Systemic and Local Leptin Resistance in Patients with Cardiovascular Diseases
Abstract
1. Introduction
2. Materials and Methods
| Gene | Sequence Direction | Sequence (5′ → 3′) | Number of Nucleotides |
|---|---|---|---|
| LEP | Forward primer | tgtccaagctgtgcccatcc | 20 |
| Reverse primer | ggtggagcccaggaatgaagt | 21 | |
| LEPR 1 NM_002303.6 (leptin receptor isoform 1 precursor) | Forward primer | ttcttggtccagcccaccatt | 21 |
| Reverse primer | agcagggatgtagctgagacaa | 22 | |
| LEPR 2 NM_001003680.3 (leptin receptor isoform 2 precursor , contains an alternate 3′ terminal exon) | Forward primer | ttcttggtccagcccaccat | 20 |
| Reverse primer | tagcagggatgtagctgagaca | 22 | |
| LEPR 2.2 NM_001198687.2 (leptin receptor isoform 2 precursor, contains alternate 5′ UTR and 3′ terminal exon) | Forward primer | tttcttggtccagcccaccat | 21 |
| Reverse primer | gcagggatgtagctgagacaat | 22 | |
| LEPR 3 NM_001003679.3 (leptin receptor isoform 3 precursor , contains an alternate 3′ terminal exon) | Forward primer | actgttgctttcggagtgagc | 21 |
| Reverse primer | agccagcactgtatgttcca | 20 | |
| LEPR 3.2 NM_001198689.2 (leptin receptor isoform 3 , precursor contains alternate 5′ UTR and 3′ terminal exon) | Forward primer | ttcttggtccagcccaccatt | 21 |
| Reverse primer | agcagggatgtagctgagacaa | 22 | |
| LEPR 4 NM_001198688.1 (leptin receptor isoform 4 precursor , contains alternate 5′ UTR and 3′ terminal exon) | Forward primer | ttcttggtccagcccaccatt | 21 |
| Reverse primer | agcagggatgtagctgagacaa | 22 |
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHD | Acquired heart disease |
| AV | Aortic valve |
| BMI | Body mass index |
| CHD | Coronary heart disease |
| CVDs | Cardiovascular diseases |
| EAT | Epicardial adipose tissue |
| FLI | Free leptin index |
| LEP | Leptin gene |
| LEPR | Leptin receptor gene |
| LR | Leptin resistance |
| MI | Myocardial infarction |
