Abstract
Background: To identify prognostic factors related to survival in patients with early-stage cervical cancer treated with radical surgery in six high-volume gynecologic oncology centers in Turkey. Methods: This retrospective analysis examined a cohort of 612 patients diagnosed with cervical cancer who underwent type II/III radical hysterectomy and pelvic lymphadenectomy, with or without para-aortic lymphadenectomy at six gynecologic oncology centers. A total of 537 patients between 1993 and 2023 were included. According to the 2009 FIGO staging system, 411 patients (76.5%) were stage IB1, 76 (14.2%) were stage IB2, 40 (4.7%) were stage IIA1, and 10 (1.9%) were stage IIA2. Patients underwent either type II or type III radical hysterectomy with pelvic lymphadenectomy, with para-aortic lymphadenectomy performed in 93.1% of cases. Among the 537 patients, 258 (48%) underwent type II radical hysterectomy and 279 (52%) underwent type III. Univariate and multivariate analyses of 5-year overall survival (OS) and 5-year disease-free survival (DFS) were performed. Results: In the entire cohort, 258 (48%) patients underwent radical surgery alone, while 279 (52%) patients underwent radical surgery followed by adjuvant therapy. The 5-year DFS and 5-year OS rates were 85.3% and 98.4%, respectively. In the multivariate logistic analysis, lymph node metastasis was identified as an independent prognostic factor for DFS and OS. Conclusions: Lymph node metastasis was the most important prognostic factor for survival in this large multicenter Turkish cohort. These findings highlight the prognostic value of nodal status, stromal invasion, margin status, and LVSI, while underscoring the importance of tailored adjuvant treatment strategies.
1. Introduction
Cervical cancer remains a major global health concern and is among the most common cancers in women worldwide, although screening programs have reduced its incidence in developed regions, recurrence and mortality rates remain considerable in global estimates [1]. The most recent GLOBOCAN 2020 report indicates approximately 604,000 new cases and 342,000 deaths worldwide, placing cervical cancer among the leading causes of cancer-related mortality in women [1]. The 5-year survival rate for cervical cancer is 80% when detected in the early stages, specifically International Federation of Gynecology and Obstetrics (FIGO) stage I-II [2]. Taken together, these figures underscore the ongoing global significance of the disease and the need for precise risk stratification.
For early-stage disease, radical hysterectomy with pelvic lymph node dissection or definitive radiotherapy, with or without concurrent chemotherapy, is regarded as the standard treatment [3]. Extensive investigations have been conducted to analyze the impact of clinical and pathological variables on survival outcomes. Reported prognostic factors include lymph node metastases, tumor size, cervical stromal invasion, lympho-vascular space invasion (LVSI), histological subtype, and direct tumor extension to the vagina, parametrium, or surgical border involvement [4,5]. Although not directly included in the current staging system, these clinicopathological factors remain crucial in determining the need for adjuvant therapy after surgery [6].
Cervical cancer is almost entirely caused by persistent infection with oncogenic HPV types, particularly HPV 16 and 18, which account for the vast majority of cases [7]. The implementation of vaccination programs developed against these viral strains has brought about a significant transformation in the field of cervical cancer prevention. Population-based data from Europe and North America have demonstrated a marked decline in high-grade cervical lesions among vaccinated cohorts; however, vaccination coverage remains suboptimal in many low- and middle-income regions, where cervical cancer continues to be a major cause of cancer-related mortality [8,9].
Screening methods such as Pap smear cytology and, more recently, HPV DNA tests have played a critical role in reducing mortality by detecting precancerous lesions before they progress [10]. However, outcomes are not the same for every patient; some patients diagnosed at an apparently early stage still experience recurrence. This highlights the need for additional prognostic indicators beyond just the classic histopathological findings [3]. Beyond traditional factors emerging evidence suggests that molecular features such as p16 overexpression, PD-L1 status, and host immune response profiles may make risk stratification in cervical cancer more sensitive [11,12]. Integrating such biomarkers with classical clinicopathological variables has the potential to refine prognostic assessment and personalize adjuvant treatment decisions.
Surgery remains the mainstay of treatment for early-stage disease, but fertility-sparing interventions such as radical trachelectomy are increasingly being performed in carefully selected young patients with small tumors [13]. In addition, organ-preserving modalities under investigation, such as photodynamic therapy (PDT) have been explored in cervical intraepithelial lesions and microinvasive cases, but have also been proposed as potential investigational options in the context of organ preservation for very early-stage cervical cancer [14]. The role of minimally invasive radical hysterectomy, which was previously widely adopted, was re-evaluated after the LACC trial reported poorer survival compared with open surgery [15]. These results highlighted the prognostic importance of the surgical approach. Overall, current therapeutic options range from definitive surgery or radiotherapy to fertility- and organ-preserving strategies in select patients, reinforcing the importance of accurate baseline risk assignment.
In this study, we aimed to analyze the clinical, surgical, and pathological characteristics of cervical cancer patients with FIGO IB1-IIA2 who underwent radical hysterectomy and lymphadenectomy with/without adjuvant therapy, as well as identify prognostic markers associated with survival outcomes. This investigation represents one of the largest contemporary multicenter cohorts in Turkey, comprising 537 patients from six high-volume gynecologic oncology centers. By integrating surgical, pathological, and survival data, it provides robust, externally valid estimates of disease-free and overall survival and clarifies the prognostic weight of nodal status alongside other clinicopathological variables within a modern treatment framework.
