Body Composition Metrics Associated with Time to Progression in Smoldering Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
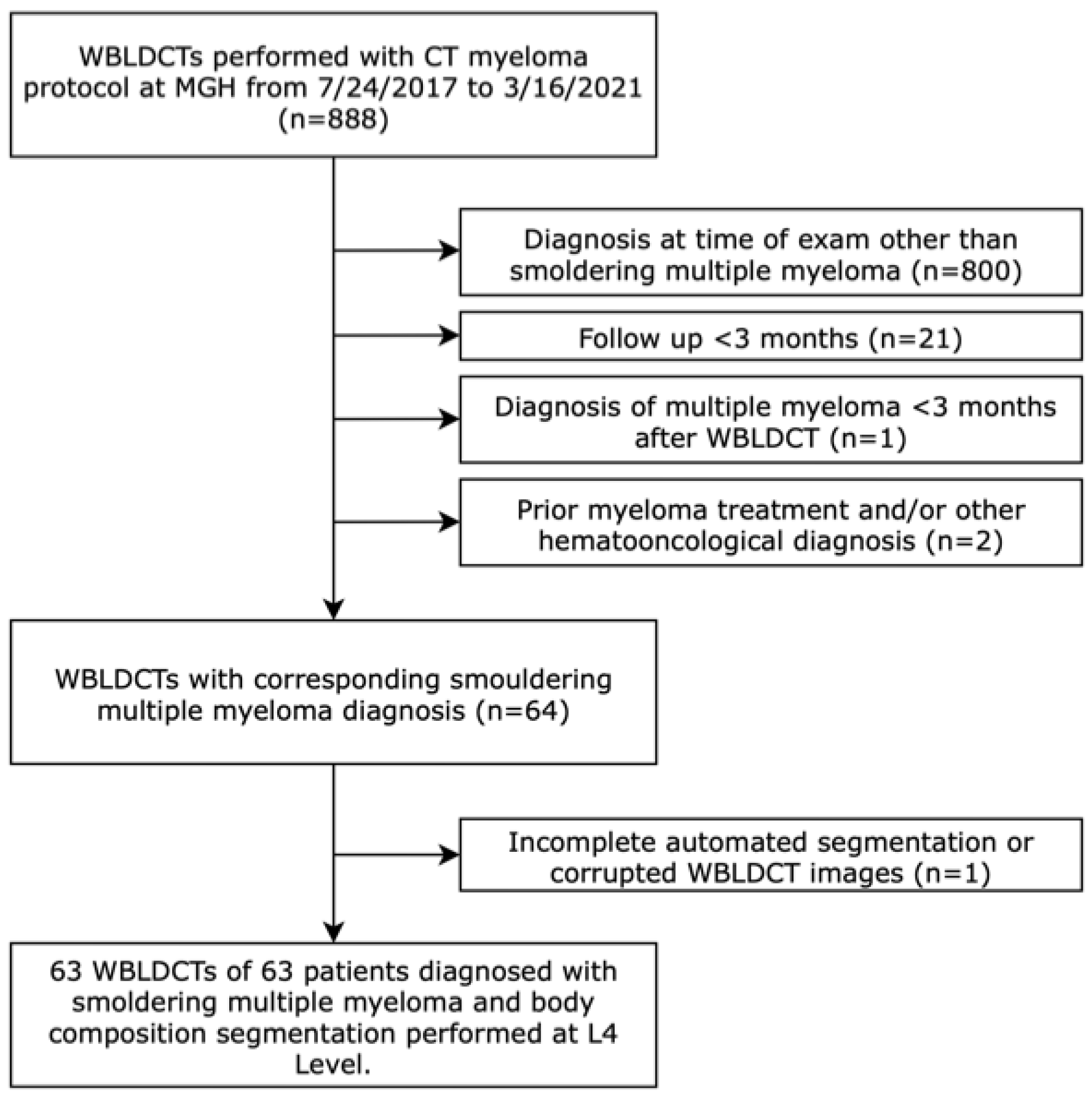
2.1. Study Cohort and Data Collection
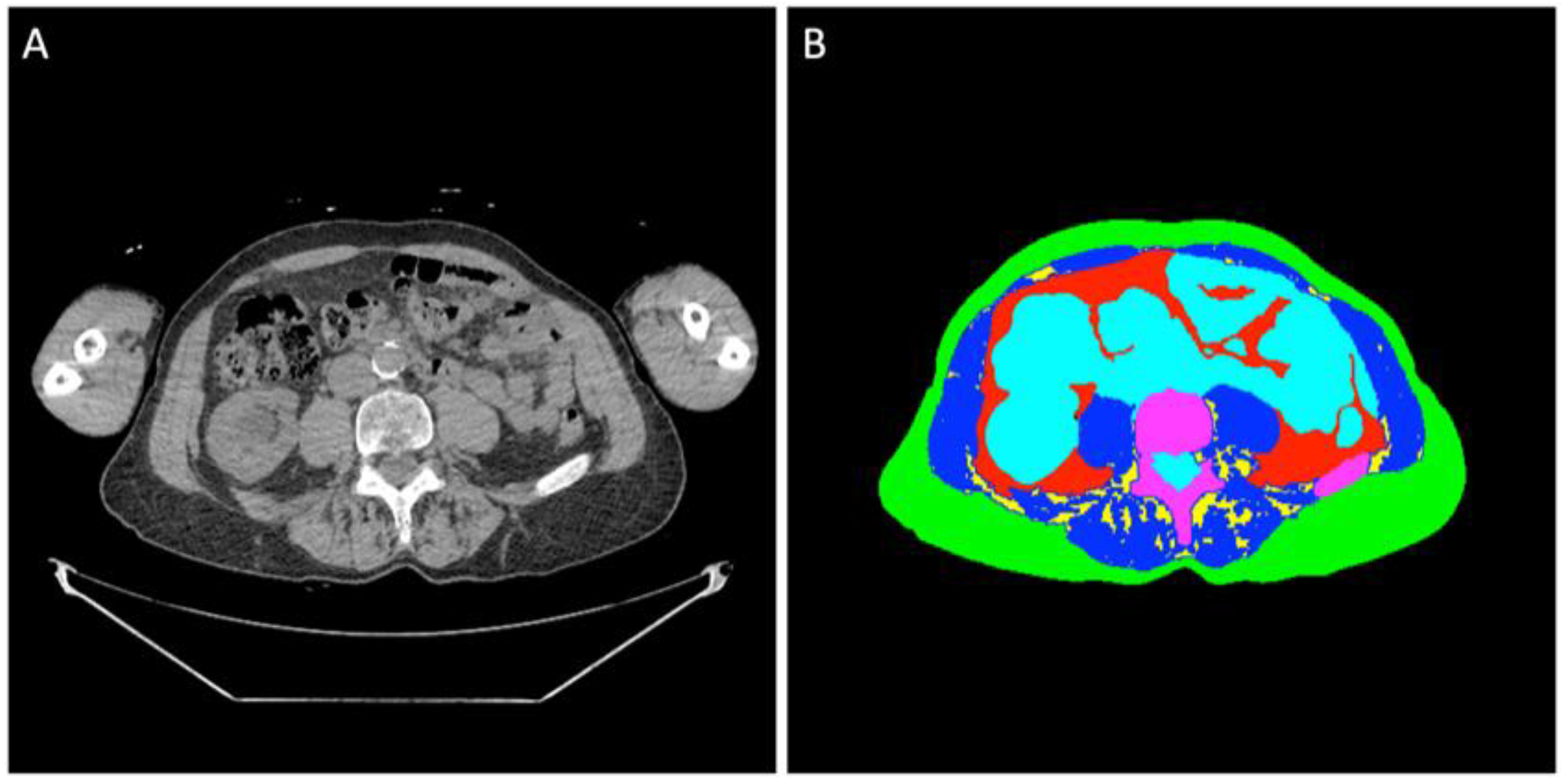
2.2. Image Analysis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
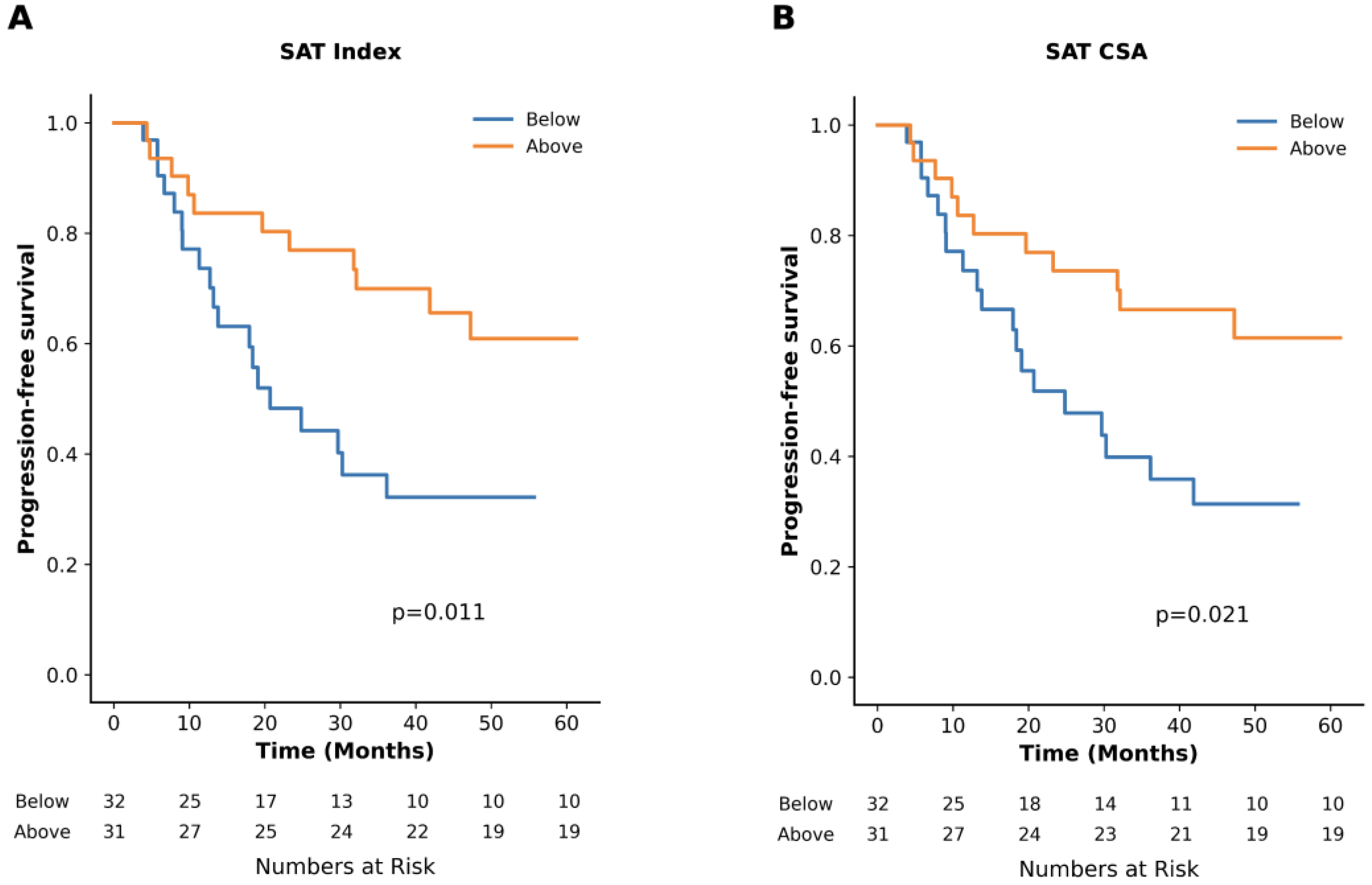
3.2. Impact of Adipose Tissue
3.3. Impact of Skeletal Muscle
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef]
- Kyle, R.A.; Remstein, E.D.; Therneau, T.M.; Dispenzieri, A.; Kurtin, P.J.; Hodnefield, J.M.; Larson, D.R.; Plevak, M.F.; Jelinek, D.F.; Fonseca, R.; et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N. Engl. J. Med. 2007, 356, 2582–2590. [Google Scholar] [CrossRef]
- Cowan, A.; Ferrari, F.; Freeman, S.S.; Redd, R.; El-Khoury, H.; Perry, J.; Patel, V.; Kaur, P.; Barr, H.; Lee, D.J.; et al. Personalised progression prediction in patients with monoclonal gammopathy of undetermined significance or smouldering multiple myeloma (PANGEA): A retrospective, multicohort study. Lancet Haematol. 2023, 10, e203–e212. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Yong, K.; Ramasamy, K.; Stern, S.; Boyle, E.; Ashcroft, J.; Basheer, F.; Rabin, N.; Pratt, G. Diagnosis and management of smouldering myeloma: A British Society for Haematology Good Practice Paper. Br. J. Haematol. 2024, 204, 1193–1206. [Google Scholar] [CrossRef]
- Kunacheewa, C.; Manasanch, E.E. High-risk smoldering myeloma versus early detection of multiple myeloma: Current models, goals of therapy, and clinical implications. Best Pract. Res. Clin. Haematol. 2020, 33, 101152. [Google Scholar] [CrossRef]
- Kyle, R.A.; Durie, B.G.; Rajkumar, S.V.; Landgren, O.; Blade, J.; Merlini, G.; Kroger, N.; Einsele, H.; Vesole, D.H.; Dimopoulos, M.; et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010, 24, 1121–1127. [Google Scholar] [CrossRef]
