The Role of 18F-FDG PET/CT in Monitoring Immunotherapy Response in Non-Small Cell Lung Cancer: Current Evidence and Challenges: A Narrative Review
Abstract
1. Introduction
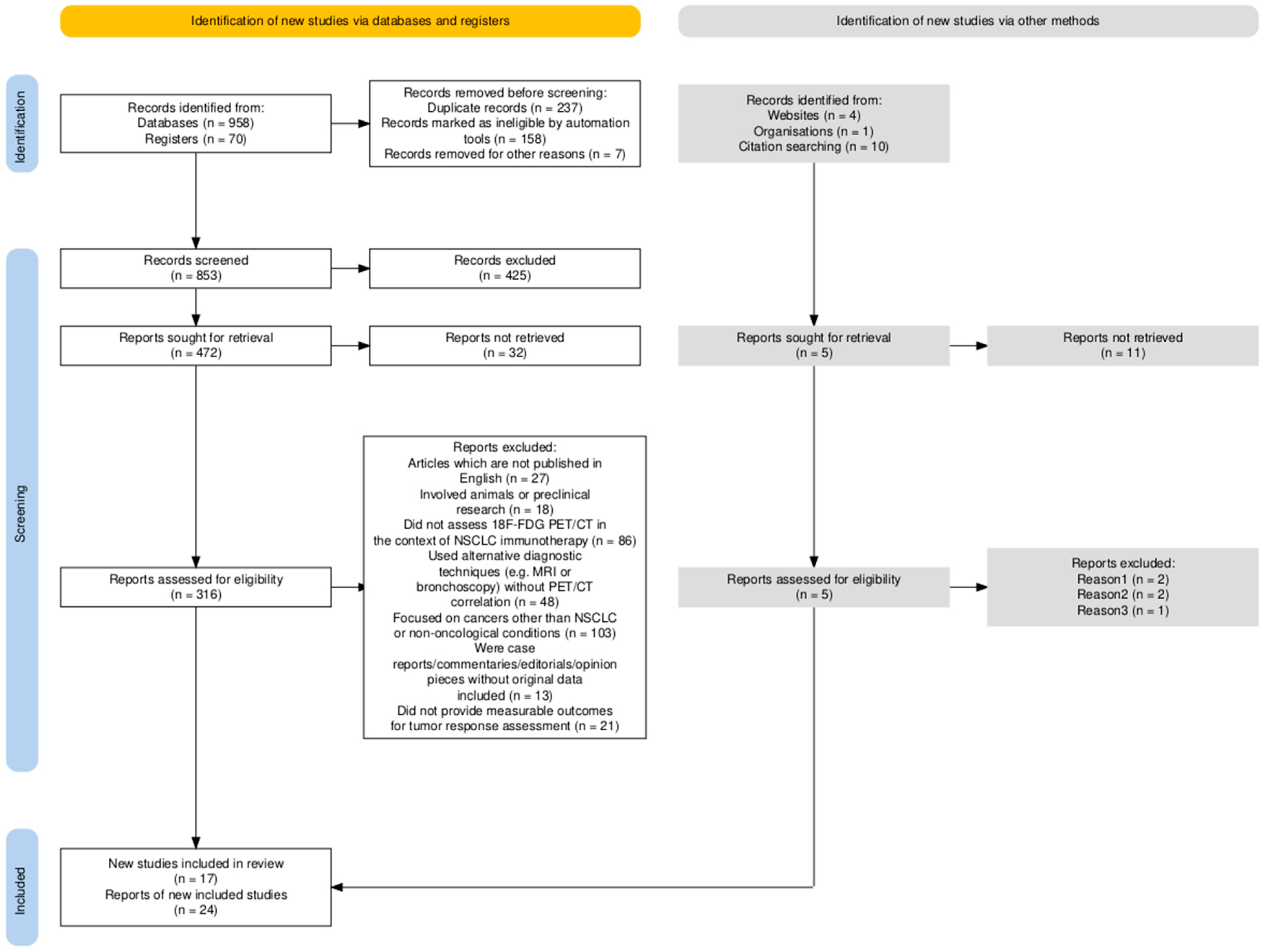
2. Methods
- Published in English;
- Conducted on human subjects;
- Evaluated the use of PET/CT for monitoring immunotherapy response in patients with NSCLC
- Clinical trials, cohort studies, comparative studies, or reviews specifically addressing metabolic imaging and response evaluation frameworks (e.g., RECIST 1.1, iRECIST, PERCIST).
- Articles which are not published in English;
- Involved animals or preclinical research;
- Did not assess 18F-FDG PET/CT in the context of NSCLC immunotherapy;
- Used alternative diagnostic techniques (e.g., CT, MRI or bronchoscopy) without PET/CT correlation;
- Focused on cancers other than NSCLC or non-oncological conditions;
- Were case reports/commentaries/editorials/opinion pieces without original data included;
- Did not provide measurable outcomes for tumor response assessment
- Reviews and meta-analyses were used only for contextual purposes and to identify relevant primary studies, without quantitative data extraction, to avoid data duplication;
- Conference abstracts were excluded from the analysis to ensure data quality and reproducibility.
3. Tumor Response Criteria: Clinical Relevance and Limitations
3.1. RECIST 1.1: Strengths and Weaknesses
3.2. iRECIST: A Partial Solution to Immunotherapy Challenges
4. iRECIST: Atypical Response Patterns in Immunotherapy: Clinical and Imaging Insights
4.1. Pseudoprogressive Disease
4.2. Hyperprogression
4.3. Dissociated Response
- ○
- Complete Response (CR) or Partial Response (PR) in some lesions;
- ○
4.4. Durable Response
5. Comparison of RECIST 1.1 vs. iRECIST: Differences and Similarities
6. What Is the Role of PET/CT in Managing Patients Undergoing Immunotherapy for Lung Cancer?
- SUV (Standardized Uptake Value): A measure of the metabolic intensity of the lesion.