2. Materials and Methods
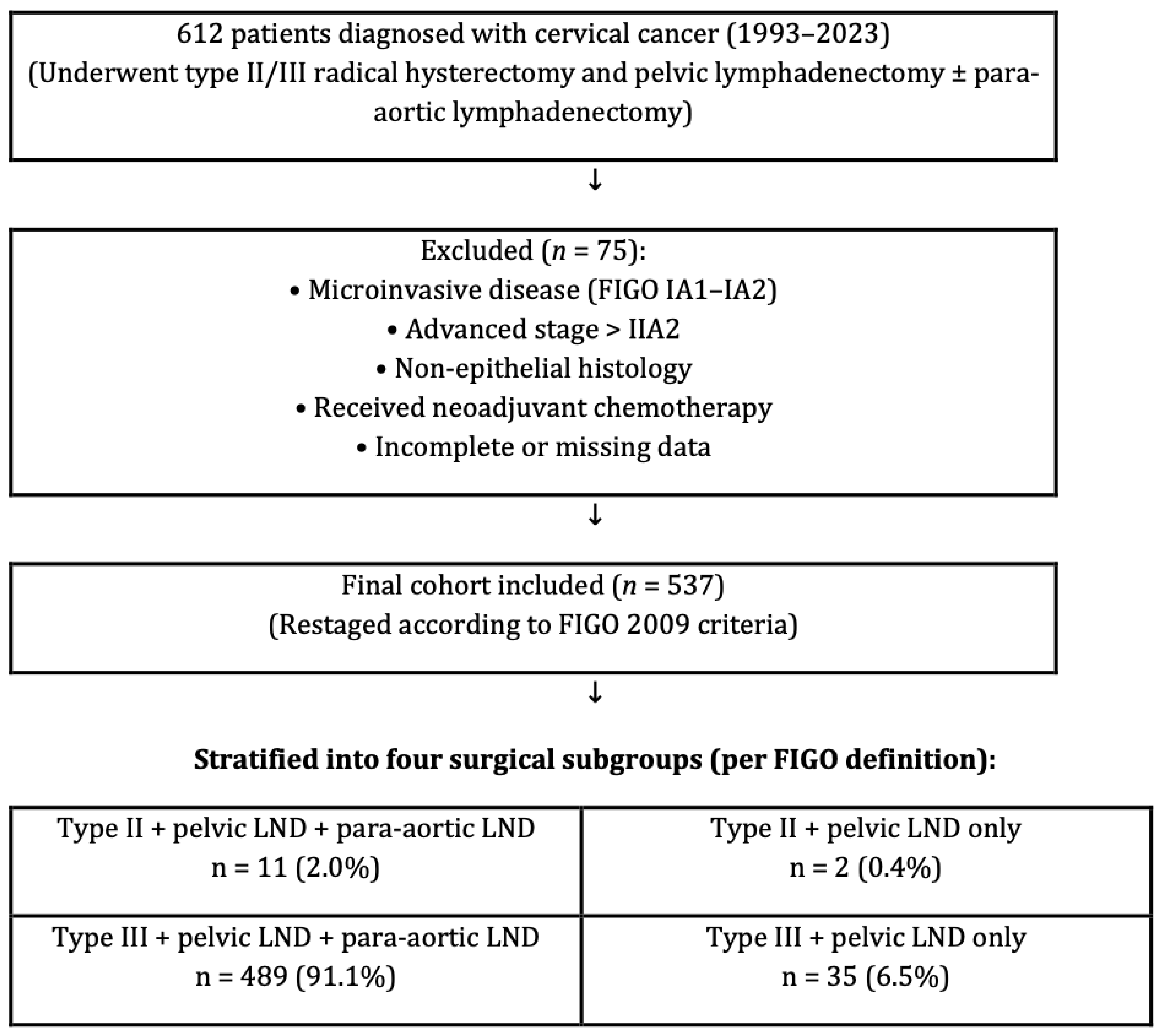
This retrospective analysis examined a cohort of 612 patients diagnosed with cervical cancer who underwent type II/III radical hysterectomy and pelvic lymphadenectomy, with or without para-aortic lymphadenectomy. The investigation was conducted at six gynecologic oncology centers between 1993 and 2023. All surgeries were performed by gynecologic oncologists trained at a single institution certification program so homogenization in surgeries could be obtained. Although this represents a relatively long inclusion period, we considered it necessary in order to achieve an adequate sample size from six high-volume institutions. To minimize potential heterogeneity arising from changes in treatment standards, surgical techniques, and diagnostic technologies over time, all patients were restaged uniformly according to the FIGO 2009 classification and pathological evaluation was standardized across centers. A total of 537 patients were eligible after exclusions, contributed from six independent centers. To ensure transparency while maintaining institutional anonymity, the distribution of patients across the centers is provided in Table 1. Patient information was obtained by utilizing the computerized database system, medical files, and pathology reports. Detailed inclusion and exclusion criteria are summarized in Table 2. In brief, eligible patients were women with histologically confirmed cervical carcinoma (FIGO 2009 stages IB1–IIA2) treated with primary radical hysterectomy and pelvic lymphadenectomy. Patients were excluded if they had microinvasive disease, advanced stage beyond IIA2, non-epithelial histology, secondary cervical involvement due to metastasis from other gynecologic malignancies (e.g., endometrial or ovarian carcinoma) synchronous primary tumors, uncertain adjuvant therapy status, receipt of neoadjuvant chemotherapy, preoperative radiotherapy, or incomplete medical records. Importantly, neoadjuvant chemotherapy prior to surgery was an exclusion criterion, and therefore no patients receiving such treatment were included in the final analysis. For clarity, “surgery” in this study refers to type II or type III radical hysterectomy with pelvic lymphadenectomy, with or without para-aortic lymphadenectomy, as illustrated in Figure 1. “Synchronous primary tumors” were defined as histologically distinct gynecologic malignancies diagnosed concurrently with cervical carcinoma. A study flow diagram summarizing patient inclusion, exclusion, and stratification into surgical subgroups (type II/III radical hysterectomy with or without para-aortic lymphadenectomy) is presented in Figure 1. The local institutional ethical board evaluated and approved the research procedure (approval: E2-23-3801/12.04.2023).

