Analysis of the Reasons for Poor Prognosis in Severe to Profound Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Criteria for Study Selection
- (1)
- Studies provide relevant data on patients with severe to profound SSNHL;
- (2)
- Prospective or retrospective studies comparing the prognosis of SSNHL;
- (3)
- Studies with clearly defined efficacy evaluation criteria;
- (4)
- Studies provide or allow the calculation of odds ratios (ORs) and 95% confidence intervals (CIs).
- (1)
- Studies with inaccessible full text despite retrieval attempts;
- (2)
- Studies with small sample sizes (<30 cases) or low quality (NOS < 5), as small-sample studies tend to produce unstable or biased effect estimates [14];
- (3)
- Basic research or animal experiments.;
- (4)
- Reviews, case reports, or conference abstracts.
2.3. Data Extraction
2.4. Hearing Loss and Prognostic Criteria
2.5. Quality Assessment
2.6. Data Analysis
3. Results
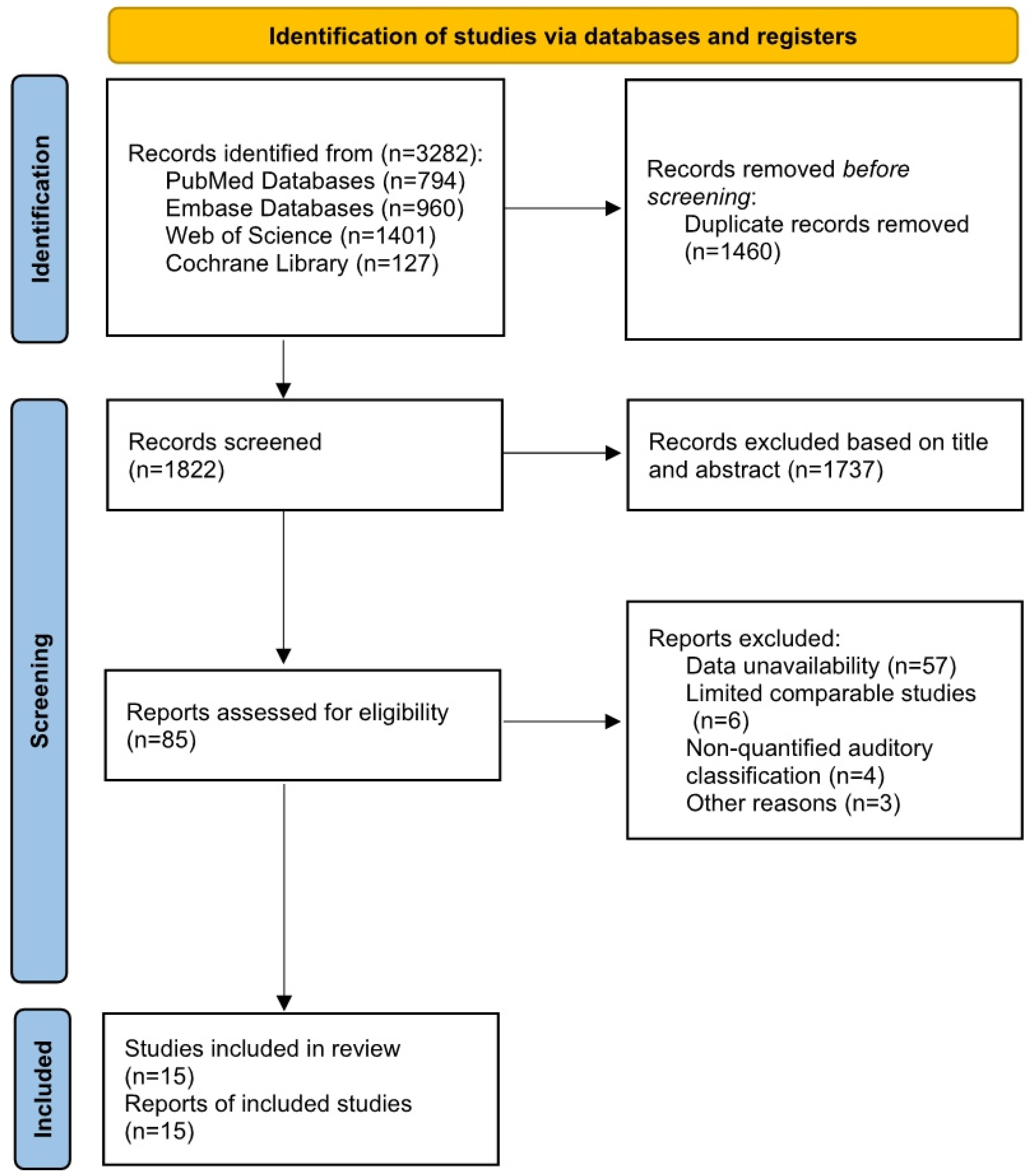
3.1. Literature Search
3.2. Included Study Characteristics
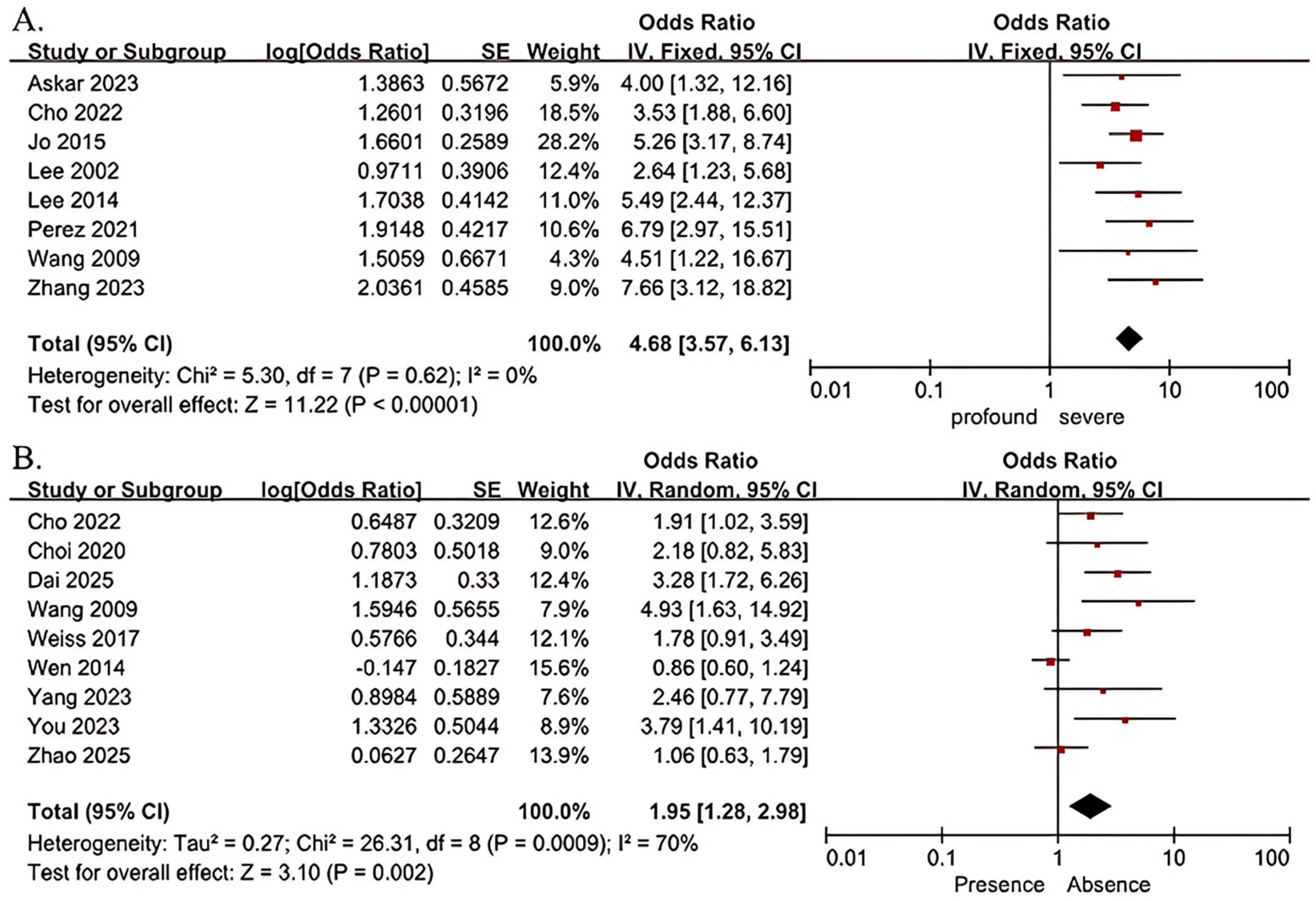
3.3. Meta-Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuhn, M.; Heman-Ackah, S.E.; Shaikh, J.A.; Roehm, P.C. Sudden sensorineural hearing loss: A review of diagnosis, treatment, and prognosis. Trends Amplif. 2011, 15, 91–105. [Google Scholar] [CrossRef]
- Alexander, T.H.; Harris, J.P. Incidence of sudden sensorineural hearing loss. Otol. Neurotol. 2013, 34, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Olzowy, B.; Osterkorn, D.; Suckfüll, M. The incidence of sudden hearing loss is greater than previously assumed. MMW-Fortschritte Der Med. 2005, 147, 37–38. [Google Scholar]
- Klemm, E.; Deutscher, A.; Mösges, R. A present investigation of the epidemiology in idiopathic sudden sensorineural hearing loss. Laryngo-Rhino-Otologie 2009, 88, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, M.; Katayama, N.; Uchida, Y.; Tominaga, M.; Nakashima, T. Thirty-year trends in sudden deafness from four nationwide epidemiological surveys in Japan. Acta Oto-Laryngol. 2007, 127, 1259–1265. [Google Scholar] [CrossRef]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol.–Head Neck Surg. 2019, 161, S1–S45. [Google Scholar] [CrossRef]
- Mandavia, R.; Joshi, N.; Hannink, G.; Ahmed, M.N.; Parmar, D.; Di Bonaventura, S.; Gomes, P.; Iqbal, I.; Lyles, J.; Schilder, A.G.M.; et al. A Prognostic Model to Predict Hearing Recovery in Patients with Idiopathic Sudden Onset Sensorineural Hearing Loss. JAMA Otolaryngol.–Head Neck Surg. 2024, 150, 896–906. [Google Scholar] [CrossRef]