| PVAT | perivascular adipose tissue |
| SAT | Subcutaneous adipose tissue |
References
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010–2022. Am. J. Prev. Med. 2024, 66, 582–589. [Google Scholar] [CrossRef]
- de Azevedo Filho, A.F.; Accorsi, T.A.; Ribeiro, H.B. Coronary Artery Disease in Patients with Aortic Stenosis and Transcatheter Aortic Valve Implantation: Implications for Management. Eur. Cardiol. 2021, 16, e49. [Google Scholar] [CrossRef] [PubMed]
- Carità, P.; Coppola, G.; Novo, G.; Caccamo, G.; Guglielmo, M.; Balasus, F.; Novo, S.; Castrovinci, S.; Moscarelli, M.; Fattouch, K.; et al. Aortic stenosis: Insights on pathogenesis and clinical implications. J. Geriatr. Cardiol. 2016, 6, 489–498. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Vilariño-García, T.; Polonio-González, M.L.; Pérez-Pérez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.C.; Gimeno-Orna, J.A.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 2338. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2018, 8, 351–369. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Cheładze, P.; Martuszewski, A.; Poręba, R.; Gać, P. The Importance of the Assessment of Epicardial Adipose Tissue in Scientific Research. J. Clin. Med. 2022, 11, 5621. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Barateiro, A.; Mahú, I.; Domingos, A.I. Leptin Resistance and the Neuro-Adipose Connection. Front. Endocrinol. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, V.; Kaur, K.; ElSharief, M.W.; Al Hajaj, S.W.; Ebrahim, A.M.; Razack, M.; Dragas, D. The New Kid on the Block: The Mechanisms of Action of Hyperleptinemia in Coronary Artery Disease and Atherosclerosis. Cureus 2021, 13, e15766. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.; Shen, Y.; Lin, B.; Li, Y.; He, X.; Zhang, Y.; Lu, L.; Shen, W.; Zhang, Q.; et al. Association Between Serum Leptin Level and Calcific Aortic Valve Disease. J. Am. Heart Assoc. 