- SUL (Standardized Uptake Value Lean Body Mass): An updated version of SUV, representing a standardized uptake value corrected for lean body mass.
- Complete Metabolic Response (CMR):
- ○
- Complete resolution of abnormal metabolic activity detected by the radiotracer 18F-FDG in the target lesion under evaluation. The SUV is comparable to that of normal tissues.
- ○
- Disappearance of all other metabolically active lesions.
- ○
- No new suspicious 18F-FDG lesions.
- Partial Metabolic Response (PMR):
- ○
- A reduction of >30% in the measurable peak metabolic activity of the tumor detected by 18F-FDG, with an absolute decrease in SUL value by at least 0.8 SUL units (indicating a >30% reduction in metabolic activity in the most active lesion SUVmax).
- ○
- No increase of >30% in SUL or lesion dimensions in any of the remaining lesions.
- ○
- No emergence of new metabolically active lesions.
- Stable Metabolic Disease (SMD):
- ○
- Changes below the diagnostic threshold for CMR, PMR, or metabolic progression.
- ○
- No appearance of new metabolically active lesions.
- Progressive Metabolic Disease (PMD):
- ○
- An increase in the SUL peak of 18F-FDG by >30% or by >0.8 SUL units compared to the baseline imaging, with characteristics consistent with cancer rather than infection or treatment effects.
- ○
- ○
- Complete response: Disappearance of all lesions and metabolic activity.
- ○
- Partial response: A reduction of >30% in the size of lesions and metabolic activity.
- ○
- Stable disease: No significant reduction or increase in the size or metabolic activity of lesions.
- ○
7. Are There Alternatives to PERCIST Criteria?
7.1. PERCIMPT (PET Response Criteria for Immunotherapy in Malignant Pleural Tumors)
7.2. imPERCIST (Immune-Modified PERCIST)
7.3. PERCIST (Immune PET Response Criteria in Solid Tumors)
7.4. Clinical Implications and Guidance for Decision-Making
7.5. Challenges of FDG Uptake Interpretation and Differentiation Strategies
7.6. Cost-Effectiveness, Availability, and Potential Risks of PET-Based Monitoring
7.7. Radiomics and Artificial Intelligence in Immunotherapy Response Assessment
8. Comparison of Tumor Response Evaluation Criteria in Major Studies Conducted over the Last Five Years
- A.
- Relevance of the criteria for immunotherapy: Classical criteria, such as RECIST 1.1, implemented in clinical practice in 2009, were designed for traditional cytotoxic and targeted therapies and are not ideal for immunotherapy due to atypical immune responses (e.g., pseudoprogression or delayed response). As an update, iRECIST was introduced to allow for follow-up monitoring of suspected progression to identify diverse immune responses. Metabolic imaging criteria, such as PERCIST and iPERCIST, provide additional information and a different perspective by analyzing tumor metabolic changes, which are often correlated with immunotherapy [66,68,69].
- B.
- Pseudoprogression refers to transient immune responses where immunotherapy-induced tumor infiltration by immune cells (e.g., T lymphocytes) and the presence of local inflammatory cells initially result in apparent tumor progression, followed by dimensional reduction. These phenomena occur more frequently in patients treated with PD-1/PD-L1 inhibitors. Studies suggest pseudoprogression rates range between 4% and 7%. The application of updated criteria facilitates the early identification of these phenomena, preventing the premature discontinuation of potentially effective treatments [66,67,69].
- C.
- Concordance Between Criteria: The studies report moderate concordance between anatomical criteria (RECIST 1.1 and iRECIST) and metabolic criteria (PERCIST and iPERCIST), particularly in early responses. Predictors of treatment response include the following:
- -
- -
- -
- D.
- E.
- Clinical decisions influenced by the choice of imaging evaluation criteria play a crucial role in determining whether to continue or discontinue immunotherapy. For instance, the use of anatomical criteria may lead to an underestimation of immune tumor responses, potentially resulting in the premature discontinuation of treatment. In contrast, metabolic criteria enable better management of complex cases, particularly those involving atypical responses such as pseudoprogression or delayed response [66,67,68,69,70]. Recent studies have shown that immune-related inflammation may mimic tumor progression on ^18F-FDG PET/CT, emphasizing the potential value of immune-specific tracers such as PD-L1 or CD8-targeted PET agents [23,74,75,76].