Table 1.
Distribution of patients across participating centers.

Table 2.
Inclusion and exclusion criteria for patient selection.
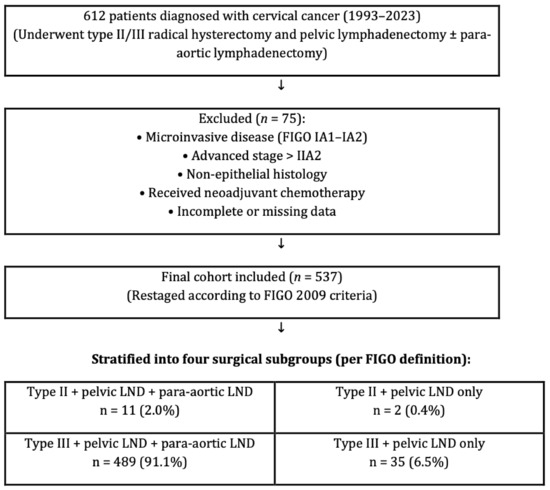
Figure 1.
Study flow diagram showing patient inclusion, exclusion, and stratification into four surgical subgroups according to the FIGO classification of radical hysterectomy (Type II or III) and the extent of lymphadenectomy (pelvic ± para-aortic).
Gynecological oncologists performed all surgical procedures. The extent of surgery was determined according to patient- and disease-related factors. Type II or type III radical hysterectomy was performed via open laparotomy or, in selected cases, minimally invasive approaches. Pelvic lymphadenectomy was systematically performed, and para-aortic lymphadenectomy was added at the discretion of the senior surgeon, typically up to the level of the inferior mesenteric artery or left renal vein. In patients with significant comorbidities or unfavorable intraoperative conditions, lymphadenectomy was limited to the pelvic region. Bilateral salpingo-oophorectomy was performed based on patient age, ovarian status, and surgeon preference.
Cervical tumors were classified as; squamous cell carcinoma (keratinizing, non-keratinizing, papillary, warty, verrucous, basaloid, lymphoepithelial), adenocarcinoma (endocervical, mucinous, endometrioid, clear cell, serous, mesonephric, villoglandular) and other (adenosquamous, undifferentiated, adenoid basal, adenoid cystic) types. Histopathological evaluation was carried out according to the 2020 World Health Organization (WHO) criteria [16]. The tumor size was determined by the tumor’s greatest diameter after fixation in paraffin blocks. Deep stromal invasion was characterized by a tumor invading the exterior half of the cervical stroma (>50% of full thickness). In hematoxylin and eosin stained pathologic sections, LVSI was described as tumor cells or cell clusters adhering to vascular walls containing both tumor and healthy tissue in the surrounding area. Surgical border involvement was defined as tumor positivity within a 5 mm margin of the pathological specimen, whereas vaginal metastasis was defined as the presence of the tumor anywhere in the vagina. The patients were staged according to the FIGO 2009 criteria [17].
Baseline demographic and clinical characteristics (age, FIGO stage, histological subtype, tumor size, depth of invasion, LVSI, margin status, etc.) were compiled into a comprehensive table (Table 3) to facilitate comparison with other studies.

Table 3.
Characteristics of entire cohort.
Additional subgroup distribution according to the extent of radical hysterectomy and lymphadenectomy is summarized below (Table 4).

Table 4.
Distribution of patients according to surgical type and para-aortic lymphadenectomy status.
Recurrence distal to the pelvic inlet was considered pelvic recurrence, whereas recurrence between the pelvic inlet and diaphragm was considered upper-abdominal recurrence. The remaining failure categories were classified as extra-abdominal recurrences such as recurrences in the liver parenchyma, skin, and bone. Among the 60 patients with recurrence, 36 cases occurred outside the pelvic cavity and were categorized as extra-abdominal recurrence.
Each center’s tumor board made judgments on adjuvant treatment. Adjuvant therapy was defined strictly as treatment administered after primary surgery; patients who had received neoadjuvant chemotherapy were not included in this category. Following surgery ± adjuvant therapy, all patients were entered into a routine surveillance program, with visits scheduled every three months for the first two years, every six months for the next three years, and every year thereafter. On the basis of their symptoms, patients with a suspicion of disease recurrence underwent additional radiological evaluation. Disease-free survival (DFS) was defined as the time between the initial surgery and recurrence, or the time between the initial surgery and the last follow-up visit in the absence of recurrence. Overall survival (OS) was defined as the length of time between initial surgery and mortality due to the disease or the last follow-up visit.