- Cvorović, L.; Deric, D.; Probst, R.; Hegemann, S. Prognostic model for predicting hearing recovery in idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 2008, 29, 464–469. [Google Scholar] [CrossRef]
- Byl, F.M., Jr. Sudden hearing loss: Eight years’ experience and suggested prognostic table. Laryngoscope 1984, 94, 647–661. [Google Scholar] [CrossRef]
- Carlsson, P.-I.; Hjaldahl, J.; Magnuson, A.; Ternevall, E.; Edén, M.; Skagerstrand, Å.; Jönsson, R. Severe to profound hearing impairment: Quality of life, psychosocial consequences and audiological rehabilitation. Disabil. Rehabil. 2015, 37, 1849–1856. [Google Scholar] [CrossRef]
- Lin, R.J.; Krall, R.; Westerberg, B.D.; Chadha, N.K.; Chau, J.K. Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope 2012, 122, 624–635. [Google Scholar] [CrossRef]
- Bhat, A.M.; Nanu, D.P.; Nguyen, S.A.; Meyer, T.A.; Labadie, R.F. Prognosis of Bilateral Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. Laryngoscope 2024, 134, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Shen, C. Avoiding Definitive Conclusions in Meta-analysis of Heterogeneous Studies with Small Sample Sizes. JAMA Otolaryngol.–Head Neck Surg. 2022, 148, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Siegel, L.G. The Treatment of Idiopathic Sudden Sensorineural Hearing Loss. Otolaryngol. Clin. N. Am. 1975, 8, 467–473. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; Mcarthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Askar, A.A.; Ghonim, M.R.; Shabana, Y.K. Discriminant Analysis of the Prognostic Factors for Hearing Outcomes in Patients with Idiopathic Sudden Sensorineural Hearing Loss. J. Int. Adv. Otol. 2023, 19, 162–168. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, J.; Oh, S.J.; Kong, S.K.; Choi, S.W. Clinical features and prognosis of severe-to-profound sudden sensorineural hearing loss. Am. J. Otolaryngol. 2022, 43, 103455. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, C.K.; Bin Kim, S.; Lee, D.Y.; Ko, S.C.; Park, K.H.; Choi, S.J. Potential benefits of salvage intratympanic dexamethasone injection in profound idiopathic sudden sensorineural hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2219–2227. [Google Scholar] [CrossRef]
- Jo, S.Y.; Lee, S.; Eom, T.H.; Jeun, E.S.; Cho, H.H.; Cho, Y.B. Outcomes of Severe to Profound Idiopathic Sudden Sensorineural Hearing Loss. Clin. Exp. Otorhinolaryngol. 2015, 8, 206–210. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, Y.J.; Kang, B.S.; Lee, B.D.; Lee, J.S. A clinical analysis of sudden sensorineural hearing loss cases. Korean J. Audiol. 