2019, 8, e012495. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mal, K.; Razaq, M.K.; Magsi, M.; Memon, M.K.; Memon, S.; Afroz, M.N.; Siddiqui, H.F.; Rizwan, A. Association of Leptin With Obesity and Insulin Resistance. Cureus 2020, 12, e12178. [Google Scholar] [CrossRef]
- Dessie, G.; Ayelign, B.; Akalu, Y.; Shibabaw, T.; Molla, M.D. Effect of Leptin on Chronic Inflammatory Disorders: Insights to Therapeutic Target to Prevent Further Cardiovascular Complication. Diabetes Metab. Syndr. Obesity 2021, 14, 3307–3322. [Google Scholar] [CrossRef] [PubMed]
- Gruzdeva, O.V.; Akbasheva, O.E.; Dyleva, Y.A.; Antonova, L.V.; Matveeva, V.G.; Uchasova, E.G.; Fanaskova, E.V.; Karetnikova, V.N.; Ivanov, S.V.; Barbarash, O.L. Adipokine and Cytokine Profiles of Epicardial and Subcutaneous Adipose Tissue in Patients with Coronary Heart Disease. Bull. Exp. Biol. Med. 2017, 163, 608–611. [Google Scholar] [CrossRef]
- Sinitsky, M.Y.; Matveeva, V.G.; Asanov, M.A.; Ponasenko, A.V. Modifications in routine protocol of RNA isolation can improve quality of RNA purified from adipocytes. Anal. Biochem. 2018, 543, 128–131. [Google Scholar] [CrossRef]
- Mazahreh, T.S.; Alfaqih, M.; Saadeh, R.; Al-Zoubi, N.A.; Hatamleh, M.; Alqudah, A.; Aleshawi, A.J.; Alzoubi, A. The Effects of Laparoscopic Sleeve Gastrectomy on the Parameters of Leptin Resistance in Obesity. Biomolecules 2019, 9, 533. [Google Scholar] [CrossRef]
- Chen, M.C.; Wang, J.H.; Lee, C.J.; Hsu, B.G. Association between hyperleptinemia and cardiovascular outcomes in patients with coronary artery disease. Ther. Clin. Risk Manag. 2018, 14, 1855–1862. [Google Scholar] [CrossRef]
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE 2010, 5, e11376. [Google Scholar] [CrossRef]