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owens, C.; Hindocha, S.; Lee, R.W.; Millard, T.P.; Sharma, B. The Lung Cancers: Staging and Response, CT, 18F-FDG PET/CT, MRI, DWI: Review and New Perspectives. Br. J. Radiol. 2023, 96, 20220339. [Google Scholar] [CrossRef]
- Léger, M.A.; Routy, B.; Juneau, D. FDG PET/CT for Evaluation of Immunotherapy Response in Lung Cancer Patients. Semin. Nucl. Med. 2022, 52, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37,513,025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Kandathil, A.; Kay, F.U.; Butt, Y.M.; Wachsmann, J.W.; Subramaniam, R.M. Role of FDG PET/CT in the Eighth Edition of TNM Staging of Non-Small Cell Lung Cancer. Radiographics 2018, 38, 2134–2149. [Google Scholar] [CrossRef]
- Rastogi, A.; Baheti, A.D.; Patra, A.; Tirumani, S.H. Tumor Response Criteria in Oncoimaging: RECIST Criteria and Beyond—Part 1. J. Gastrointest. Abdom. Radiol. 2019, 2, 98–106. [Google Scholar] [CrossRef]
- Baheti, A.D.; Rastogi, A.; Natarajan, A.; Patra, A.; Tirumani, S.H. Tumor Response Criteria in Oncoimaging: RECIST Criteria and Beyond—Part 2. J. Gastrointest. Abdom. Radiol. 2019, 2, 107–115. [Google Scholar] [CrossRef]
- Berz, A.M.; Dromain, C.; Vietti-Violi, N.; Boughdad, S.; Duran, R. Tumor response assessment on imaging following immunotherapy. Front. Oncol. 2022, 12, 982983, Erratum in Front. Oncol. 2023, 13, 1175321. https://doi.org/10.3389/fonc.2023.1175321. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic Virus Immunotherapy: Future Prospects for Oncology. J. Immunother. Cancer 2018, 6, 140. [Google Scholar] [CrossRef]
- Haslam, A.; Gill, J.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw. Open 2020, 3, e200423. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Wang, Y. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29 (Suppl. 4), iv264–iv266. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Evangelista, L.; Sepulcri, M.; Pasello, G. PET/CT and the Response to Immunotherapy in Lung Cancer. Curr. Radiopharm. 2020, 13, 177–184. [Google Scholar] [CrossRef]
- Farsad, M. FDG PET/CT in the Staging of Lung Cancer. Curr. Radiopharm. 2020, 13, 195–203. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Ikeda, N. Updates on Minimally Invasive Surgery in Non-Small Cell Lung Cancer. Curr. Treat. Options Oncol. 2019, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, G.; Dai, J. Tumor Therapeutics in the Era of “RECIST”: Past, Current Insights, and Future Prospects. Oncol. Rev. 2024, 18, 1435922. [Google Scholar] [CrossRef]
- Zettler, M.; Basch, E.; Nabhan, C. Surrogate End Points and Patient-Reported Outcomes for Novel Oncology Drugs Approved between 2011 and 2017. JAMA Oncol. 2019, 5, 1358–1359. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Brezun, J.; Aide, N.; Peroux, E.; Lamboley, J.-L.; Gutman, F.; Lussato, D.; Helissey, C. [18F]FDG PET/CT Integration in Evaluating Immunotherapy for Lung Cancer: A Clinician’s Practical Approach. Diagnostics 2024, 14, 2104. [Google Scholar] [CrossRef]
- The Radiology Assistant: RECIST 1.1—And More. Available online: https://radiologyassistant.nl/more/recist-1-1/recist-1-1 (accessed on 7 November 2020).
- Yu, Y.; Knipe, H.; Bell, D.; Jones, S. Response Evaluation Criteria in Solid Tumors. Radiopaedia.org. Available online: https://radiopaedia.org/articles/response-evaluation-criteria-in-solid-tumours?lang=us (accessed on 28 December 2024).
- The Radiology Assistant: RECIST 1.1—The Basics. Radiologyassistant.nl. 2020. Available online: https://radiologyassistant.nl/more/recist-1-1/recist-1-1-1 (accessed on 18 December 2024).
- Mulkey, F.; Theoret, M.R.; Keegan, P.; Pazdur, R.; Sridhara, R. Comparison of iRECIST versus RECIST v.1.1 in Patients Treated with an Anti-PD-1 or PD-L1 Antibody: Pooled FDA Analysis. J. Immunother. Cancer 2020, 8, e000146. [Google Scholar] [CrossRef]
- Remacha, E.; Álvarez Fernández, M.C.; Morbelli, J.A.; Luceño Ros, M.Á.; Coma García, M.; Álvarez Fernández, C.; Pacios Llorca, P.; Yusta Santamaría, D. RECIST 1.1 and iRECIST Response Criteria: Update and Analysis. In Proceedings of the European Congress of Radiology (ECR 2024), Vienna, Austria, 28 February–3 March 2024. [Google Scholar] [CrossRef]
- Ferrara, R.; Caramella, C.; Besse, B.; Champiat, S. Pseudoprogression in Non–Small Cell Lung Cancer upon Immunotherapy: Few Drops in the Ocean? J. Thorac. Oncol. 2019, 14, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, C.; Nie, J.; Sun, Y.; Xu, Y.; Wu, F.; Huang, Y.; Li, S.; Wang, Y.; Zhou, Y.; et al. Response Evaluation and Survival Prediction Following PD-1 Inhibitor in Patients with Advanced Hepatocellular Carcinoma: Comparison of the RECIST 1.1, iRECIST, and mRECIST Criteria. Front. Oncol. 2021, 11, 764189. [Google Scholar] [CrossRef]
- Mönch, S.; Heimer, M.M.; Winkelmann, M.; Guertler, A.; Schlaak, M.; Tufman, A.; Khaled, N.B.; de Toni, E.; Westphalen, C.B.; von Bergwelt-Baildon, M.; et al. Patterns of Pseudoprogression across Different Cancer Entities Treated with Immune Checkpoint Inhibitors. Cancer Imaging 2023, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; Nandikolla, A.; Long, G.; Goel, S.; Le Tourneau, C. Patterns of Response and Progression to Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Ismail, R.; Puzanov, I. Intratumoral Immunotherapy–Update 2019. Oncologist 2020, 25, e423–e438. [Google Scholar] [CrossRef]
- Chen, M.Y.; Zeng, Y.C. Pseudoprogression in Lung Cancer Patients Treated with Immunotherapy. Crit. Rev. Oncol. Hematol. 2022, 169, 103531. [Google Scholar] [CrossRef]
- Ferté, C.; Marabelle, A. iRECIST: A Clarification of Tumour Response Assessment in the Immunotherapy Era. Eur. J. Cancer 2017, 77, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Persigehl, T.; Lennartz, S.; Schwartz, L.H. iRECIST: How to Do It. Cancer Imaging 2020, 20, 2. [Google Scholar] [CrossRef]