- Lakshman, A.; Rajkumar, S.V.; Buadi, F.K.; Binder, M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Dingli, D.; Fonder, A.L.; Hayman, S.R.; et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Kumar, S.; Dimopoulos, M.A.; Gonzalez-Calle, V.; Kastritis, E.; Hajek, R.; De Larrea, C.F.; Morgan, G.J.; Merlini, G.; Goldschmidt, H.; et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020, 10, 102. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Mutis, T.; Poddighe, P.J.; Lokhorst, H.M.; Zweegman, S. Diagnosis, risk stratification and management of monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Int. J. Lab. Hematol. 2016, 38 (Suppl. S1), 110–122. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.V.; Lonial, S.; Joao, C.; Anderson, K.C.; Garcia-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Shah, U.A.; Ballinger, T.J.; Bhandari, R.; Dieli-Cornwright, C.M.; Guertin, K.A.; Hibler, E.A.; Kalam, F.; Lohmann, A.E.; Ippolito, J.E. Imaging modalities for measuring body composition in patients with cancer: Opportunities and challenges. J. Natl. Cancer Inst. Monogr. 2023, 2023, 56–67. [Google Scholar] [CrossRef]
- Boutin, R.D.; Lenchik, L. Value-Added Opportunistic CT: Insights Into Osteoporosis and Sarcopenia. AJR Am. J. Roentgenol. 2020, 215, 582–594. [Google Scholar] [CrossRef]
- Boutin, R.D.; Yao, L.; Canter, R.J.; Lenchik, L. Sarcopenia: Current Concepts and Imaging Implications. AJR Am. J. Roentgenol. 2015, 205, W255–W266. [Google Scholar] [CrossRef]
- Hanna, P.E.; Ouyang, T.; Tahir, I.; Katz-Agranov, N.; Wang, Q.; Mantz, L.; Strohbehn, I.; Moreno, D.; Harden, D.; Dinulos, J.E.; et al. Sarcopenia, adiposity and large discordance between cystatin C and creatinine-based estimated glomerular filtration rate in patients with cancer. J. Cachexia Sarcopenia Muscle 2024, 15, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.D.; Fuchs, G.; El-Jawahri, A.; Mario, J.; Troschel, F.M.; Greer, J.A.; Gallagher, E.R.; Jackson, V.A.; Kambadakone, A.; Hong, T.S.; et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist 2018, 23, 97–104. [Google Scholar] [CrossRef]
- Rier, H.N.; Jager, A.; Sleijfer, S.; Maier, A.B.; Levin, M.D. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist 2016, 21, 1396–1409. [Google Scholar] [CrossRef]
- van Seventer, E.; Marquardt, J.P.; Troschel, A.S.; Best, T.D.; Horick, N.; Azoba, C.; Newcomb, R.; Roeland, E.J.; Rosenthal, M.; Bridge, C.P.; et al. Associations of Skeletal Muscle With Symptom Burden and Clinical Outcomes in Hospitalized Patients With Advanced Cancer. J. Natl. Compr. Cancer Netw. 2021, 19, 319–327. [Google Scholar] [CrossRef]