- Ayeser, T.; Basak, M.; Arslan, K.; Sayan, I. Investigating the correlation of the number of diagnostic criteria to serum adiponectin, leptin, resistin, TNF-alpha, EGFR levels and abdominal adipose tissue. Diabetes Metab. Syndr. 2016, 10 (Suppl. S1), 165–169. [Google Scholar] [CrossRef]
- Inokuchi, J.I.; Inamori, K.I.; Kabayama, K.; Nagafuku, M.; Uemura, S.; Go, S.; Suzuki, A.; Ohno, I.; Kanoh, H.; Shishido, F. Biology of GM3 Ganglioside. Prog. Mol. Biol. Transl. Sci. 2018, 156, 151–195. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.B.; Silva, I.B.D.; Zampieri, T.T.; Totola, L.T.; Moreira, T.S.; Taniguti, A.P.T.; Diniz, G.P.; Barreto-Chaves, M.L.M.; Donato, J., Jr. SOCS3 Ablation in Leptin Receptor-Expressing Cells Causes Autonomic and Cardiac Dysfunctions in Middle-Aged Mice despite Improving Energy and Glucose Metabolism. Int. J. Mol. Sci. 2022, 23, 6484. [Google Scholar] [CrossRef]
- Liu, R.; Mathieu, C.; Berthelet, J.; Zhang, W.; Dupret, J.M.; Rodrigues Lima, F. Human Protein Tyrosine Phosphatase 1B (PTP1B): From Structure to Clinical Inhibitor Perspectives. Int. J. Mol. Sci. 2022, 23, 7027. [Google Scholar] [CrossRef]
- Mizia-Stec, K.; Bochenek, T.; Kusz, B.; Mizia-Szubryt, M.; Sikora-Puz, A.; Gieszczyk-Strózik, K. Severe degenerative aortic stenosis with preserved ejection fraction does not change adipokines serum levels. Cardiol. J. 2019, 26, 483–492. [Google Scholar] [CrossRef]
- Kolasa-Trela, R.; Miszalski-Jamka, T.; Grudzień, G.; Wypasek, E.; Kostkiewicz, M. Adiponectin, leptin, and resistin in patients with aortic stenosis without concomitant atherosclerotic vascular disease. Pol. Arch. Med. Wewn. 2011, 121, 352–359. [Google Scholar]
- Rosa, M.; Paris, C.; Sottejeau, Y.; Corseaux, D.; Robin, E.; Tagzirt, M.; Juthier, F.; Jashari, R.; Rauch, A.; Vincentelli, A.; et al. Leptin induces osteoblast differentiation of human valvular interstitial cells via the Akt and ERK pathways. Acta Diabetol. 2017, 6, 551–560. [Google Scholar] [CrossRef]
