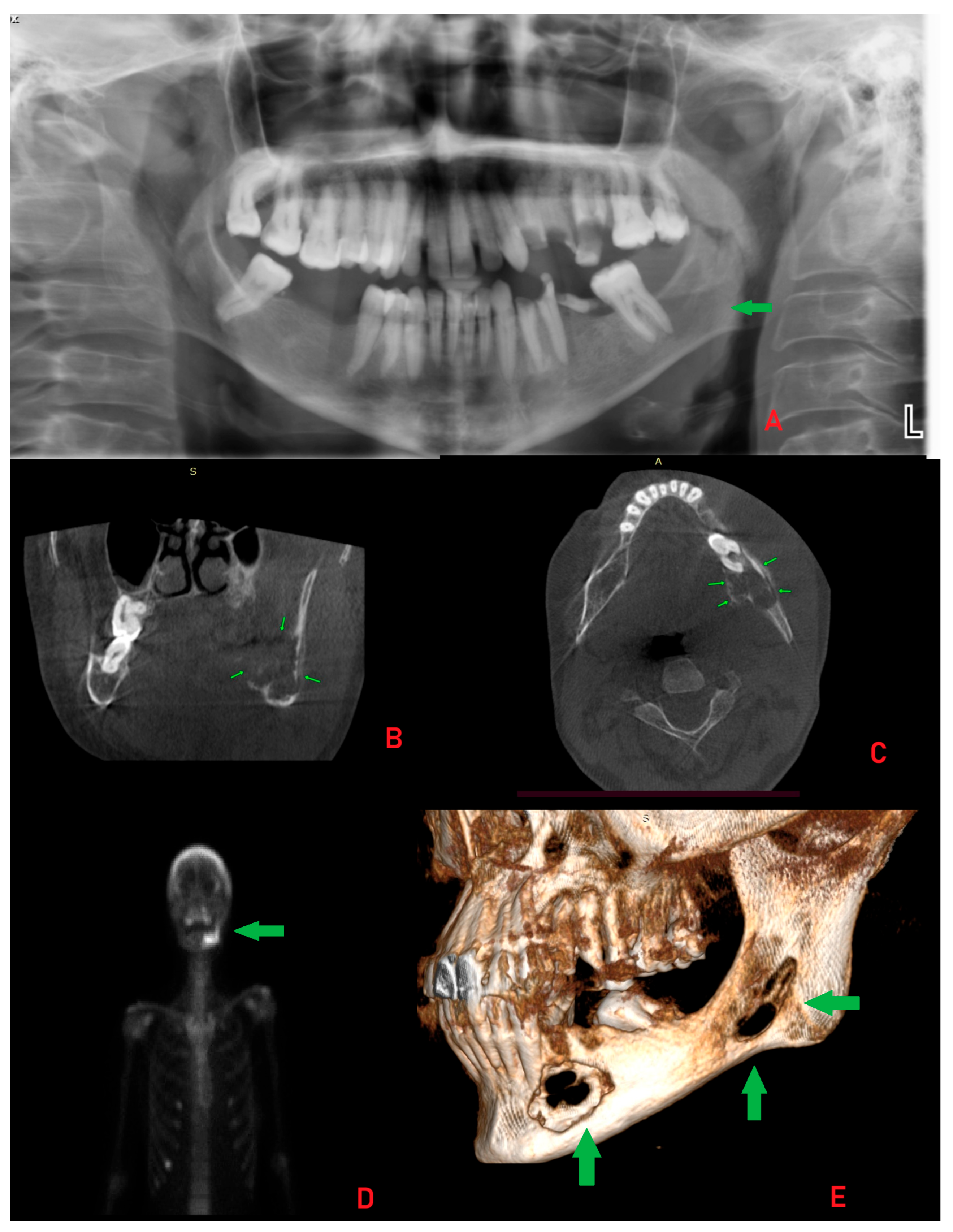
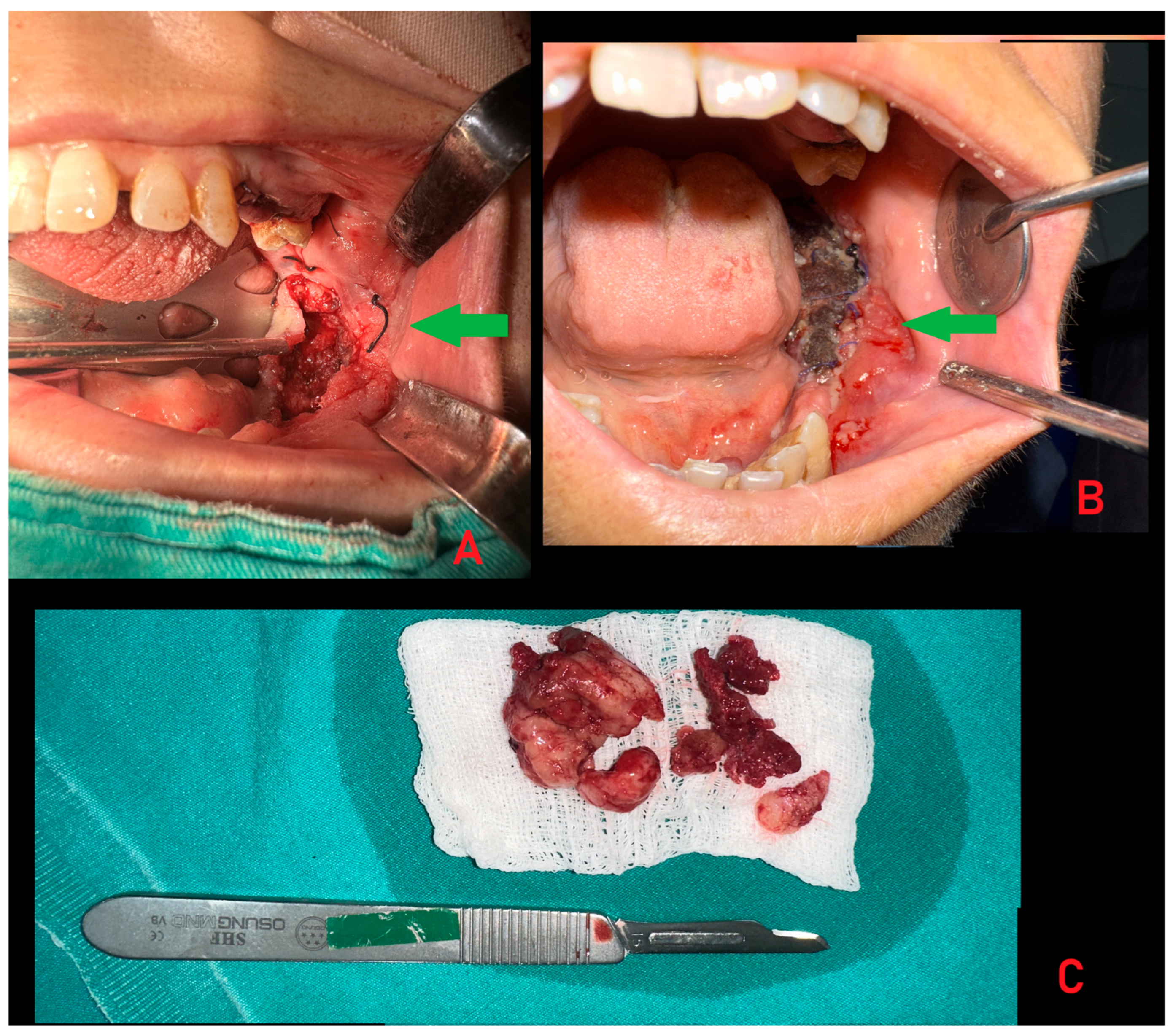
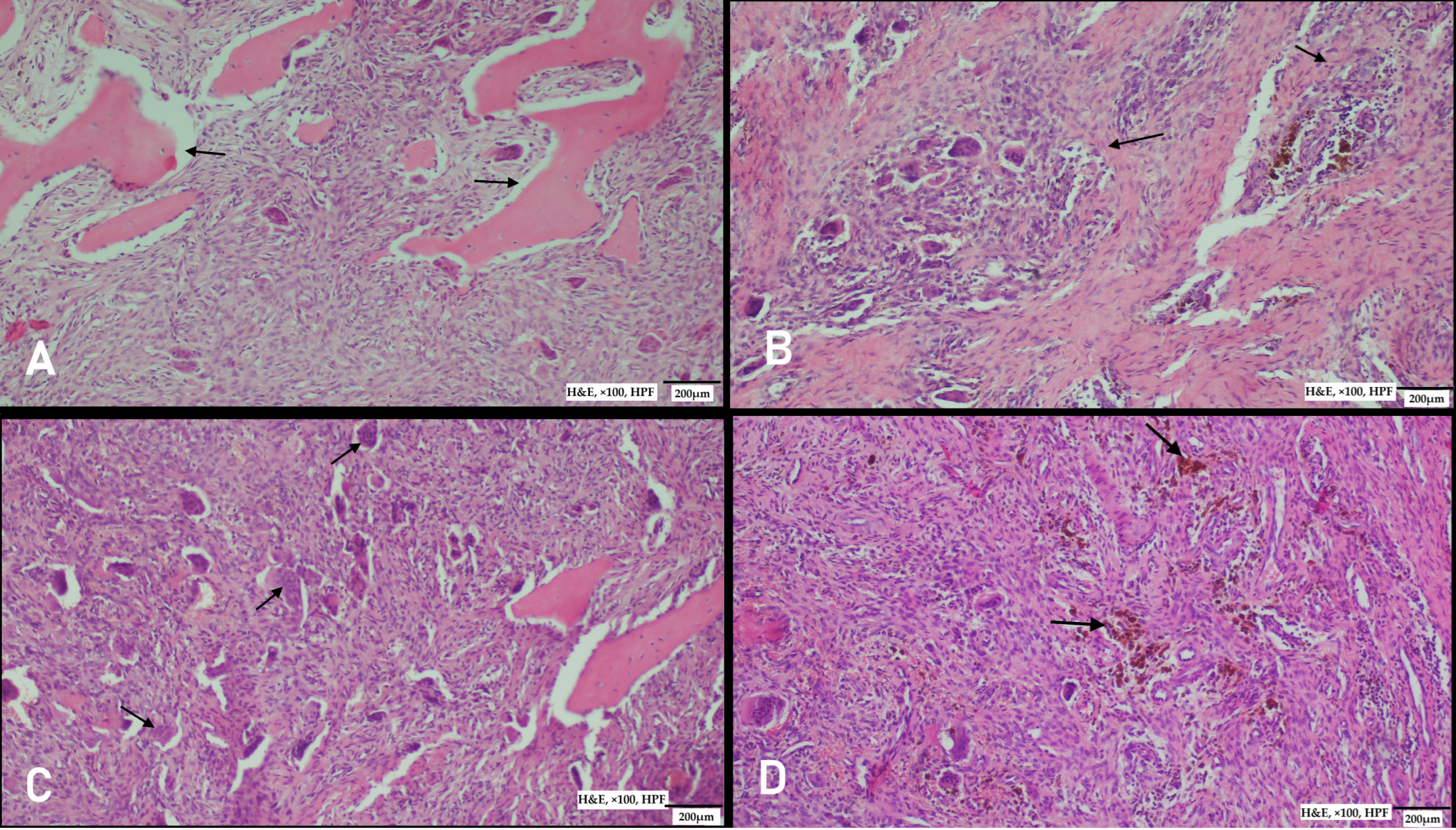
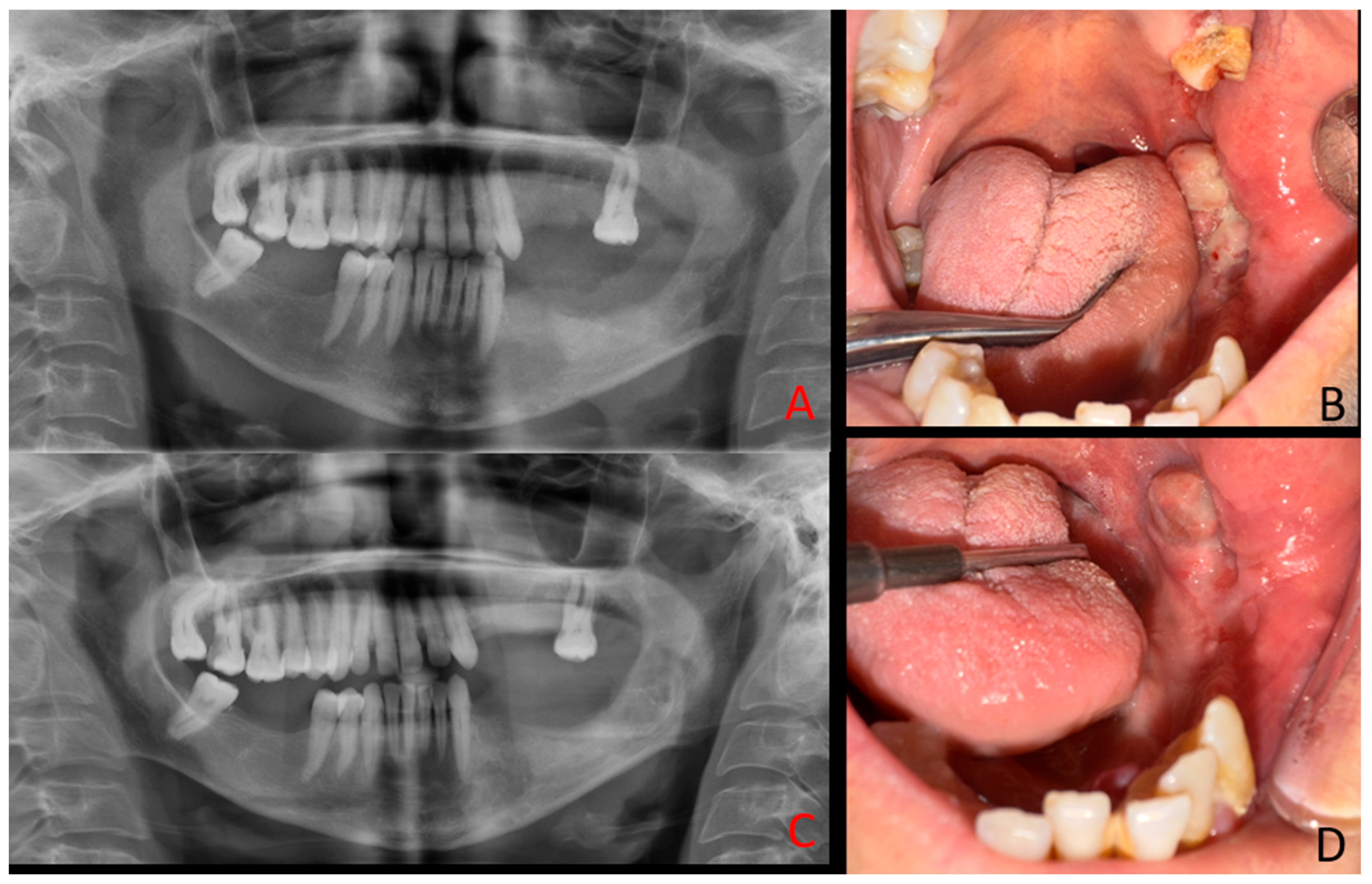
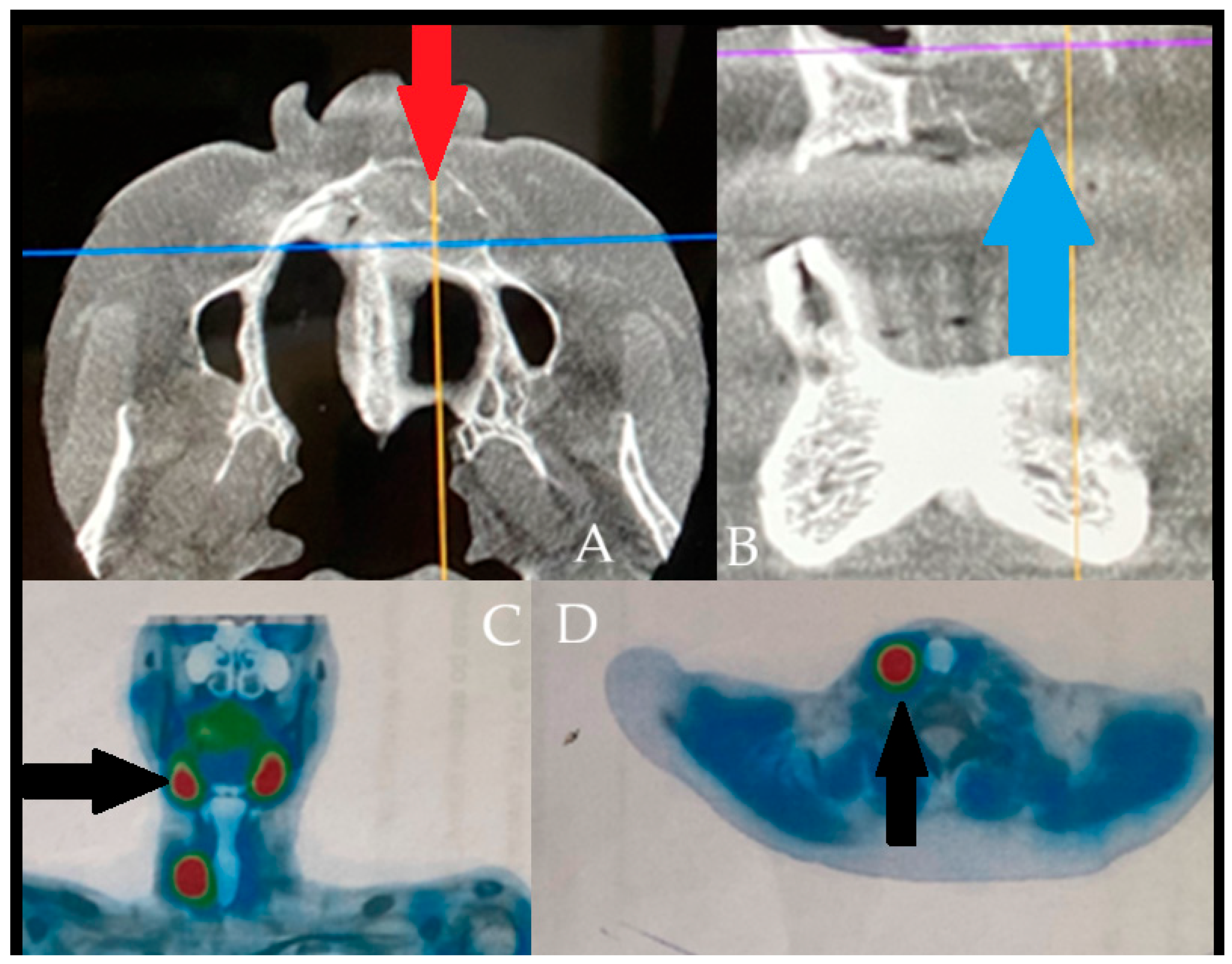
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, A.; Shahid, F.; Ain, N.U. Primary hyperparathyroidism with brown tumor of the mandible misdiagnosed as a giant cell tumor: A case report. Cureus 2024, 16, e56153. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, P. Management of central giant cell granulomas of the jaws: An unusual case report with critical appraisal of existing literature. Ann. Maxillofac. Surg. 2019, 9, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, H.; Adams, A.; Richards, P.; Whitley, S. Hyperparathyroidism jaw tumour syndrome: A pictoral review. Insights Imaging 2016, 7, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Piciu, D.; Piciu, A.; Barbus, E.; Pestean, C.; Larg, M.I.; Fetica, B. Primary hyperparathyroidism-jaw tumor syndrome: A confusing and forgotten diagnosis. Clujul Med. 2016, 89, 555. [Google Scholar] [CrossRef] [PubMed]
- Nelke, K.H.; Lysenko, L.; Leszczyszyn, J.; Gerber, H. Human papillomavirus and its influence on head and neck cancer predisposition. Postep. Hig. Med. Dosw. (Online) 2013, 67, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.; Ahmed, F.; Mihai, R.; Rattan, A.; Humayun, M.A. The unforeseen diagnosis: Hyperparathyroidism-Jaw Tumour Syndrome case report and review of the literature. Case Rep. Endocrinol. 2021, 2021, 5551203. [Google Scholar] [CrossRef] [PubMed]
- Ozbey, F.; Uranbey, O.; Kaygisiz, O.F.; Sadik, E.; Ayranci, F. Exploring the Landscape of Salivary Gland Disorders: A Comprehensive Bibliometric Analysis. J. Maxillofac. Oral Surg. 2025, 24, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Moka, D.; Voth, E.; Dietlein, M.; Larena-Avellaneda, A.; Schicha, H. Technetium 99m-MIBI-SPECT: A highly sensitive diagnostic tool for localization of parathyroid adenomas. Surgery 2000, 128, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lis, E.; Gontarz, M.; Marecik, T.; Wyszyńska-Pawelec, G.; Bargiel, J. Residual Cyst Mimicking an Aggressive Neoplasm—A Life-Threatening Condition. Oral 2024, 4, 354–361. [Google Scholar] [CrossRef]
- Dilek TÜZÜ, N.; İrfan, K.; Murat, Ş.A.; Abdulkadir Yasir, B. Brown Tumor Related to Primary Hyperparathyroidism Mimicking Malignity in the Mandible: Case Report and Literature Review. Endocrinol. Res. Pract. 2023, 27, 93–97. [Google Scholar]