Statistical Analysis
All statistical analyses were performed using SPSS version 22.0 (IBM SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation or median (range), and categorical variables as number (percentage). Survival estimates for DFS and OS were calculated with the Kaplan–Meier method, and differences were compared with the log-rank test [18]. Variables significant in univariate analyses, or deemed clinically important, were entered into a Cox proportional hazards regression model to identify independent prognostic factors, with hazard ratios (HRs) and 95% confidence intervals (CIs) reported [19]. A p-value < 0.05 was considered statistically significant. In addition to whole-cohort analyses, patients were stratified into four surgical subgroups (type II ± para-aortic, type III ± para-aortic) for exploratory survival comparisons.
3. Results
3.1. Patient’s Characteristics
3.2. Adjuvant Therapy and Survival
In the entire cohort, 258 (48%) patients underwent radical surgery alone, while 279 (52%) patients underwent radical surgery followed by adjuvant therapy. Adjuvant treatment consisted of concomitant chemoradiotherapy (CCRT; n: 146, 27.2%), radiotherapy alone (RT; n: 125, 23.3%), and chemotherapy (CT) followed by RT (n: 3, 0.6%) (Table 3).
The median follow-up period was 36 months (range 1–300 months). The 5-year DFS and 5-year OS rates were 85.3% and 98.4%, respectively. Recurrence was detected in 60 (11.2%) patients, and 28 (5.2%) patients died because of the disease. In patients with recurrence, the mean time between surgery and recurrence was 22 months (range: 1–75 months). In 27 (5%) patients, recurrences were limited to the pelvic region, in 2 (0.4%) patients to the upper abdominal region, and in 12 (2.2%) patients to the extra abdominal region only. Hepatic recurrence, pulmonary recurrence, bone recurrence, and brain recurrence were detected in 8 (1.5%) patients, 21 (3.9%) patients, 6 (1.1%) patients, and 1 (0.2%) patient, respectively. Data on adjuvant treatment and recurrence patterns are shown in Table 5.

Table 5.
Adjuvant Treatment, Recurrence and Recurrence Pattern.
3.3. Disease-Free Survival Analysis
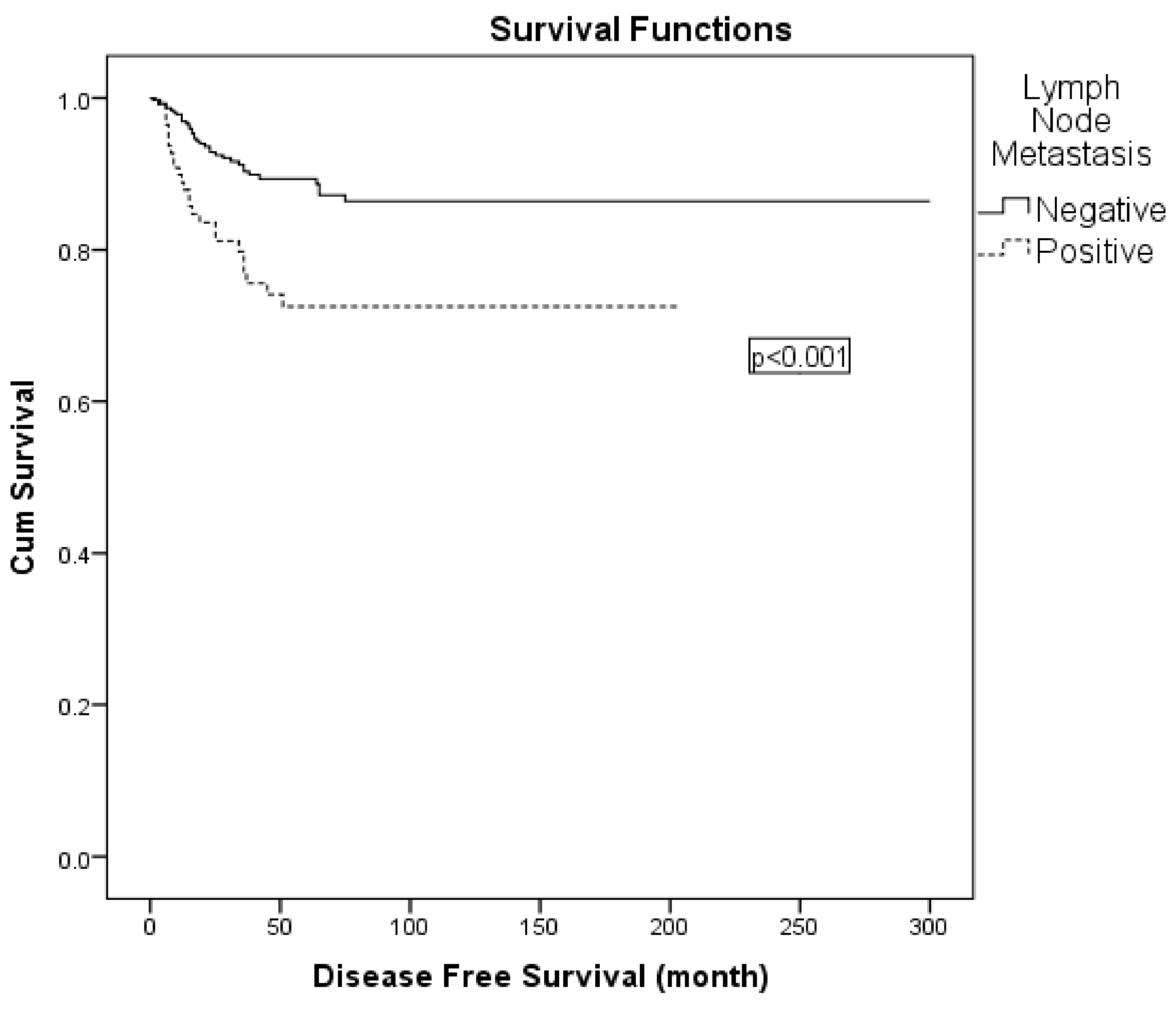