- Abdallah, N.H.; Nagayama, H.; Takahashi, N.; Gonsalves, W.; Fonder, A.; Dispenzieri, A.; Dingli, D.; Buadi, F.K.; Lacy, M.Q.; Hobbs, M.; et al. Muscle and fat composition in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2023, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Barajas Ordonez, F.; Wolleschak, D.; Zeller, Y.; Hinnerichs, M.; Rodriguez-Feria, P.; Aghayev, A.; Mikusko, M.; Borggrefe, J.; Mougiakakos, D.; Surov, A. Parameters of body composition do not predict survival in patients with multiple myeloma undergoing autologous stem cell transplantation. Leuk. Lymphoma 2024, 65, 825–832. [Google Scholar] [CrossRef]
- Diallo, T.D.; Blessing, A.I.L.; Ihorst, G.; Moller, M.D.; Jungmann, P.M.; Bamberg, F.; Herget, G.; Wasch, R.; Engelhardt, M.; Neubauer, J. Myosteatosis in multiple myeloma: A key determinant of survival beyond sarcopenia. Skelet. Radiol. 2024, 54, 275–285. [Google Scholar] [CrossRef]
- Nandakumar, B.; Baffour, F.; Abdallah, N.H.; Kumar, S.K.; Dispenzieri, A.; Buadi, F.K.; Dingli, D.; Lacy, M.Q.; Hayman, S.R.; Kapoor, P.; et al. Sarcopenia identified by computed tomography imaging using a deep learning-based segmentation approach impacts survival in patients with newly diagnosed multiple myeloma. Cancer 2023, 129, 385–392. [Google Scholar] [CrossRef]
- Park, S.S.; Kwag, D.; Lee, J.Y.; Jeon, Y.W.; Yahng, S.A.; Shin, S.H.; Youn, S.Y.; Min, C.K. Prognostic value of low muscle mass at the 12(th) thoracic vertebral level in multiple myeloma treated with transplantation: CAREMM-2101 study. Diagn. Interv. Radiol. 2023, 29, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Benkert, F.; Ponisch, W.; Meyer, H.J. CT-defined body composition as a prognostic factor in multiple myeloma. Hematology 2023, 28, 2191075. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Rossi, F.; Bignotti, B.; Torri, L.; Bonsignore, A.; Belgioia, L.; Domineitto, A. CT-derived relationship between low relative muscle mass and bone damage in patients with multiple myeloma undergoing stem cells transplantation. Br. J. Radiol. 2022, 95, 20210923. [Google Scholar] [CrossRef]