- Rosa, M.; Ahmed, E.; Anaïs, A.Y.M.; Tagzirt, M.; Roma, C.; Lorenzi, R.; Corseaux, D.; Zawadzki, C.; Juthier, F.; Vincentelli, A.; et al. Leptin and its receptor in human calcific aortic valve: Expression and functional implications. Arch. Cardiovasc. Dis. Suppl. 2014, 6, 72. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N. There and Back Again: Leptin Actions in White Adipose Tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Abu-Asab, M.; Glasow, A.; Päth, G.; Hauner, H.; Tsokos, M.; Chrousos, G.P.; Scherbaum, W.A. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells Iи8MИHфцкншщхэъn primary culture. Diabetes 2000, 49, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Guo, W.; Yang, Y.; Wu, J. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPβ transcription. J. Cell Biochem. 2011, 12, 488–497. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Salgado-Somoza, A.; Teijeira-Fernández, E.; Fernández, A.L.; González-Juanatey, J.R.; Eiras, S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H202–H209. [Google Scholar] [CrossRef]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 2014, 1842, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Yeung, J.; Tseung, E.; Karmazyn, M. Leptin-induced cardiomyocyte hypertrophy is associated with enhanced mitochondrial fission. Mol. Cell Biochem. 2019, 454, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.E.; Harmancey, R.; Stec, D.E. Lean heart: Role of leptin in cardiac hypertrophy and metabolism. World J. Cardiol. 2015, 7, 511–524. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, K.; Li, P.; Sheng, Y. The emerging role of leptin in obesity-associated cardiac fibrosis: Evidence and mechanism. Mol. Cell Biochem. 2023, 478, 991–1011. [Google Scholar] [CrossRef]
- Kim, J.I.; Huh, J.Y.; Sohn, J.H.; Choe, S.S.; Lee, Y.S.; Lim, C.Y.; Jo, A.; Park, S.B.; Han, W.; Kim, J.B. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol. Cell Biol. 2015, 35, 1686–1699. [Google Scholar] [CrossRef]