2014, 18, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Perez Ferreira Neto, A.; Da Costa Monsanto, R.; Dore Saint Jean, L.; Sonzzini Ribeiro De Souza, L.; De Oliveira Penido, N. Clinical Profile of Patients with Unilateral Sudden Sensorineural Hearing Loss: Correlation with Hearing Prognosis. Otolaryngol.–Head Neck Surg. 2021, 165, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Huang, T.W.; Kuo, S.W.; Cheng, P.W. Correlation between audiovestibular function tests and hearing outcomes in severe to profound sudden sensorineural hearing loss. Ear Hear. 2009, 30, 110–114. [Google Scholar] [CrossRef]
- Weiss, D.; Böcker, A.J.; Koopmann, M.; Savvas, E.; Borowski, M.; Rudack, C. Predictors of hearing recovery in patients with severe sudden sensorineural hearing loss. J. Otolaryngol.-Head Neck Surg. 2017, 46, 27. [Google Scholar] [CrossRef]
- Wen, Y.H.; Chen, P.R.; Wu, H.P. Prognostic factors of profound idiopathic sudden sensorineural hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 1423–1429. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, D.; Ma, X.; Shen, J.; Chen, X.; Zhang, Q.; Jin, Y.; Chen, J.; Duan, M.; Yang, J. Abnormal posterior semicircular canal function may predict poor prognosis in patients with severe and profound ISSNHL. Front. Neurol. 2023, 14, 1123165. [Google Scholar] [CrossRef] [PubMed]
- You, D.; You, Q.; Liu, Y. Analysis of Clinical Prognosis of Totally Deaf Idiopathic Sudden Sensorineural Hearing Loss in Different Ages. Altern. Ther. Health Med. 2023, 29, 506–511. [Google Scholar]
- Zhang, S.; Li, P.; Fan, F.; Zheng, Y.; Chen, X.; Chen, Y.; Cui, X. Nomogram for predicting the prognosis of sudden sensorineural hearing loss patients based on clinical characteristics: A retrospective cohort study. Ann. Transl. Med. 2023, 11, 104. [Google Scholar] [CrossRef]
- Lee, J.K.; Seo, D.J.; Cho, H.H.; Cho, Y.; Kim, H.J.; Cho, Y.B. A Study on the Hearing Recovery Patterns in Sudden Sensorineural Hearing Loss Patients. Korean J. Otorhinolaryngol.-Head Neck Surg. 2002, 45, 656–661. [Google Scholar]
- Dai, Q.L.; Xiong, W.P.; Wang, Y.J.; Hu, N.; Sun, X.; Fan, Z.M.; Wang, H.B.; Wang, M.M. Application of 3D-Flair MRI and vestibular function assessment in profound sudden sensorineural hearing loss patients. Chin. J. Otorhinolaryngol. Head Neck Surg. 2025, 60, 2–9. [Google Scholar]
- Zhao, K.; Ma, H. The prognostic value of systemic immune-inflammation index and lymphocyte-to-monocyte ratio in cases with profound sudden sensorineural hearing loss. Am. J. Otolaryngol. 2025, 46, 104671. [Google Scholar] [CrossRef] [PubMed]
- Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guideline of diagnosis and treatment of sudden deafness. Chin. J. Otorhinolaryngol. Head Neck Surg. 2015, 50, 443–447. [Google Scholar]
- Ceylan, A.; Celenk, F.; Kemaloğlu, Y.K.; Bayazit, Y.A.; Göksu, N.; Ozbilen, S. Impact of prognostic factors on recovery from sudden hearing loss. J. Laryngol. Otol. 2007, 121, 1035–1040. [Google Scholar] [CrossRef]
- Russolo, M.; Poli, P. Acute idiopathic auditory failure: Prognosis: A review of 65 cases. Audiology 1980, 19, 422–433. [Google Scholar] [CrossRef]
- Liebau, A.; Pogorzelski, O.; Salt, A.N.; Plontke, S.K. Hearing Changes After Intratympanically Applied Steroids for Primary Therapy of Sudden Hearing Loss: A Meta-analysis Using Mathematical Simulations of Drug Delivery Protocols. Otol. Neurotol. 2017, 38, 19–30. [Google Scholar] [CrossRef]
- Nakashima, T.; Sato, H.; Gyo, K.; Hato, N.; Yoshida, T.; Shimono, M.; Teranishi, M.; Sone, M.; Fukunaga, Y.; Kobashi, G.; et al. Idiopathic sudden sensorineural hearing loss in Japan. Acta Oto-Laryngol. 2014, 134, 1158–1163. [Google Scholar] [CrossRef]
- World Health Organization. Programme for the Prevention of Deafness and Hearing Impairment. In Proceedings of the Future Programme Developments for Prevention of Deafness and Hearing Impairments: Report of the First Informal Consultation, Geneva, Switzerland, 23–24 January 1997; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Bogaz, E.A.; Maranhão, A.S.; Inoue, D.P.; Suzuki, F.A.; Penido Nde, O. Variables with prognostic value in the onset of idiopathic sudden sensorineural hearing loss. Braz. J. Otorhinolaryngol. 2015, 81, 520–526. [Google Scholar] [CrossRef]
- Tiong, T.S. Prognostic indicators of management of sudden sensorineural hearing loss in an Asian hospital. Singap. Med. J. 2007, 48, 45–49. [Google Scholar]
- Kim, J.; Jeong, J.; Ha, R.; Sunwoo, W. Heparin therapy as adjuvant treatment for profound idiopathic sudden sensorineural hearing loss. Laryngoscope 2020, 130, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, R.-H.; Liu, B.; Leng, Y.-M.; Liu, J.-J.; Liu, D.-D.; Zhang, S.-L.; Kong, W.-J. Assessment of balance and vestibular functions in patients with idiopathic sudden sensorineural hearing loss. J. Huazhong Univ. Sci. Technol. 2017, 37, 264–270. [Google Scholar] [CrossRef]
- Kitoh, R.; Nishio, S.-Y.; Ogawa, K.; Kanzaki, S.; Hato, N.; Sone, M.; Fukuda, S.; Hara, A.; Ikezono, T.; Ishikawa, K.; et al. Nationwide epidemiological survey of idiopathic sudden sensorineural hearing loss in Japan. Acta Oto-Laryngol. 2017, 137, S8–S16. [Google Scholar] [CrossRef]