- Takeoka, Y.; Sakatoku, K.; Miura, A.; Yamamura, R.; Araki, T.; Seura, H.; Okamura, T.; Koh, H.; Nakamae, H.; Hino, M.; et al. Prognostic Effect of Low Subcutaneous Adipose Tissue on Survival Outcome in Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2016, 16, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Baruah, D.; Patel, J.; Szabo, A.; Chhabra, S.; Dhakal, B.; Hari, P.; Janz, S.; Stolley, M.; D’Souza, A. Prevalence and significance of sarcopenia in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Bone Marrow Transpl. 2021, 56, 225–231. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, A.D.J.; Silveira, M.N.; Takahashi, M.E.S.; de Souza, E.M.; Mosci, C.; Ramos, C.D.; Brambilla, S.R.; Pericole, F.V.; Prado, C.M.; Mendes, M.C.S.; et al. Adipose tissue radiodensity: A new prognostic biomarker in people with multiple myeloma. Nutrition 2021, 86, 111141. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Nachit, M.; Horsmans, Y.; Summers, R.M.; Leclercq, I.A.; Pickhardt, P.J. AI-based CT Body Composition Identifies Myosteatosis as Key Mortality Predictor in Asymptomatic Adults. Radiology 2023, 307, e222008. [Google Scholar] [CrossRef]
- Schmidt, B.; Debold, E.; Frank, M.; Arendt, M.; Dragano, N.; Dürig, J.; Dührsen, U.; Moebus, S.; Erbel, R.; Jöckel, K.-H.; et al. Socioeconomic Position is Positively Associated with Monoclonal Gammopathy of Undetermined Significance in a Population-based Cohort Study. Ann. Hematol. 2019, 98, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Hillengass, J.; Fechtner, K.; Weber, M.A.; Bauerle, T.; Ayyaz, S.; Heiss, C.; Hielscher, T.; Moehler, T.M.; Egerer, G.; Neben, K.; et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J. Clin. Oncol. 2010, 28, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Veld, J.; O’Donnell, E.K.; Reagan, M.R.; Yee, A.J.; Torriani, M.; Rosen, C.J.; Bredella, M.A. Abdominal adipose tissue in MGUS and multiple myeloma. Skelet. Radiol. 2016, 45, 1277–1283. [Google Scholar] [CrossRef]
- Thordardottir, M.; Lindqvist, E.K.; Lund, S.H.; Costello, R.; Burton, D.; Korde, N.; Mailankody, S.; Eiriksdottir, G.; Launer, L.J.; Gudnason, V.; et al. Obesity and risk of monoclonal gammopathy of undetermined significance and progression to multiple myeloma: A population-based study. Blood Adv. 2017, 1, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, Y.N.; O’Donnell, E.K. Oncologist perspective: Role of imaging in myeloma. Skelet. Radiol. 2022, 51, 123–133. [Google Scholar] [CrossRef]