- Huan, J.N.; Li, J.; Han, Y.; Chen, K.; Wu, N.; Zhao, A.Z. Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J. Biol. Chem. 2003, 278, 45638–45650. [Google Scholar] [CrossRef] [PubMed]
- Tsirigotaki, A.; Dansercoer, A.; Verschueren, K.H.G.; Marković, I.; Pollmann, C.; Hafer, M.; Felix, J.; Birck, C.; Van Putte, W.; Catteeuw, D.; et al. Mechanism of receptor assembly via the pleiotropic adipokine Leptin. Nat. Struct. Mol. Biol. 2023, 30, 551–563. [Google Scholar] [CrossRef]
- Polyakova, E.A.; Kolodina, D.A.; Miroshnikova, V.V.; Razgildina, N.D.; Bogdanova, E.O.; Lyapina, E.N.; Belyaeva, O.D.; Pchelina, S.N.; Berkovich, O.A.; Baranova, E.I. Subcutaneous and Epicardial Adipose Tissue Leptin Gene Expression in Coronary Artery Disease Patient. Transl. Med. 2019, 6, 25–35. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, P.; Li, T.; Gao, J.; Zhang, Y. Leptin Expression in Human Epicardial Adipose Tissue Is Associated with Local Coronary Atherosclerosis. Med. Sci. Monit. 2019, 25, 9913–9922. [Google Scholar] [CrossRef]
- Baker, A.R.; Silva, N.F.; Quinn, D.W.; Harte, A.L.; Pagano, D.; Bonser, R.S.; Kumar, S.; McTernan, P.G. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 2006, 5, 1. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Konja, D.; Wang, Y. Adipose tissue secretory profile and cardiometabolic risk in obesity. Endocr. Metab. Sci. 2020, 1, 100061. [Google Scholar] [CrossRef]
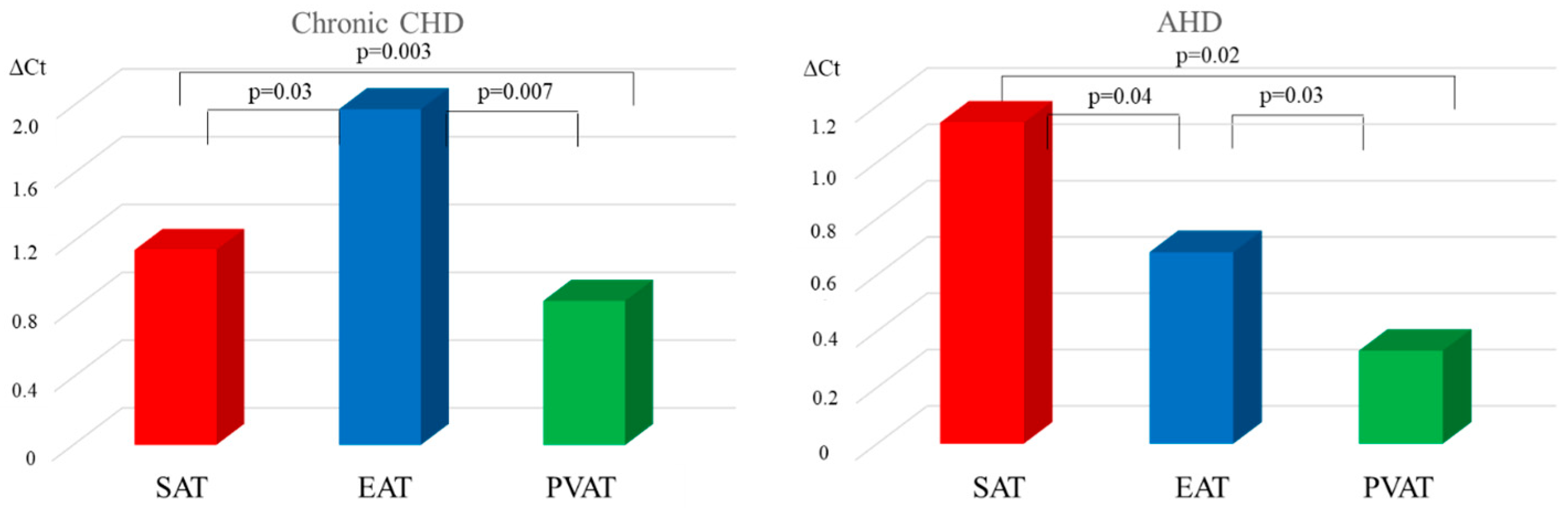
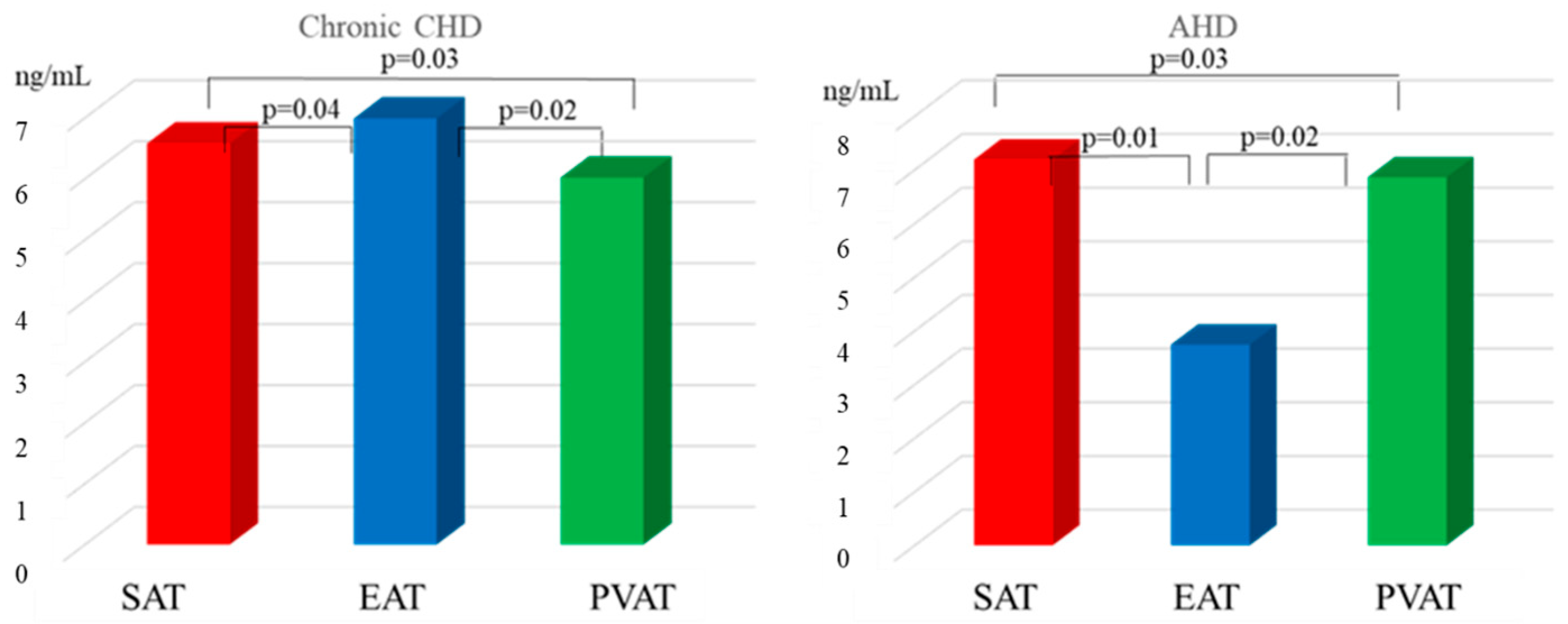
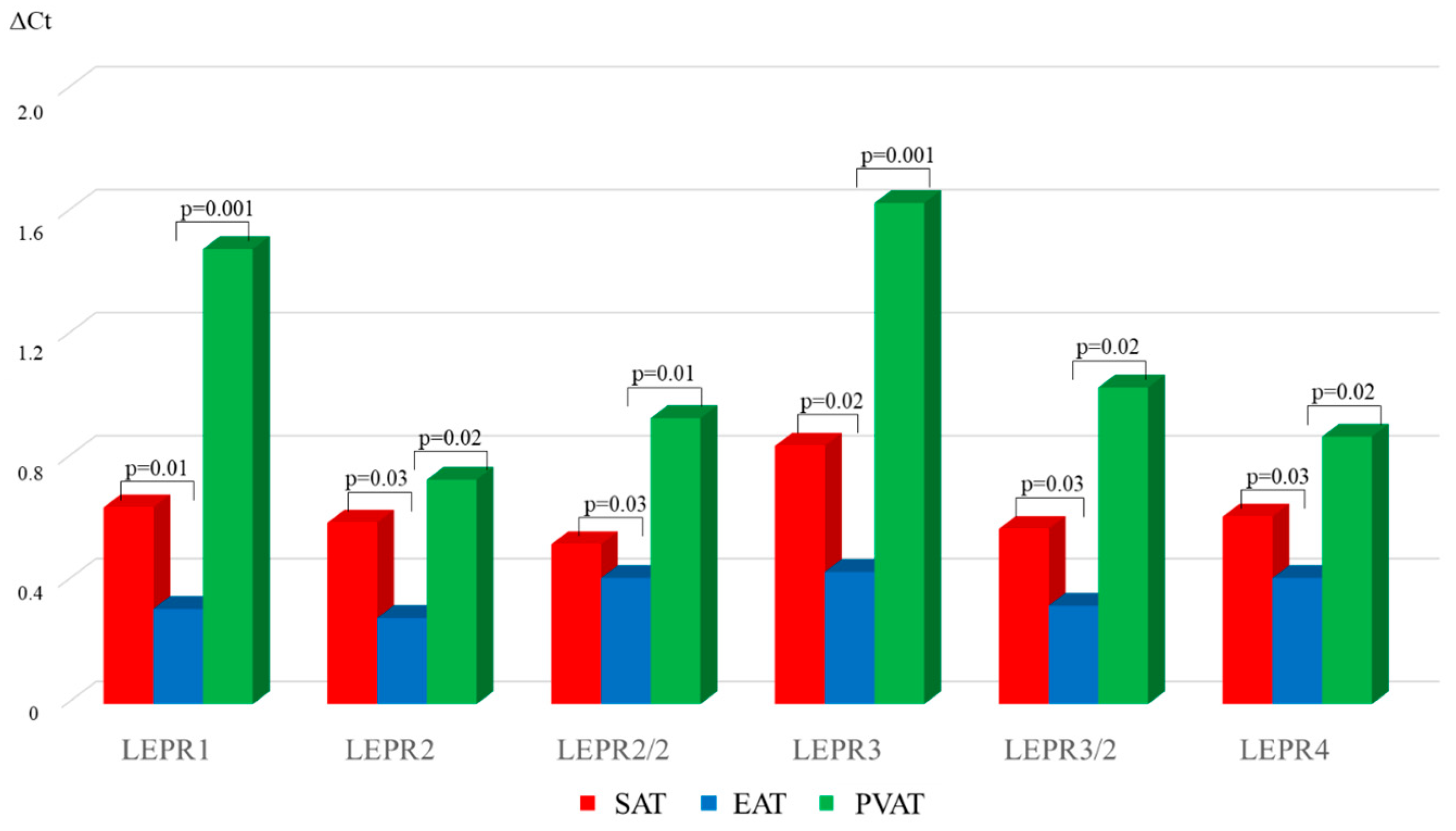
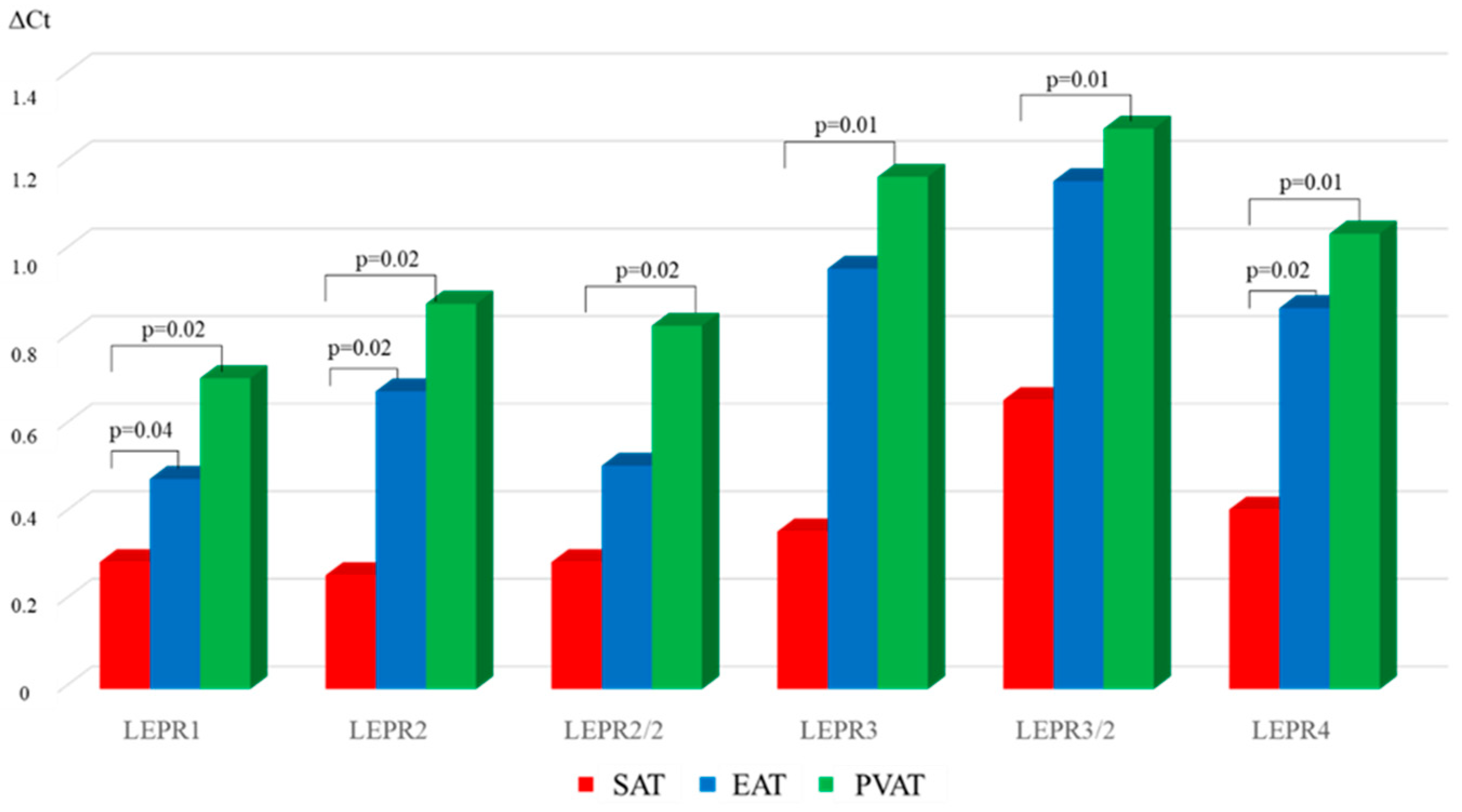
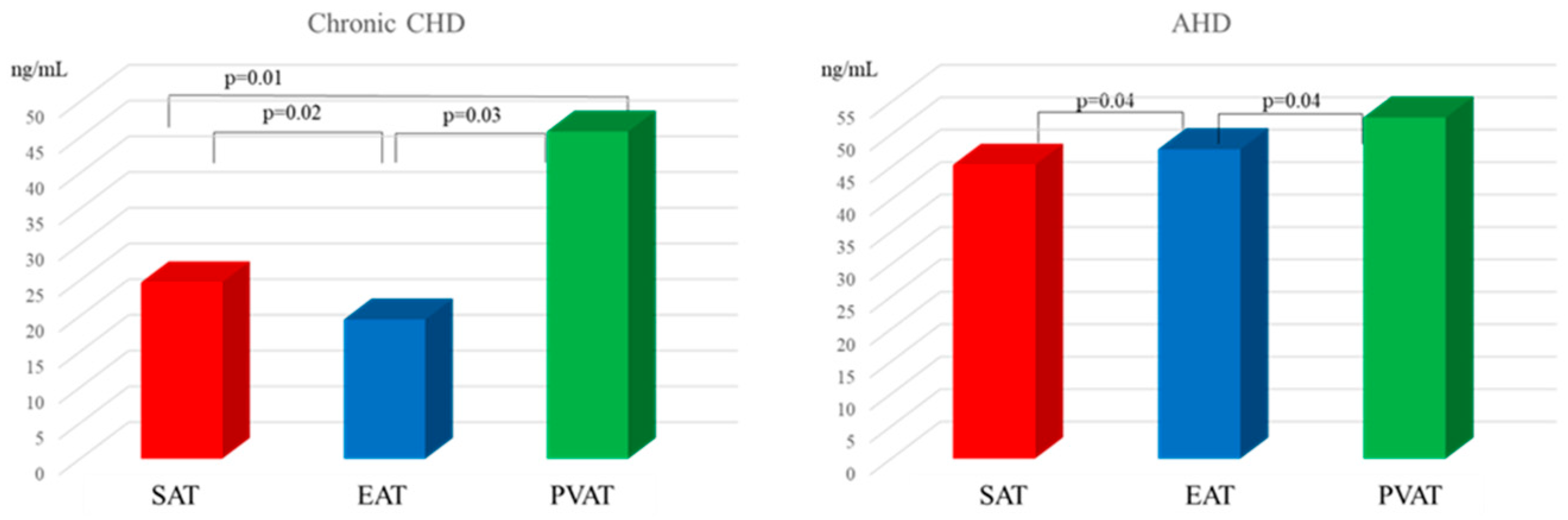

| Parameter | MI n = 108 | Chronic CHD n = 96 | AHD n = 96 | Control Group n = 40 |
|---|---|---|---|---|
| Male gender, n (%) | 108 (100) | 96 (100) | 96 (100) | 40 (100) |
| Age, years | 61 | 64 | 65 | 58 |