Variables with statistical or clinical relevance were subsequently entered into a multivariable Cox regression model. Given the strong association between nodal positivity and receipt of adjuvant radiotherapy, the latter was excluded from the multivariable model to avoid collinearity. The final model included FIGO 2009 stage (IIA vs. IB), lymph node metastasis (positive vs. negative), surgical margin status (positive vs. negative), and depth of stromal invasion (>50% vs. ≤50%). In this analysis, lymph node metastasis was identified as an independent prognostic factor for DFS (hazard ratio: 2.049; 95% CI: 1.192–3.522; p = 0.009) (Table 6; Figure 2). When patients were stratified into four surgical subgroups (type II plus pelvic LND with para-aortic, type II plus pelvic LND without para-aortic, type III plus pelvic LND with para-aortic, and type III plus pelvic LND without para-aortic lymphadenectomy), no statistically significant differences in 5-year DFS were observed between the groups (Table 6). In the comparison of surgical subgroups, adjuvant radiotherapy was administered in 52.8% of all patients, most frequently in those who underwent type III radical hysterectomy with para-aortic lymphadenectomy. However, the difference in adjuvant RT rates between surgical types was not statistically significant (χ2 = 4.978, p = 0.173).

Table 6.
Factors related with disease-free survival.
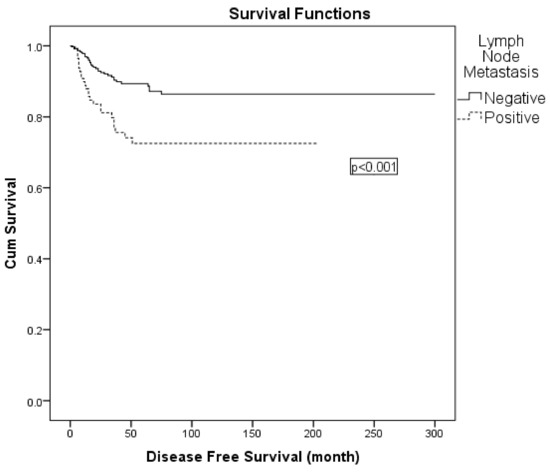
Figure 2.
Kaplan–Meier curve for disease-free survival according to lymph node status.
A subgroup analysis was performed on 127 patients with lymph node metastases. The 5-year DFS rates for negative and positive metastatic paraaortic lymph nodes were 76% and 56%, respectively (p = 0.223). Analyzing the relationship between the number of metastatic lymph nodes and survival in this group, patients with single lymph node metastases had a 5-year DFS of 84%, while those with two or more lymph node metastases had a DFS of 63%. Although there was a tendency for a difference, there was no statistically significant difference between the groups (p = 0.061).
3.4. Overall Survival Analysis
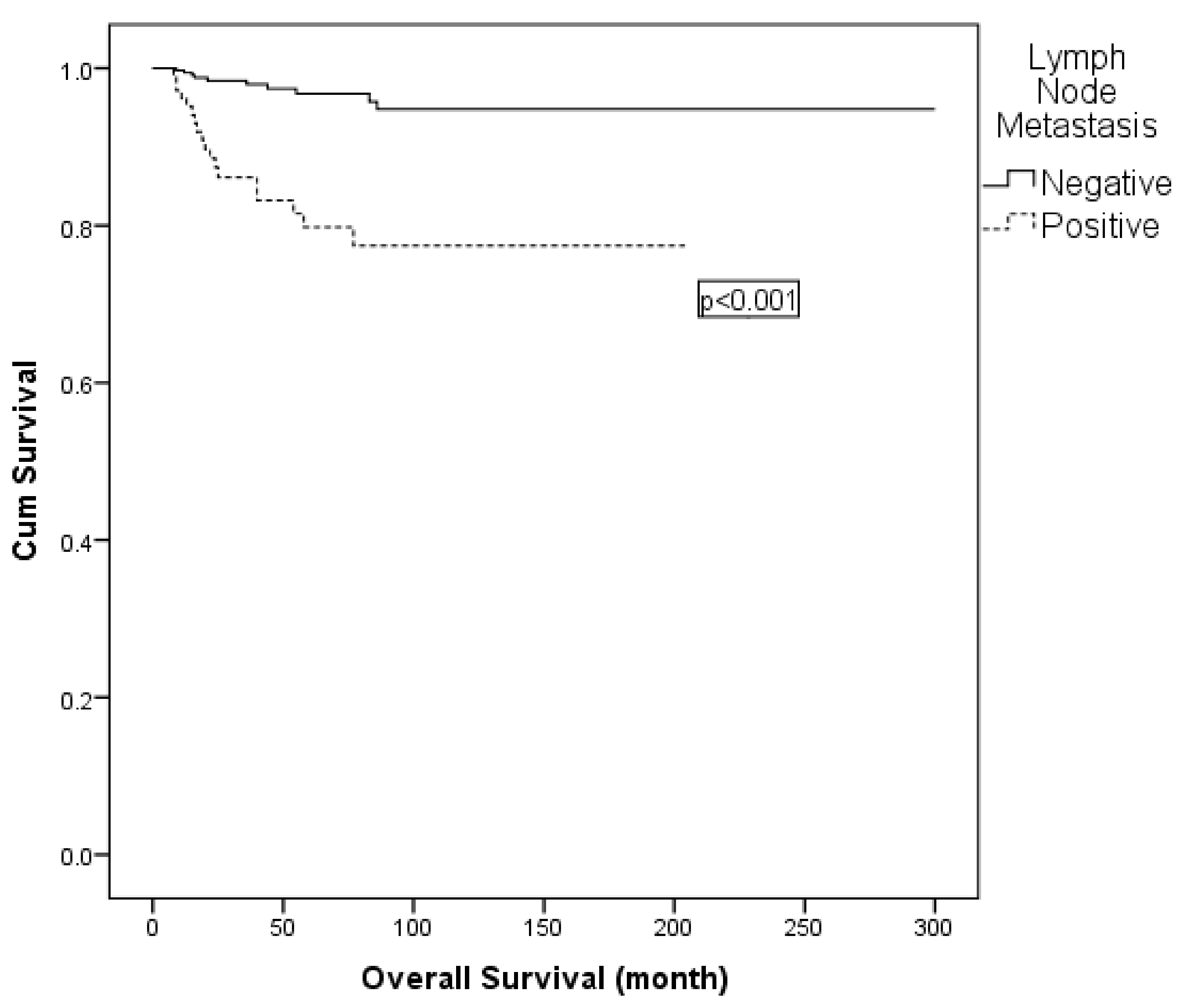
On univariate analysis, lymph node metastasis, LVSI and adjuvant radiotherapy were found to be effective for OS. In multivariable Cox regression, adjuvant radiotherapy was excluded due to collinearity with nodal status. The final model comprised lymph node metastasis (positive vs. negative) and LVSI (positive vs. negative). Lymph node metastasis remained an independent prognostic factor for OS (hazard ratio: 4.529; 95% CI: 1.974–10.394; p < 0.001) (Table 7; Figure 3). Similarly, subgroup comparisons according to surgical type (type II vs. type III, with or without para-aortic lymphadenectomy) did not reveal significant differences in 5-year OS (Table 7).