- Vicentini, J.R.T.; Bredella, M.A. Whole body imaging in musculoskeletal oncology: When, why, and how. Skelet. Radiol. 2023, 52, 281–295. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Himbert, C.; Delphan, M.; Scherer, D.; Bowers, L.W.; Hursting, S.; Ulrich, C.M. Signals from the Adipose Microenvironment and the Obesity-Cancer Link-A Systematic Review. Cancer Prev. Res. 2017, 10, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Matsuzawa, Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 131–141. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Liao, L.M.; Pollak, M.N.; Wang, Y.; Pfeiffer, R.M.; Baris, D.; Andreotti, G.; Lan, Q.; Landgren, O.; Rothman, N.; et al. A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood 2012, 120, 4418–4420. [Google Scholar] [CrossRef]
- Groß, J.P.; Nattenmüller, J.; Hemmer, S.; Tichy, D.; Krzykalla, J.; Goldschmidt, H.; Bertsch, U.; Delorme, S.; Kauczor, H.-U.; Hillengass, J.; et al. Body fat composition as predictive factor for treatment response in patients with newly diagnosed multiple myeloma—Subgroup analysis of the prospective GMMG MM5 trial. Oncotarget 2017, 8, 68460–68471. [Google Scholar] [CrossRef]
- Panaroni, C.; Fulzele, K.; Mori, T.; Siu, K.T.; Onyewadume, C.; Maebius, A.; Raje, N. Multiple myeloma cells induce lipolysis in adipocytes and uptake fatty acids through fatty acid transporter proteins. Blood 2022, 139, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Marinac, C.R.; Birmann, B.M.; Lee, I.M.; Rosner, B.A.; Townsend, M.K.; Giovannucci, E.; Rebbeck, T.R.; Buring, J.E.; Colditz, G.A. Body mass index throughout adulthood, physical activity, and risk of multiple myeloma: A prospective analysis in three large cohorts. Br. J. Cancer 2018, 118, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Luo, S.; Thomas, T.S.; O’Brian, K.K.; Colditz, G.A.; Carlsson, N.P.; Carson, K.R. Obesity and the Transformation of Monoclonal Gammopathy of Undetermined Significance to Multiple Myeloma: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2017, 109, djw264. [Google Scholar] [CrossRef]