- Niu, X.; Zhang, Y.; Zhang, Q.; Xu, X.; Han, P.; Cheng, Y.; Gao, Y.; Zhang, R.; Yang, Y.; Chen, Z.; et al. The relationship between hearing loss and vestibular dysfunction in patients with sudden sensorineural hearing loss. Acta Oto-Laryngol. 2016, 136, 225–231. [Google Scholar] [CrossRef]
- Yu, H.; Li, H. Association of Vertigo with Hearing Outcomes in Patients with Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-analysis. AMA Otolaryngol.–Head Neck Surg. 2018, 144, 677–683. [Google Scholar] [CrossRef]
- Zou, T.; Xu, J.; Lu, H.; Yu, M. The Relationship between the Characteristics of Tinnitus and the Hearing Curative Effect of Sudden Deafness. Audiol. Neurotol. 2023, 28, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Danino, J.; Joachims, H.Z.; Eliachar, I.; Podoshin, L.; Ben-David, Y.; Fradis, M. Tinnitus as a prognostic factor in sudden deafness. Am. J. Otolaryngol. 1984, 5, 394–396. [Google Scholar] [CrossRef]
- Mamak, A.; Yilmaz, S.; Cansiz, H.; Inci, E.; Güçlü, E.; Dereköylü, L. A study of prognostic factors in sudden hearing loss. Ear Nose Throat J. 2005, 84, 641–644. [Google Scholar] [CrossRef]
- Xiao, L.; Su, S.; Liang, J.; Jiang, Y.; Shu, Y.; Yao, H.; Ding, L. Clinical features and prognostic factors of children with profound sudden sensorineural hearing loss. Front. Pediatr. 2022, 10, 1023781. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, J.J.; Sunwoo, W.S.; Koo, J.-W.; Oh, S.-H.; Park, M.-H.; Kim, Y.H. Sudden sensorineural hearing loss in children and adolescents: Clinical characteristics and age-related prognosis. Auris Nasus Larynx 2018, 45, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Huy, P.T.; Sauvaget, E. Idiopathic sudden sensorineural hearing loss is not an otologic emergency. Otol. Neurotol. 2005, 26, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, E.; Morawski, K.; Telischi, F.F.; Özdamar, Ö.; Delgado, R.E.; Manns, F.; Parel, J.-M. Simultaneous measurement of electrocochleography and cochlear blood flow during cochlear hypoxia in rabbits. J. Neurosci. Methods 2005, 147, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Raphael, Y.; Kim, Y.H.; Osumi, Y.; Izumikawa, M. Non-sensory cells in the deafened organ of Corti: Approaches for repair. Int. J. Dev. Biol. 2007, 51, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Zand, V.; Dadgarnia, M.; Baradaranfar, M.; Meybodian, M.; Vaziribozorg, S.; Fazilati, M. The association between metabolic syndrome and the prognosis of idiopathic sudden sensorineural hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Chung, J.H.; Byun, H.; Jeong, J.H.; Lee, S.H. Effect of Diabetes on the Prognosis of Sudden Sensorineural Hearing Loss: Propensity Score Matching Analysis. Otolaryngol.–Head Neck Surg. 2020, 162, 346–352. [Google Scholar] [CrossRef]