Table 7.
Factors related to overall survival.
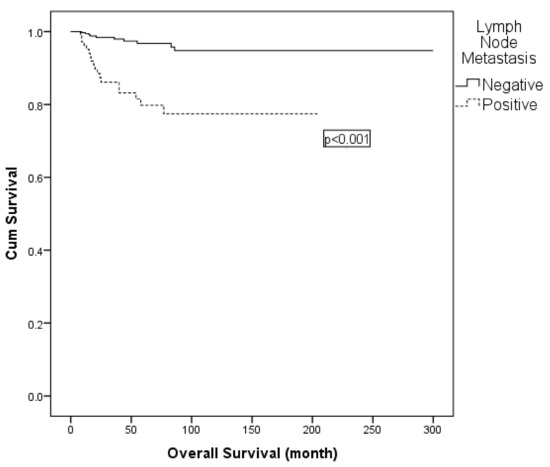
Figure 3.
Kaplan–Meier curve for overall survival according to lymph node status.
A subgroup analysis was performed on 127 patients with lymph node metastasis. The 5-year OS rates for metastatic paraaortic lymph nodes that were negative and positive were 80% and 74%, respectively (p = 0.383). Analyzing the relationship between the number of metastatic lymph nodes and survival in this group, patients with single lymph node metastases had a 5-year OS of 89%, while patients with two or more lymph node metastases had a 5-year OS of 73%. Although there appeared to be a distinction between the groups, this was not statistically significant (p = 0.079).
4. Discussion
In this study, we analyzed the clinical, surgical, and pathological characteristics of 537 patients with stage IB1-IIA2 cervical cancer who underwent radical hysterectomy and lymphadenectomy +/− adjuvant therapy. Additionally, the study sought to investigate the potential association between survival outcomes and these aforementioned variables.
Our results show the 5-year DFS and OS rates to be 85.3% and 98.4%, respectively. Recurrence was observed in 11.2% of the patients and 5.2% died as a result of the disease. Lymph node metastasis was found to be an independent prognostic factor for DFS and OS.
Many studies have confirmed a significant correlation between lymph node metastasis and cervical cancer patient prognosis [20,21,22]. Our study revealed that the 5-year DFS and OS for patients with lymph node metastasis were 73% and 80%, compared to 89% and 97% for patients without lymph node metastasis, respectively. In a study of 197 patients with FIGO stage IB-II cervical cancer presented by Ho et al., 5-year DFS was found to be 67.2% in patients with lymph node metastasis whereas individuals without lymph node metastasis exhibited a higher DFS rate of 87.3%. Similarly to our report, lymph node metastasis was determined to be an independent prognostic factor for DFS in that study [23]. In a multivariate analysis of 102 patients with stage IA2-IIB cervical cancer, Obrzut et al. also reported lymph node metastasis as an independent and significant variable for DFS and OS [24].