| (57.0; 71.0) | (58.0; 69.0) | (55.0; 72.0) | (45.0; 66.0) | |
| Anamnesis | ||||
| Family history of CAD, n (%) | 49 (45.4) | 44 (45.8) | 31 (32.0) | 0 |
| Smoking, n (%) | 58 (53.7) | 78 (81.3) | 80 (83.3) | 0 |
| Arterial hypertension, n (%) | 97 (89.8) | 84 (87.5) | 64 (66.7) | 0 |
| Hypercholesterolemia, n (%) | 15 (13.9) | 12 (12.5) | 16 (16.7) | 0 |
| History of MI, n (%) | 33 (30.5) | 39 (40.6) | 8 (8.3) | 0 |
| Body mass index, kg/m2 | ||||
| <25, n (%) | 53 (49.1) | 45 (46.9) | 48 (50.0) | 40 (100) |
| 25.0–29.9, n (%) | 45 (41.6) | 42 (43.7) | 40 (41.7) | 0 |
| 30.0–39.9, n (%) | 10 (9.3) | 9 (9.4) | 8 (8.3) | 0 |
| Coronary artery disease | ||||
| Atherosclerosis of the 1st coronary artery, n (%) | 50 (46.3) | 9 (9.4) | 0 | 0 |
| Atherosclerosis of the 2nd coronary artery, n (%) | 36 (33.3) | 21 (21.8) | 0 | 0 |
| Atherosclerosis of three or more coronary artery, n (%) | 22 (20.4) | 66 (68.8) | 0 | 0 |
| Ejection fraction, % | ||||
| >50, n (%) | 76 (70.4) | 90 (93.8) | 88 (91.7) | 40 (100) |
| 40–49, n (%) | 28 (25.9) | 3 (3.1) | 8 (8.3) | 0 |
| <40, n (%) | 4 (3.7) | 3 (3.1) | 0 | 0 |
| Treatment strategy/group of drugs | ||||
| Aspirin, n (%) | 112 (98.2) | 114 (95.0) | 0 | 0 |
| Clopidogrel, n (%) | 114 (100) | 18 (15.0) | 0 | 0 |
| Warfarin, n (%) | 0 | 0 | 80 (83.3) | 0 |
| Heparin, n (%) | 114 (100) | 120 (100) | 0 | 0 |
| β-blockers, n (%) | 114 (100) | 108 (90) | 86 (89.6) | 0 |
| ACE inhibitors, n (%) | 102 (89.4) | 90 (75) | 74 (77.1) | 0 |
| Statins, n (%) | 114 (100) | 120 (100) | 70 (72.9) | 0 |
| Calcium channel Blocker, n (%) | 101 (88.6) | 90 (75) | 70 (72.9) | 0 |
| Nitrates, n (%) | 20 (17.5) | 6 (5.0) | 10 (10.4) | 0 |
| Diuretics, n (%) | 36 (31.6) | 96 (80.0) | 82 (85.4) | 0 |
| Variable | MI | Chronic CHD | AHD | Control Group | p |
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Leptin, ng/mL | 11.31 [6.8; 22.6] | 16.37 [7.0; 20.5] | 3.54 [3.3; 9.1] | 3.2 [2.7; 5.6] | p (1–2) = 0.4 |