- Adashek, J.J.; Subbiah, I.M.; Matos, I.; Garralda, E.; Menta, A.K.; Ganeshan, D.M.; Meric-Bernstam, F. Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact? Trends Cancer 2020, 6, 181–191. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, L. Hyperprogressive Disease in Malignant Carcinoma with Immune Checkpoint Inhibitor Use: A Review. Front. Nutr. 2022, 9, 810472. [Google Scholar] [CrossRef]
- Kanjanapan, Y.; Day, D.; Wang, L.; Al-Sawaihey, H.; Abbas, E.; Namini, A.; Siu, L.L.; Hansen, A.; Razak, A.A.; Spreafico, A.; et al. Hyperprogressive Disease in Early-Phase Immunotherapy Trials: Clinical Predictors and Association with Immune-Related Toxicities. Cancer 2019, 125, 1341–1349. [Google Scholar] [CrossRef]
- Frelaut, M.; Le Tourneau, C.; Borcoman, E. Hyperprogression under immunotherapy. Int. J. Mol. Sci. 2019, 20, 2674. [Google Scholar] [CrossRef]
- Shen, P.; Han, L.; Ba, X.; Qin, K.; Tu, S. Hyperprogressive Disease in Cancers Treated with Immune Checkpoint Inhibitors. Front. Pharmacol. 2021, 12, 678409. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Mazziotti, E.; Toschi, L.; Lopci, E. Hyperprogressive Disease in Patients with Non-Small Cell Lung Cancer Treated with Checkpoint Inhibitors: The Role of 18F-FDG PET/CT. J. Nucl. Med. 2020, 61, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Humbert, O.; Chardin, D. Dissociated Response in Metastatic Cancer: An Atypical Pattern Brought into the Spotlight with Immunotherapy. Front. Oncol. 2020, 10, 566297. [Google Scholar] [CrossRef]
- Vaflard, P.; Paoletti, X.; Servois, V.; Tresca, P.; Pons-Tostivint, E.; Sablin, M.P.; Le Tourneau, C. Dissociated Responses in Patients with Metastatic Solid Tumors Treated with Immunotherapy. Drugs RD 2021, 21, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Maino, C.; Ragusi, M.; Porta, M.; Gandola, D.; Franzesi, C.T.; Giandola, T.P.; Sironi, S. Immune Response Evaluation Criteria in Solid Tumors for Assessment of Atypical Responses after Immunotherapy. World J. Clin. Oncol. 2021, 12, 323–334. [Google Scholar] [CrossRef]
- Tozuka, T.; Kitazono, S.; Sakamoto, H.; Koda, K.; Yoshida, T.; Tamiya, A. Dissociated Responses at Initial Computed Tomography Evaluation Is a Good Prognostic Factor in Non-Small Cell Lung Cancer Patients Treated with Anti-Programmed Cell Death-1/Ligand 1 Inhibitors. BMC Cancer 2020, 20, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, Y.; Xiu, W.; Qiu, P.; Li, W.; Pan, Y. Overall Survival Benefit of Continuing Immune Checkpoint Inhibitors Treatment Post Dissociated Response in Patients with Advanced Lung Cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E. Immunotherapy Monitoring with Immune Checkpoint Inhibitors Based on [18F]FDG PET/CT in Metastatic Melanomas and Lung Cancer. J. Clin. Med. 2021, 10, 5160. [Google Scholar] [CrossRef]
- Castello, A.; Lopci, E. Update on Tumor Metabolism and Patterns of Response to Immunotherapy. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 175–185. [Google Scholar] [CrossRef]
- Reinhorn, D.; Jacobi, O.; Icht, O.; Dudnik, E.; Rotem, O.; Zer, A.; Goldstein, D.A. Treatment Beyond Progression with Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Immunotherapy 2020, 12, 235–243. [Google Scholar] [CrossRef]
- Humbert, O.; Cadour, N.; Paquet, M.; Schiappa, R.; Poudenx, M.; Chardin, D.; Marquette, C.H. 18F-FDG PET/CT in the Early Assessment of Non-Small Cell Lung Cancer Response to Immunotherapy: Frequency and Clinical Significance of Atypical Evolutive Patterns. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1158–1167. [Google Scholar] [CrossRef]
- Tazdait, M.; Mezquita, L.; Lahmar, J.; Ferrara, R.; Bidault, F.; Ammari, S.; Balleyguier, C.; Planchard, D.; Gazzah, A.; Soria, J.C.; et al. Patterns of Responses in Metastatic NSCLC during PD-1 or PDL-1 Inhibitor Therapy: Comparison of RECIST 1.1, irRECIST and iRECIST Criteria. Eur. J. Cancer 2018, 88, 38–47. [Google Scholar] [CrossRef]
- Sato, Y.; Morimoto, T.; Hara, S.; Tsuda, K.; Nishida, S.; Okamoto, N.; Ito, M.; Araki, N. Dissociated Response and Clinical Benefit in Patients Treated with Nivolumab Monotherapy. Investig. New Drugs 2021, 39, 1170–1178. [Google Scholar] [CrossRef]
- Pons-Tostivint, E.; Latouche, A.; Vaflard, P.; Daste, A.; Brosse, D.; Coquan, E.; Boudy, N.; Hescot, S.; Postel-Vinay, S.; Italiano, A. Comparative Analysis of Durable Responses on Immune Checkpoint Inhibitors Versus Other Systemic Therapies: A Pooled Analysis of Phase III Trials. JCO Precis. Oncol. 2019, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manitz, J.; D’Angelo, S.P.; Apolo, A.B.; Postow, M.A.; Wong, D.J.L.; Redman, M.W.; Singh, H.; Rubin, E.H.; McKee, A.E. Comparison of Tumor Assessments Using RECIST 1.1 and irRECIST, and Association with Overall Survival. J. Immunother. Cancer 2022, 10, e003302. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, J.; Jarraya, H.; Lebellec, L.; Penel, N. irRECIST and iRECIST: The Devil Is in the Details. Ann. Oncol. 2017, 28, 1676–1678. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, G.H.; Kim, K.W.; Lee, C.W.; Yoon, S.; Chae, Y.K.; Tirumani, S.H.; Ramaiya, N.H. Comparison of RECIST 1.1 and iRECIST in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 120. [Google Scholar] [CrossRef]
- Houdek, Š.; Büchler, T.; Kindlová, E. Comparison of RECIST 1.1 and iRECIST for Response Evaluation in Solid Tumours. Klin. Onkol. 2017, 30 (Suppl. 3), 32–39. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.L. From RECIST to PERCIST: Navigating the Landscape of Tumor Response Assessment. Eur. Radiol. 2024, 34, 3656–3658. [Google Scholar] [CrossRef]
- Nikolaev, A.; Moore, C.; Sharma, R.; Adams, L.; Gaillard, F. Positron Emission Tomography Response Criteria in Solid Tumours (PERCIST). Radiopaedia.org. Available online: https://radiopaedia.org/articles/positron-emission-tomography-response-criteria-in-solid-tumors-percist-1?lang=us (accessed on 4 January 2025).