Recent analyses have also highlighted that the number of positive lymph nodes has an impact on prognosis and that survival loss is associated with nodal burden [25]. Additionally, some studies have reported that the anatomic location of lymph node metastasis (pelvic vs. paraaortic) is prognostically important, but our results did not show a significant difference. This difference may be due to differences in surgical extent and adjuvant treatment approaches between centers [26]. Although some studies have suggested that the extent of surgery (type II vs. type III radical hysterectomy, with or without para-aortic lymphadenectomy) may influence survival outcomes, we were unable to demonstrate such an association in our cohort. This may be related to heterogeneity in surgical practice across centers and time periods, as well as incomplete documentation of surgical details in earlier cases [27]. In our updated subgroup analysis (Table 6 and Table 7), no statistically significant differences were observed between pelvic-only and pelvic + para-aortic lymph-node dissection groups in terms of 5-year DFS (87% vs. 85%, p = 0.506) and OS (100% vs. 92%, p = 0.232). The slightly lower OS in the type III + PALND subgroup likely reflects that patients with poorer prognostic features such as LVSI, deep stromal invasion, or larger tumor size were more frequently selected for para-aortic dissection. This finding suggests that the extent of lymph-node dissection itself does not independently affect prognosis once baseline risk factors are considered. In the present study, 127 (23.6%) patients had metastatic lymph nodes and the median number of metastatic lymph nodes was 2 (range, 1–37). Our study showed that the 5-year DFS and OS were 84% and 89% for those with only one positive node and 63% and 73% for those with two or more positive nodes, respectively. In a study presented by Ho et al., the 5-year DFS was 60.4% for those with a single positive lymph node, 58.6% for those with two or three positive nodes, 45.9% for those with four or more positive nodes, and 80.4% for those without any positive nodes [23]. Unlike our study, in this study, a statistically significant correlation was found between the number of metastatic lymph nodes and survival. In a study of 2222 patients with FIGO 2009 stage IA-IIB cervical cancer, Zhou et al. reported that patients with 1–2 positive lymph nodes had better OS and DFS outcomes than patients with > 2 positive lymph nodes [28].
Other pathological parameters, such as lymphovascular space invasion (LVSI), depth of stromal invasion, and positive surgical margins, also remain important determinants of recurrence risk. Several studies have shown that LVSI is strongly associated with lymph node metastasis and poorer survival, thus serving as a potential marker of tumor aggressiveness [29,30]. Similarly, tumor size > 4 cm has consistently been reported as a factor increasing the risk of recurrence, as reflected in adjuvant treatment guidelines such as ESGO and NCCN [31]. Furthermore, the ability to achieve complete tumor removal at the initial surgery is a recognized prognostic factor in cervical cancer. However, as R0/R1 status was not systematically recorded in all centers, its impact could not be assessed in this analysis. This is a limitation that warrants consideration in future prospective studies [32]. Recent meta-analyses have further confirmed the prognostic significance of these parameters, particularly in early-stage disease [33].
In our cohort, adjuvant radiotherapy was administered to approximately half of the patients (52.8%), most commonly among those undergoing type III radical hysterectomy with para-aortic lymphadenectomy. Although adjuvant RT rates varied across surgical subgroups, the difference was not statistically significant (p = 0.173). This finding likely reflects the higher prevalence of high-risk pathological features in patients selected for extended lymphadenectomy, rather than differences in institutional treatment preferences.
The findings of our study indicate that there was no correlation between the location of the positive lymph nodes (pelvic vs. paraaortic) and DFS or OS. In contrast to our findings, Takeda et al. analyzed the outcomes of 36 lymph node-positive patients and found a correlation between the location of positive lymph nodes (pelvic node excluding common iliac nodes or common iliac nodes vs. paraaortic nodes) and DFS [34].
In the present study, the 5-year OS rate was considerably higher among patients without LVSI than among women diagnosed with LVSI (97 vs. 89%; p = 0.030), according to the univariate analysis. However, the results of the multivariate test showed no significance. While no statistically significant difference was seen, those without LVSI also had a higher 5-year DFS rate with a tendency towards significance. Similarly, Obrzut et al. reported in a study that although DFS and OS were higher in patients without LVSI, it was not an independent and significant variables [24]. An unexpected finding in our series was that patients who did not receive adjuvant radiotherapy had higher 5-year OS rates compared to those who did receive radiotherapy. This should not be considered a detrimental effect of radiotherapy. We believe this result is primarily due to the fact that patients who received radiotherapy had high-risk features such as lymph node involvement, margin positivity, or deep stromal invasion. The poorer initial prognosis of this group explains why their survival rates were lower despite receiving additional treatment.
Finally, although molecular prognostic markers such as p16INK4a and PD-L1 were discussed in the context of recent literature, it should be noted that these biomarkers were not analyzed within the present dataset. Their inclusion serves only to contextualize our findings within emerging molecular prognostic frameworks.
Moreover, emerging evidence suggests that additional histopathological and immune-related factors, such as tumor budding, cell nesting, and tumor-infiltrating lymphocytes, may further refine risk stratification in cervical cancer patients [35]. Similarly, scoping reviews focusing on Asian populations have confirmed that classical prognostic factors like histology, LVSI, and nodal status remain relevant even in diverse clinical contexts [36].