| p (1–3) = 0.001 | |||||
| p (1–4) = 0.001 | |||||
| p (2–3) = 0.001 | |||||
| p (2–4) = 0.001 | |||||
| p (3–4) = 0.89 | |||||
| Leptin receptor, ng/mL | 40.49 [29.3; 46.1] | 34.82 [27.3; 47.8] | 57.06 [41.6; 65.7] | 58.06 [45.6; 67.7] | p (1–2) = 0.68 |
| p (1–3) = 0.003 | |||||
| p (1–4) = 0.003 | |||||
| p (2–3) = 0.001 | |||||
| p (2–4) = 0.001 | |||||
| p (3–4) = 0.91 | |||||
| FLI | 31.91 [12.9; 65.5] | 39.08 [19.1; 83.6] | 6.04 [5.1; 22.4] | 5.05 [4.2; 25.0] | p (1–2) = 0.3 |
| p (1–3) = 0.004 | |||||
| p (1–4) = 0.003 | |||||
| p (2–3) = 0.001 | |||||
| p (2–4) = 0.001 | |||||
| p (3–4) = 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruzdeva, O.; Gorbatovskaya, E.; Dyleva, Y.; Dolmatova, S.; Romanova, A.; Fanaskova, E.; Tarasov, R.; Stasev, A.; Barbarash, O. Systemic and Local Leptin Resistance in Patients with Cardiovascular Diseases. Diagnostics 2025, 15, 2772. https://doi.org/10.3390/diagnostics15212772
Gruzdeva O, Gorbatovskaya E, Dyleva Y, Dolmatova S, Romanova A, Fanaskova E, Tarasov R, Stasev A, Barbarash O. Systemic and Local Leptin Resistance in Patients with Cardiovascular Diseases. Diagnostics. 2025; 15(21):2772. https://doi.org/10.3390/diagnostics15212772
Chicago/Turabian StyleGruzdeva, Olga, Evgeniya Gorbatovskaya, Yulia Dyleva, Sofya Dolmatova, Anastasiya Romanova, Elena Fanaskova, Roman Tarasov, Aleksandr Stasev, and Olga Barbarash. 2025. "Systemic and Local Leptin Resistance in Patients with Cardiovascular Diseases" Diagnostics 15, no. 21: 2772. https://doi.org/10.3390/diagnostics15212772
APA StyleGruzdeva, O., Gorbatovskaya, E., Dyleva, Y., Dolmatova, S., Romanova, A., Fanaskova, E., Tarasov, R., Stasev, A., & Barbarash, O. (2025). Systemic and Local Leptin Resistance in Patients with Cardiovascular Diseases. Diagnostics, 15(21), 2772. https://doi.org/10.3390/diagnostics15212772