- Cheng, J.; Bell, D.; Deng, F.; Gaillard, F. Lugano Classification (PET-CT Treatment Response). Radiopaedia.org. 2025. Available online: https://radiopaedia.org/articles/lugano-classification-pet-ct-treatment-response?lang=us (accessed on 4 January 2025).
- Annovazzi, A.; Ferraresi, V.; De Rimini, M.L.; Sciuto, R. 18F-FDG PET/CT in the Clinical-Diagnostic Workup of Patients Treated with Immunotherapy: When and How? Clin. Transl. Imaging 2022, 10, 325–329. [Google Scholar] [CrossRef]
- Ayati, N.; Lee, S.T.; Zakavi, S.R.; Cheng, M.; Lau, W.F.E.; Parakh, S.; Pathmaraj, K.; Scott, A.M. Response Evaluation and Survival Prediction After PD-1 Immunotherapy in Patients with Non–Small Cell Lung Cancer: Comparison of Assessment Methods. J. Nucl. Med. 2021, 62, 926–933. [Google Scholar] [CrossRef]
- Goldfarb, L.; Duchemann, B.; Chouahnia, K.; Zelek, L.; Soussan, M. Monitoring Anti-PD-1-Based Immunotherapy in Non-Small Cell Lung Cancer with FDG PET: Introduction of iPERCIST. EJNMMI Res. 2019, 9, 8. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.S.; Jain, P.; Zakavi, S.R.; Ayati, N.; Lee, S.T.; Scott, A.M. Molecular Response Assessment with Immune Adaptive PERCIST in Lung Cancer Patients Treated with Nivolumab: Is It Better Than iRECIST? World J. Nucl. Med. 2022, 21, 34–43. [Google Scholar] [CrossRef]
- Nelles, C.; Gräf, M.; Bernard, P.; Persigehl, T.; Hokamp, N.G.; Zopfs, D.; Maintz, D.; Kreuzberg, N.; Wolf, J.; Bröckelmann, P.J.; et al. Real-World Response Assessment of Immune Checkpoint Inhibition: Comparing iRECIST and RECIST 1.1 in Melanoma and Non-Small Cell Lung Cancer Patients. Eur. Radiol. 2024, 35, 2084–2093. [Google Scholar] [CrossRef]
- Guégan, J.-P.; Peyraud, F.; Dadone-Montaudie, B.; Teyssonneau, D.; Palmieri, L.-J.; Clot, E.; Cousin, S.; Roubaud, G.; Cabart, M.; Leroy, L.; et al. Analysis of PD1, LAG3, TIGIT, and TIM3 Expression in Human Lung Adenocarcinoma Reveals a 25-Gene Signature Predicting Immunotherapy Response. Cell Rep. Med. 2024, 5, 101831. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.S.; Rao, S.A.; Jain, P.; Sharma, M.; Babu, V.P.; Goyal, S.; Pasricha, S.; Batra, U. A Comparative Study of Immune RECIST and Immune Adaptive PERCIST in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Nucl. Med. 2020, 61 (Suppl. S1), 242. [Google Scholar]
- Castello, A.; Toschi, L.; Grizzi, F.; Rossi, S.; Qehajaj, D.; Lopci, E. Added Value of Metabolic Response with 18F-FDG PET/CT versus iRECIST in Patients with NSCLC Treated with ICIs. J. Nucl. Med. 2019, 60 (Suppl. S1), 148. [Google Scholar]
- Beer, L.; Hochmair, M.; Haug, A.R.; Schwabel, B.; Kifjak, D.; Wadsak, W.; Fuereder, T.; Fabikan, H.; Fazekas, A.; Schwab, S.B.; et al. Comparison of RECIST, iRECIST, and PERCIST for the Evaluation of Response to PD-1/PD-L1 Blockade Therapy in Patients with Non–Small Cell Lung Cancer. Clin. Nucl. Med. 2019, 44, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Guariglia, M.; Pasello, G.; Lopci, E. PET Radiomics and Response to Immunotherapy in Lung Cancer. Cancers 2023, 15, 3258. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, S.; Wang, Y.; Zhang, J.; Liu, L.; Liu, X.; Huang, B.; Li, K.; Chen, Y. The Role of Artificial Intelligence Based on PET/CT Radiomics in Non-Small Cell Lung Cancer. Front. Oncol. 2023, 13, 1133164. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.; Zhang, X.; Chen, D.; Liu, Z.; Zhang, Y.; Ma, X.; Xie, C. Applications of CT-Based Radiomics for the Prediction of Immunotherapy Benefits in Non-Small Cell Lung Cancer. Front. Immunol. 2024, 15, 1434171. [Google Scholar] [CrossRef]