- Landgren, O.; Rajkumar, S.V.; Pfeiffer, R.M.; Kyle, R.A.; Katzmann, J.A.; Dispenzieri, A.; Cai, Q.; Goldin, L.R.; Caporaso, N.E.; Fraumeni, J.F.; et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood 2010, 116, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; El-Khoury, H.; Tramontano, A.C.; Alberge, J.B.; Perry, J.; Davis, M.I.; Horowitz, E.; Redd, R.; Sakrikar, D.; Barnidge, D.; et al. Mass spectrometry-detected MGUS is associated with obesity and other novel modifiable risk factors in a high-risk population. Blood Adv. 2024, 8, 1737–1746. [Google Scholar] [CrossRef]
- Boursi, B.; Weiss, B.M.; Haynes, K.; Mamtani, R.; Yang, Y.-X. Reappraisal of risk factors for monoclonal gammopathy of undetermined significance. Am. J. Hematol. 2016, 91, 581–584. [Google Scholar] [CrossRef]
- Beason, T.S.; Chang, S.H.; Sanfilippo, K.M.; Luo, S.; Colditz, G.A.; Vij, R.; Tomasson, M.H.; Dipersio, J.F.; Stockerl-Goldstein, K.; Ganti, A.; et al. Influence of body mass index on survival in veterans with multiple myeloma. Oncologist 2013, 18, 1074–1079. [Google Scholar] [CrossRef]
- Shah, U.A.; Whiting, K.; Devlin, S.; Ershler, R.; Kanapuru, B.; Lee, D.J.; Tahri, S.; Gwise, T.; Rustad, E.H.; Mailankody, S.; et al. Extreme body mass index and survival in newly diagnosed multiple myeloma patients. Blood Cancer J. 2023, 13, 13. [Google Scholar] [CrossRef]
- Wennmann, M.; Goldschmidt, H.; Mosebach, J.; Hielscher, T.; Bäuerle, T.; Komljenovic, D.; McCarthy, P.L.; Merz, M.; Schlemmer, H.P.; Raab, M.S.; et al. Whole-body magnetic resonance imaging plus serological follow-up for early identification of progression in smouldering myeloma patients to prevent development of end-organ damage. Br. J. Haematol. 2022, 199, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Nanni, C.; Gay, F.; Pezzi, A.; Patriarca, F.; Bello, M.; Rambaldi, I.; Tacchetti, P.; Hillengass, J.; Gamberi, B.; et al. 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia 2016, 30, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Thormann, M.; Wienke, A.; Ricke, J.; Seidensticker, M. Different cutoff values of the skeletal muscle mass and myosteatosis result in different clinical impact on overall survival in oncology. A subanalysis of a clinical trial. J. Cancer Res. Clin. Oncol. 2025, 151, 141. [Google Scholar] [CrossRef] [PubMed]