- Rikhotso, R.E.; Khan, F. Lytic brown tumour of the mandible as the initial presentation of hyperparathyroidism in a 7-year old girl: A case report. Int. J. Surg. Case Rep. 2024, 119, 109735. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Bahrami, R.; Amiri, M.A.; Nikparto, N.; Zangooei Booshehri, M.; Keshvari, H.; Daneste, H. Aggressive Brown tumors of the maxilla and mandible post-parathyroidectomy in chronic renal failure: A case report and literature review. Oral Maxillofac. Surg. Cases 2024, 10, 100370. [Google Scholar] [CrossRef]
- Majumdar, S.; Uppala, D.; Kotina, S.; Alekhya, B. Brown tumor of hyperparathyroidism with multiple lesions. J. Oral Maxillofac. Pathol. 2022, 26, S111–S115. [Google Scholar] [CrossRef] [PubMed]
- Fahimy, M.A.M.; Merican, S.R.H.I.; Yahya, M.M.; Othman, M.F. A rare occurrence of primary hyperparathyroidism with brown tumor in the left maxilla. Gulhane Med. J. 2024, 66, 146–148. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, Y.; Luo, J.; Ye, Y. Misdiagnosis of brown tumour caused by primary hyperparathyroidism: A case report with literature review. BMC Endocr. Disord. 2022, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gutiérrez, V.M.; Macías-Jiménez, J.B.; Molotla-Fragoso, A.; Mejía-Velázquez, C.P.; Estévez-González, G.L.; Jacinto-Alemán, L.F. Brown Tumor in Jaw Associated with Hyperparathyroidism: A Case Report. Oral 2025, 5, 59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uranbey, Ö.; Diri, F.; Ekinci, B.; Gontarz, M.; Kuropka, P.; Dobrzyński, M.; Nelke, K. Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism—Radiological and Clinical Pitfalls and Dilemmas. Diagnostics 2025, 15, 2798. https://doi.org/10.3390/diagnostics15212798
Uranbey Ö, Diri F, Ekinci B, Gontarz M, Kuropka P, Dobrzyński M, Nelke K. Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism—Radiological and Clinical Pitfalls and Dilemmas. Diagnostics. 2025; 15(21):2798. https://doi.org/10.3390/diagnostics15212798
Chicago/Turabian StyleUranbey, Ömer, Furkan Diri, Büşra Ekinci, Michał Gontarz, Piotr Kuropka, Maciej Dobrzyński, and Kamil Nelke. 2025. "Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism—Radiological and Clinical Pitfalls and Dilemmas" Diagnostics 15, no. 21: 2798. https://doi.org/10.3390/diagnostics15212798
APA StyleUranbey, Ö., Diri, F., Ekinci, B., Gontarz, M., Kuropka, P., Dobrzyński, M., & Nelke, K. (2025). Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism—Radiological and Clinical Pitfalls and Dilemmas. Diagnostics, 15(21), 2798. https://doi.org/10.3390/diagnostics15212798