| Type | Hearing Recovery |
|---|---|
| Complete recovery (CR) | Final hearing threshold < 25 dB HL |
| Partial recovery (PR) | Hearing gain ≥ 15 dB HL and final threshold 25–45 dB HL |
| Slight Improvement (SI) | Hearing gain ≥ 15 dB HL and final threshold > 45 dB HL |
| No improvement (NI) | Hearing gain < 15 dB HL and final threshold > 75 dB HL |
| Study | Country | Design | Severe (n) | Profound (n) | Treatment | Hearing Improvement Criteria | Prognostic Factor |
|---|---|---|---|---|---|---|---|
| Askar (2023) [18] | Egypt | CS | 45 | 22 | ITS | Siegel’s criteria (≥15 dB HL hearing gain) | Severity of hearing loss |
| Cho (2022) [19] | Korea | CS | 84 | 94 | OS | Siegel’s criteria | Severity of hearing loss, Vertigo, Delayed treatment |
| Choi (2020) [20] | Korea | RC | - | 103 | SST/SST + ITS, salvage ITS | Siegel’s criteria (≥15 dB HL hearing gain and a final hearing better than 45 dB HL) | Gender, Vertigo, Hypertension, Diabetes |
| Jo (2015) [21] | Korea | CS | 77 | 225 | IM, P, MS, PE1, C, ITS | Siegel’s criteria | Severity of hearing loss |
| Lee (2014) [22] | Korea | CS | 66 | 54 | IM, OS, GFG, SGB | Siegel’s criteria (≥15 dB HL hearing gain) | Severity of hearing loss |
| Perez (2021) [23] | Brazil | RC | 48 | 71 | OS | AAO-HNSF (≥10 dB HL improvement in PTA, PTA ≤ 50 dB HL and WRS ≥ 50%) | Severity of hearing loss |
| Wang (2009) [24] | China | RC | 88 (Specific subgroup numbers not provided) | OS, RR | Complete recovery (hearing improvement > 30 dB HL and side difference ≤ 10 dB HL) marked recovery (hearing improvement > 30 dB HL) mild recovery (10 dB HL < hearing improvement ≤ 30 dB HL) no recovery (hearing improvement ≤ 10 dB HL) | Gender, Hypertension, Diabetes, Vertigo, Tinnitus, Age | |
| Weiss (2017) [25] | Germany | RC | 198 | - | PE, LS, HS | Siegel’s criteria (≥15 dB HL hearing gain) | Gender, Vertigo, Tinnitus |
| Wen (2014) [26] | China | RC | - | 576 | OS, salvage ITS | Siegel’s criteria (≥15 dB HL hearing gain) | Gender, Hypertension, Diabetes, Vertigo, Tinnitus |
| Yang (2023) [27] | China | CS | 14 | 35 | IV, ITS, HBO | PTA improvement > 30 dB HL | Gender, Vertigo, Delayed treatment |
| You (2023) [28] | China | CS | - | 75 | OS/IV, I, EP, CP, HBO | PTA improvement > 30 dB HL | Age, Vertigo |
| Zhang (2023) [29] | China | RC | 61 | 60 | SST/LST, M, GBE, B, HBO | Siegel’s criteria (≥15 dB HL hearing gain) | Severity of hearing loss |
| Lee (2002) [30] | Korea | CS | 50 | 75 | IP | Siegel’s criteria (≥15 dB HL hearing gain) | Severity of hearing loss |