| Total | Not Progressed | Progressed | p-Value | |
|---|---|---|---|---|
| WBLDCT | 63 | 33 | 30 | |
| Subjects | 63 | 33 | 30 | |
| Male sex | 26 (41) | 11 (33) | 15 (50) | 0.21 |
| Age in years | 69 (64–75) | 68 (63–75) | 71 (66–75) | 0.42 |
| BMI in kg/m2 | 27 (25–30) | 28 (25–31) | 27 (25–29) | 0.44 |
| Diabetes mellitus type II | 12 (19) | 9 (27) | 3 (10) | 0.11 |
| Smoking ≥ 5 pack-years | 24 (38) | 12 (36) | 12 (40) | 0.80 |
| SFLCR | 13 (4–63) | 9 (3–18) | 27 (8–79) | 0.44 |
| Serum creatinine in mg/dL | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 0.9 (0.8–1.2) | 0.46 |
| Hemoglobin in g/dL | 12.6 (11.7–13.6) | 12.7 (11.8–13.6) | 12.6 (11.7–13.6) | 0.60 |
| M-protein in g/L [n missing values] | 1.3 (0.6–1.9) [13] | 0.8 (0.3–1.4) [20] | 1.6 (1.1–2.2) [20] | 0.33 |
| PCI in % [n missing values] | 15 (10–20) [31] | 15 (8–20) [5] | 18 (10–20) [32] | 0.26 |
| Body Composition Metric | Median Males (IQR) | Median Females (IQR) | TTP Based on Median in Months (95%CI): Below vs. Above |
|---|---|---|---|
| Adipose Tissue | |||
| SAT CSA in cm2 | 213.2 (187.5, 271.1) | 273.1 (185.6, 336.2) | 24.8 (13.8–41.9) vs. NR (32.1-NR), p = 0.02 |
| SAT Index in cm2/m2 | 74.4 (63.7, 93.7) | 101.6 (72.9, 125.0) | 20.7 (13.2–36.1) vs. NR (41.9-NR), p = 0.01 |
| SAT Radiodensity in HU | −89.0 (−95.7, −79.1) | −100.5 (−104.0, −93.4) | 36.1 (19.1-NR) vs. NR (18.4-NR), p = 0.47 |
| VAT CSA in cm2 | 170.5 (114.4, 222.4) | 128.8 (55.7, 177.0) | 36.1 (19.6-NR) vs. NR (12.7-NR), p = 0.61 |
| VAT Index in cm2/m2 | 59.3 (36.3, 76.2) | 45.8 (20.4, 71.7) | 36.1 (19.6-NR) vs. NR (12.7-NR), p = 0.61 |
| VAT Radiodensity in HU | −96.2 (−101.6, −93.6) | −96.5 (102.0, −87.9) | 36.1 (11.3-NR) vs. NR (19.6-NR), p = 0.38 |
| IMAT CSA in cm2 | 3.2 (1.7, 7.1) | 3.0 (1.6, 7.0) | 47.2 (24.8-NR) vs. NR (12.7-NR), p = 0.73 |
| IMAT Index in cm2/m2 | 1.3 (0.5, 2.5) | 1.0 (0.7, 2.6) | 47.2 (24.8-NR) vs. NR (12.7-NR), p = 0.76 |
| TAT CSA in cm2 | 399.6 (337.7, 518.5) | 435.9 (258.6, 494.9) | 29.7 (18.4-NR) vs. NR (23.2-NR), p = 0.18 |
| TAT Index in cm2/m2 | 133.6 (108.7, 162.2) | 161.9 (101.2, 193.6) | 30.3 (19.1-NR) vs. NR (13.2-NR), p = 0.42 |