- Niemeijer, A.-L.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.A.M.S.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole-body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef]
- Mederle, A.L.; Dima, M.; Stoicescu, E.R.; Căpăstraru, B.F.; Levai, C.M.; Hațegan, O.A.; Maghiari, A.L. Impact of Gut Microbiome Interventions on Glucose and Lipid Metabolism in Metabolic Diseases: A Systematic Review and Meta-Analysis. Life 2024, 14, 1485. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Postow, M.A.; Hellmann, M.D.; Harding, J.J.; Barker, C.A.; O’Donoghue, J.A.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.K.; Tsai, F.; et al. First-in-Humans Imaging with ^89Zr-Df-IAB22M2C Anti-CD8 Minibody in Patients with Solid Malignancies. J. Nucl. Med. 2020, 61, 1187–1195. [Google Scholar] [CrossRef]
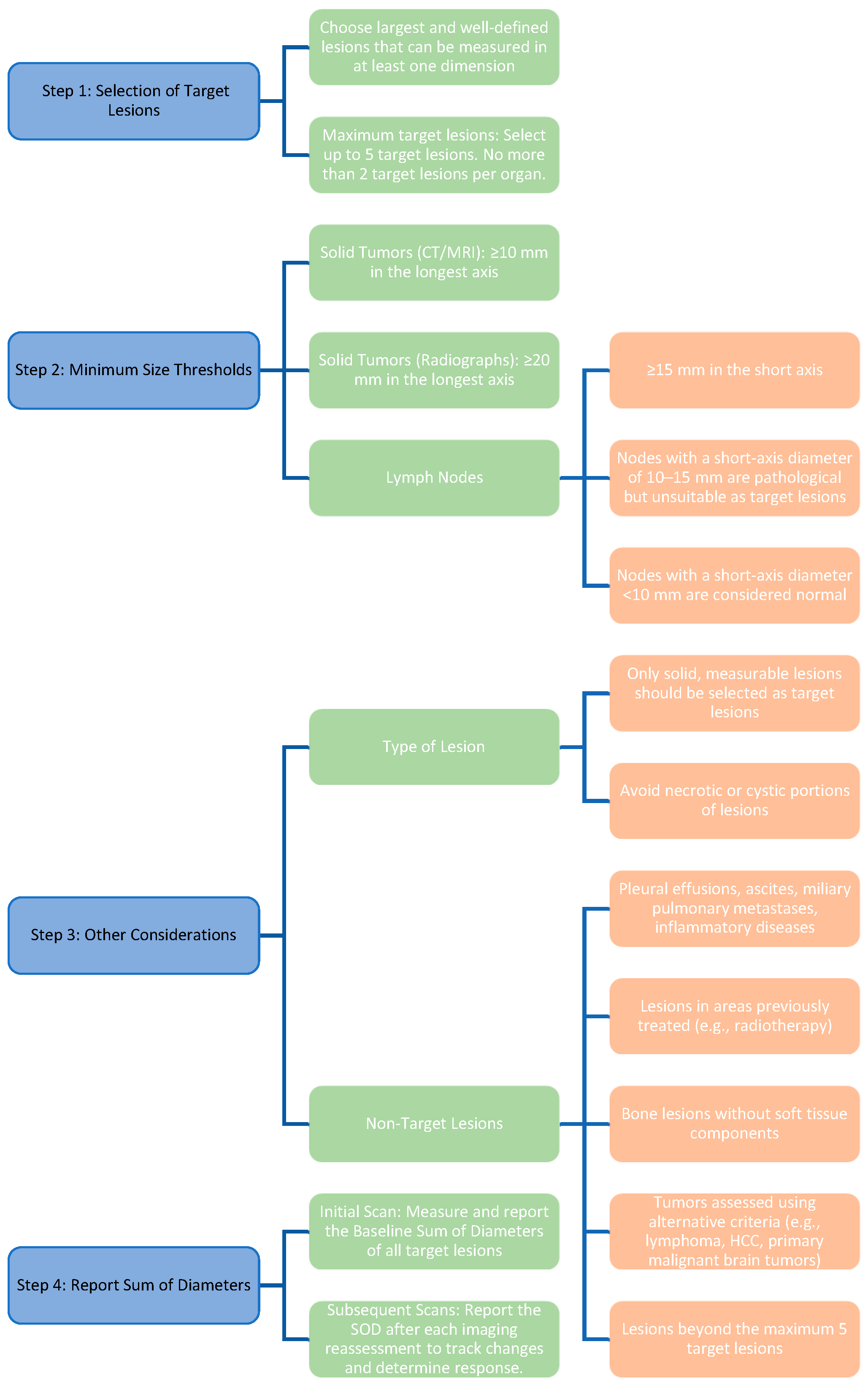
| Category | Criteria/Description |
|---|---|
| Number of target lesions |
|
| Minimum size thresholds |
|
| Type of lesion |
|
| Non-target lesions | Lesions that do not meet criteria for target selection, including:
|
| Response Category | Criteria |
|---|---|
| Complete Response (CR) | All of the following conditions must be met:
|
| Partial Response (PR) | All of the following conditions must be met:
|
| Progressive Disease (PD) | Progression is indicated by any of the following criteria:
|
| Stable Disease (SD) | Criteria for PD or PR are not met |
| Response Category | Acronym | Definition | Imaging Criteria | Recommended Action | Additional Notes |
|---|---|---|---|---|---|
| Immune Unconfirmed Progressive Disease | iUPD | Apparent disease progression compared to the nadir, but not meeting RECIST 1.1 criteria for PD |
| Continue treatment if clinically appropriate; repeat imaging within 4–8 weeks | Allows for the detection of pseudoprogression |
| Immune Confirmed Progressive Disease | iCPD | Apparent disease progression compared to the nadir, but not meeting RECIST 1.1 criteria for PD |
| Discontinue treatment if clinically indicated | Confirms that initial iUPD was true progression |
| Immune Complete Response | iCR | Disappearance of all target, non-target, and new lesions |
| Continue treatment or consider discontinuation based on protocol | Equivalent to CR per RECIST 1.1 |
| Immune Partial Response | iPR | Reduction of ≥30% in total tumor burden of target lesions |
| Continue treatment | Same as PR per RECIST 1.1 |
| Immune Partial Response | iSD | Does not meet criteria for iCR, iPR, or iCPD |
| Continue treatment and monitor | Same as SD per RECIST 1.1 |
| Characteristic | Description | Clinical Relevance |
|---|---|---|
| Persistence over time | The tumor response (e.g., complete remission or significant tumor reduction) remains stable over a prolonged period, confirmed through serial imaging | Indicates sustained antitumor activity and the potential for long-term disease control |
| Long-term efficacy | After discontinuation of immunotherapy, the immune system continues to exert antitumor effects, maintaining the response | Suggests durable immunologic memory and prolonged therapeutic benefit without continuous treatment |
| Lack of progression | No emergence of new lesions or growth of existing ones during the entire follow-up period | Demonstrates ongoing disease stability and supports continued benefit from the initial response |
| Clinical impact | Patients experience prolonged clinical benefit, including improved survival outcomes and quality of life | Highlights the broader value of durable responses beyond imaging metrics alone |