| Dai (2025) [31] | China | RC | - | 191 | IV, ITS, B, GBE, M | Chinese Medical Association of Otolaryngology criteria | Vertigo |
| Zhao (2025) [32] | China | RC | - | 252 | Not reported | AAO-HNSF (PTA ≤ 50 dB HL and WRS ≤ 50%) | Gender, Hypertension, Diabetes, Tinnitus, Vertigo |
| Risk Factor | Studies | Sample Size | Heterogeneity | Meta-Analysis Model | OR (95% CI) | Z | p | |
|---|---|---|---|---|---|---|---|---|
| I2 (%) | Ph | |||||||
| Severity of hearing loss (profound vs. severe) | 8 | 1120 | 0 | 0.62 | Fixed-effect model | 4.68 (3.57, 6.13) | 11.22 | <0.001 |
| Gender (male vs. female) | 6 | 1266 | 1 | 0.41 | Fixed-effect model | 0.80 (0.63,1.02) | 1.78 | 0.08 |
| Age (>60 vs. ≤60 years) | 2 | 163 | 0 | 0.42 | Fixed-effect model | 0.94 (0.42, 2.11) | 0.15 | 0.88 |
| Tinnitus (presence vs. absence) | 3 | 838 | 0 | 0.66 | Fixed-effect model | 0.84 (0.61, 1.15) | 1.08 | 0.28 |
| Delayed treatment (>7 vs. ≤7 days) | 2 | 191 | 0 | 0.9 | Fixed-effect model | 1.08 (0.26, 4.51) | 0.11 | 0.91 |
| Vertigo (presence vs. absence) | 9 | 1014 | 70 | 0.0009 | Random-effects model | 1.95 (1.28, 2.98) | 3.10 | 0.002 |
| Hypertension (presence vs. absence) | 4 | 434 | 67 | 0.03 | Random-effects model | 1.02 (0.49, 2.14) | 0.06 | 0.95 |
| Diabetes (presence vs. absence) | 4 | 452 | 55 | 0.09 | Random-effects model | 1.15 (0.62, 2.11) | 0.44 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Qiu, J.; Zhang, Q.; Xi, Z.; Wu, Q.; Xu, M.; Zhang, Q.; Jin, Y.; Yang, J.; Duan, M.; et al. Analysis of the Reasons for Poor Prognosis in Severe to Profound Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 2770. https://doi.org/10.3390/diagnostics15212770
Chen L, Qiu J, Zhang Q, Xi Z, Wu Q, Xu M, Zhang Q, Jin Y, Yang J, Duan M, et al. Analysis of the Reasons for Poor Prognosis in Severe to Profound Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(21):2770. https://doi.org/10.3390/diagnostics15212770
Chicago/Turabian StyleChen, Linrui, Jianhui Qiu, Qianqian Zhang, Zian Xi, Qiong Wu, Mingwei Xu, Qin Zhang, Yulian Jin, Jun Yang, Maoli Duan, and et al. 2025. "Analysis of the Reasons for Poor Prognosis in Severe to Profound Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 21: 2770. https://doi.org/10.3390/diagnostics15212770
APA StyleChen, L., Qiu, J., Zhang, Q., Xi, Z., Wu, Q., Xu, M., Zhang, Q., Jin, Y., Yang, J., Duan, M., Zhang, Q., & Zhang, Z. (2025). Analysis of the Reasons for Poor Prognosis in Severe to Profound Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. Diagnostics, 15(21), 2770. https://doi.org/10.3390/diagnostics15212770