| Muscle Tissue | |||
| Skeletal Muscle Index a in cm2/m2 | 59.8 (53.6, 66.5) | 48.5 (43.6, 53.8) | 18.4 (5.8–41.9) vs. NR (29.7-NR), p = 0.07 |
| Skeletal Muscle Radiodensity b in HU | 22.5 (18.2, 30.8) | 16.2 (7.8, 20.9) | 41.9 (13.2-NR) vs. 47.2 (19.6-NR), p = 0.59 |
| SAT Index | SAT CSA | |||||
|---|---|---|---|---|---|---|
| Below | Above | p-Value | Below | Above | p-Value | |
| Age in years | 73.0 | 68.0 | 0.03 | 72.0 | 68.0 | 0.05 |
| Serum creatinine in mg/dL | 0.9 | 0.9 | 0.84 | 0.91 | 9.0 | 0.91 |
| Hemoglobin in g/dL | 12.0 | 13.2 | <0.01 | 12.0 | 13.2 | 0.01 |
| SFLCR | 13.9 | 11.4 | 0.14 | 13.1 | 12.5 | 0.24 |
| M-protein in g/L | 1.3 | 1.4 | 0.57 | 13.3 | 14.0 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, F.; Huber, F.A.; Galdamez, M.E.; Zorgno, I.; Habibollahi, S.; El Kandoussi, A.; Fintelmann, F.J.; Tonnesen, P.E.; Dietrich, A.-S.W.; Wang, Z.; et al. Body Composition Metrics Associated with Time to Progression in Smoldering Multiple Myeloma. Diagnostics 2025, 15, 2760. https://doi.org/10.3390/diagnostics15212760
Bauer F, Huber FA, Galdamez ME, Zorgno I, Habibollahi S, El Kandoussi A, Fintelmann FJ, Tonnesen PE, Dietrich A-SW, Wang Z, et al. Body Composition Metrics Associated with Time to Progression in Smoldering Multiple Myeloma. Diagnostics. 2025; 15(21):2760. https://doi.org/10.3390/diagnostics15212760
Chicago/Turabian StyleBauer, Fabian, Florian A. Huber, Marilyn E. Galdamez, Ivanna Zorgno, Sina Habibollahi, Amine El Kandoussi, Florian J. Fintelmann, P. Erik Tonnesen, Anna-Sophia W. Dietrich, Zhe Wang, and et al. 2025. "Body Composition Metrics Associated with Time to Progression in Smoldering Multiple Myeloma" Diagnostics 15, no. 21: 2760. https://doi.org/10.3390/diagnostics15212760
APA StyleBauer, F., Huber, F. A., Galdamez, M. E., Zorgno, I., Habibollahi, S., El Kandoussi, A., Fintelmann, F. J., Tonnesen, P. E., Dietrich, A.-S. W., Wang, Z., Graeber, A., Boutin, R. D., Lenchik, L., Gustine, J. N., Staffa, S. J., Raje, N., & Chang, C. Y. (2025). Body Composition Metrics Associated with Time to Progression in Smoldering Multiple Myeloma. Diagnostics, 15(21), 2760. https://doi.org/10.3390/diagnostics15212760