| Pseudoprogression followed by response | An initial increase in tumor size due to immune cell infiltration is followed by true tumor shrinkage, with response sustained over time | Requires careful monitoring and interpretation to avoid premature discontinuation of effective therapy |
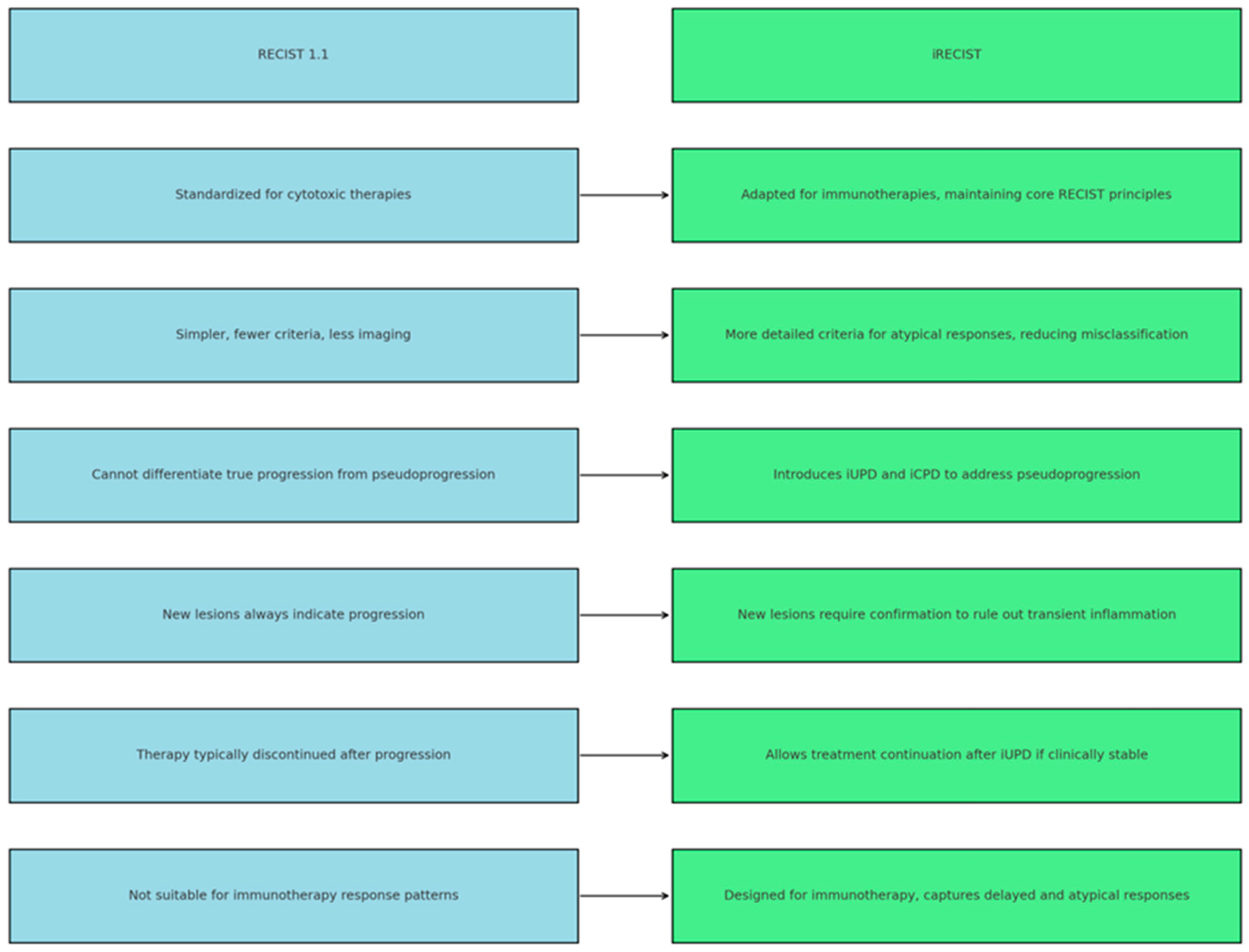
| Aspect | RECIST 1.1 | iRECIST | Strengths/Limitations Summary |
|---|---|---|---|
| Origin | Developed in 2000 (updated in 2009—RECIST 1.1) to standardize tumor response evaluation for solid tumors treated with cytotoxic therapies | Developed in 2017 as an improvement to RECIST 1.1, addressing atypical response patterns such as pseudoprogression and delayed responses | iRECIST builds upon RECIST 1.1, adapting it for immunotherapy-specific phenomena |
| Main Objective | Evaluates changes in tumor burden to determine tumor response or progression | Evaluates immune response patterns, including pseudoprogression | iRECIST expands applicability beyond cytotoxic therapies |
| Lesion Categories |
|
| Structural consistency ensures comparability |
| Definition of Target Lesions |
| Same as RECIST 1.1 (up to 5 lesions, maximum of 2 per organ) | Maintains methodological uniformity |
| Definition of Non-Target Lesions | Non-measurable lesions monitored for significant progression or complete response (e.g., ascites, lymphatic spread) | Same definition, but progression requires confirmation with additional imaging | Enhances reliability for immune-related cases |
| Impact of new lesions | Impact of New Lesions | New lesions are recorded, but do not immediately confirm progression; further confirmation is required to rule out pseudoprogression | Reduces false positives due to inflammatory activity |
| Complete Response (CR) | Disappearance of all lesions | Same as RECIST 1.1 | - |
| Partial Response (PR) | Reduction >30% in the sum of the diameters of target lesions | Same as RECIST 1.1 | - |
| Stable Disease (SD) | Criteria not meeting CR or PR | Same as RECIST 1.1 | - |
| Progressive Disease (PD) | Increase >20% in the sum of diameters of target lesions or clear progression of target lesions/new lesions | Initial progression is labeled as iUPD and requires imaging confirmation for iCPD | Prevents premature discontinuation of effective immunotherapy |
| Handling of Pseudoprogression | Not addressed. Progressive disease is diagnosed immediately |
| Improves diagnostic accuracy under immunotherapy |
| Additional Immune Categories | Not applicable | Adds immune categories:
| Allows for refined classification of immune responses |
| Confirmation of PD |
|
| Enables continuation of therapy in clinically stable patients |
| Commonly Applied Therapies |
|
| Each criterion suits specific treatment classes |
| Clinical Decisions | Therapy is often discontinued after confirmation of progressive disease | Therapy may continue after iUPD if the patient’s clinical condition permits, as pseudoprogression or delayed response is possible | Improves management of atypical immune responses |
| Use in Immunotherapy | Not suitable for immune checkpoint inhibitor treatments | Specifically designed for immune therapies to characterize delayed responses and atypical patterns | iRECIST provides superior clinical applicability |
| Advantages | Standardized, simple, and reproducible; broadly applicable in conventional oncology | Addresses atypical immune responses; reduces risk of misclassification | Both contribute to consistent evaluation; iRECIST offers higher sensitivity to immune phenomena |
| Limitations | Cannot distinguish true progression from pseudoprogression; may lead to premature treatment discontinuation | More complex and resource-demanding; confirmation delays may prolong evaluation timelines | Trade-off between simplicity and immunologic precision |
| Authors (Publication Year) | Study Location | Number of Patients | Total Duration | ICI Treatment | Criteria Compared | Key Findings | Reported Pseudoprogressions | Response Predictors | Overall Survival (OS) | Progression-Free Survival (PFS) | Imaging Modality Used | Criteria Concordance | Response Type Analyzed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nelles et al., 2024 [66] | Germany | 252 | 4 years | Immune checkpoint inhibitors | RECIST 1.1 iRECIST | iRECIST better captured atypical responses. Time to progression (TTP) was significantly longer with iRECIST | Not reported | Immune response patterns | 618.3 ± 626.9 days | 538.1 ± 617.9 days | CT | High | Atypical immune response |
| Ayati et al., 2021 [63] | Iran | 72 | 30 months | PD-1 inhibitors | RECIST 1.1 iRECIST PERCIST1.0 | All criteria correlated with OS. iRECIST was superior for atypical immune responses | 7% | SUL (Standardized Uptake Value) | Significant correlation with OS | Significantly improved | PET/CT | Moderate | Metabolic response |
| Gupta et al., 2022 [65,68] | India | 20 | 24 months | Nivolumab | iRECIST imPERCIST | High concordance between criteria. iRECIST and imPERCIST better predicted PFS than RECIST 1.1. | Not reported | Tumor size | Not reported | Significantly higher in imPERCIST | PET/CT | High | Complete/partial response |
| Castello et al., 2019 [69] | Italy | 52 | 36 months | PD-1/PD-L1 inhibitors | RECIST 1.1 iRECIST iPERCIST | iPERCIST provided better predictions for OS and PFS than anatomical criteria. Moderate concordance. | Not reported | Tumor metabolism | Longer OS for iPERCIST responders | Significantly improved | PET/CT | Moderate | Complete/partial response |
| Beer et al., 2019 [70] | Austria | 42 | 24 months | PD-1/PD-L1 inhibitors | RECIST 1.1 iRECIST PERCIST | Moderate concordance between methods. All predicted OS with no significant differences between them. | Not reported | Tumor size and metabolism | Moderate correlation with OS | Moderate correlation | PET/CT | Moderate | Metabolic response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladin, R.; Oancea, C.; Stoicescu, E.R.; Pusztai, A.M.; Constantinescu, A.; Poplicean, E.; Manolescu, D. The Role of 18F-FDG PET/CT in Monitoring Immunotherapy Response in Non-Small Cell Lung Cancer: Current Evidence and Challenges: A Narrative Review. Diagnostics 2025, 15, 2754. https://doi.org/10.3390/diagnostics15212754
Mladin R, Oancea C, Stoicescu ER, Pusztai AM, Constantinescu A, Poplicean E, Manolescu D. The Role of 18F-FDG PET/CT in Monitoring Immunotherapy Response in Non-Small Cell Lung Cancer: Current Evidence and Challenges: A Narrative Review. Diagnostics. 2025; 15(21):2754. https://doi.org/10.3390/diagnostics15212754
Chicago/Turabian StyleMladin, Roxana, Cristian Oancea, Emil Robert Stoicescu, Agneta Maria Pusztai, Amalia Constantinescu, Emanuel Poplicean, and Diana Manolescu. 2025. "The Role of 18F-FDG PET/CT in Monitoring Immunotherapy Response in Non-Small Cell Lung Cancer: Current Evidence and Challenges: A Narrative Review" Diagnostics 15, no. 21: 2754. https://doi.org/10.3390/diagnostics15212754
APA StyleMladin, R., Oancea, C., Stoicescu, E. R., Pusztai, A. M., Constantinescu, A., Poplicean, E., & Manolescu, D. (2025). The Role of 18F-FDG PET/CT in Monitoring Immunotherapy Response in Non-Small Cell Lung Cancer: Current Evidence and Challenges: A Narrative Review. Diagnostics, 15(21), 2754. https://doi.org/10.3390/diagnostics15212754