Molecular biomarkers are increasingly being evaluated to complement traditional histopathological risk factors. For example, p16INK4a overexpression has been shown in meta-analyses to be associated with improved disease-free and overall survival, as it may be linked to HPV-associated oncogenesis [12]. PD-L1 expression and tumor immune microenvironment profiles are also being investigated for predicting survival and response to adjuvant or immunotherapies [37]. Recent prognostic nomograms designed for younger cervical cancer populations also highlight the role of integrating molecular and clinical factors into predictive models [38]. Integrating these biomarkers into clinical models may increase prognostic accuracy and contribute to the individualization of adjuvant treatment decisions.
Importantly, this multicentric design allows validation of established prognostic factors under diverse real-world surgical and institutional conditions, bridging the gap between controlled single-center studies and everyday clinical practice. By including data from six tertiary gynecologic oncology centers, the study enhances the external validity and generalizability of its findings across heterogeneous treatment settings.
The primary limitation of this study is its retrospective design. The study’s strengths are its multicentric design and large patient population. Secondly, all surgical procedures were conducted by gynecologic oncologists, and then pathologic assessments were evaluated by expert gyneco-pathologists. Compared with more recent multicenter series, our findings remain consistent in demonstrating the prognostic importance of nodal involvement and LVSI, while also underlining the challenges of treatment heterogeneity across institutions [33,38].
Another limitation of the study is the heterogeneity in adjuvant treatment strategies across centers and over time. While this reflects real-world practice, it may account for some variability in the results. However, the multicenter nature of the cohort and long-term follow-up strengthen the external validity of our findings. In addition, detailed data regarding the specific surgical methods (type II vs. type III radical hysterectomy with or without para-aortic lymphadenectomy) were not uniformly available across all centers and time periods. All radical hysterectomies were performed by certified gynecologic oncologists in accordance with FIGO-based standards (Type II or III radical hysterectomy with systematic pelvic lymphadenectomy ± para-aortic dissection). Although R0/R1 status was not uniformly documented in earlier years, all included patients met the criteria for macroscopically complete resection at the time of surgery. For this reason, these parameters could not be included in the multivariate analysis, and we acknowledge this as a limitation. Similarly, information on the completeness of tumor resection (R0 vs. R1 status) was not consistently documented, although it is recognized as an important prognostic factor. Future prospective multicenter studies that include molecular biomarkers and standardize adjuvant treatment protocols will help confirm these observations.
5. Conclusions
In summary, our study confirms that lymph node metastasis remains an independent prognostic factor for DFS and OS in patients with early-stage cervical cancer. Although additional clinicopathological variables such as tumor size, depth of invasion, LVSI, and surgical margin status also influence outcomes, their independent predictive value is limited in multivariate models. These findings underscore the importance of accounting for potential collinearity between nodal status and adjuvant treatment variables when interpreting prognostic analyses. Our findings, consistent with recent multicenter studies, reinforce the continued importance of careful surgical staging and comprehensive risk assessment in guiding adjuvant treatment decisions.
Author Contributions
Conceptualization, Y.O.U., G.T.G., F.C. and T.T. (Taner Turan); Methodology, Y.O.U., O.A., H.E.K.Y., M.U., A.A.T., F.K., I.S., I.U. and D.B.; Software, M.U., B.E., C.C., C.K., T.T. (Tayfun Toptas), I.U., D.B. and T.T. (Tolga Tasci); Validation, G.T.G., C.C., I.S. and S.K.; Formal analysis, Y.O.U., H.E.K.Y., G.K.C., F.K. and C.K.; Investigation, O.O., G.T.G., M.G., M.U., F.C., D.Y., T.T. (Taner Turan) and T.T. (Tolga Tasci); Resources, N.Y., M.G., M.U., A.A.T., F.C., B.E., S.K.K., D.Y., C.C., T.T. (Taner Turan), T.T. (Tayfun Toptas), S.K., A.K., I.U., D.B. and T.T. (Tolga Tasci); Data curation, Y.O.U., O.A., N.Y., H.E.K.Y., G.K.C., G.T.G., F.K., B.E., S.K.K. and A.K.; Writing—original draft, Y.O.U.; Writing—review and editing, Y.O.U., G.T.G. and T.T. (Taner Turan); Visualization, O.O., M.G., C.C., C.K. and S.K.; Supervision, T.T. (Taner Turan). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ankara Bilkent City Hospital ethical committee evaluated and approved the research procedure in accordance with the Declaration of Helsinki (IRB: E2-23-3801/12 April 2023).
Informed Consent Statement
Our study is a retrospective study. Patient data were anonymized in our study. Patient consent was not obtained after our study was approved by the Ethics Committee Review Board (approval number: E2-23-3801, 12 April 2023).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
| OS | overall survival |
| DFS | disease-free survival |
| FIGO | International Federation of Gynecology and Obstetrics |
| LVSI | lympho-vascular space invasion |
| WHO | World Health Organization |
| CCRT | concomitant chemo-radiotherapy |
| RT | radiotherapy |
| CT | chemotherapy |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Zhao, D.; Li, B.; Wang, Y.; Liu, S.; Li, P.; Zuo, J.; Bai, P.; Zhang, R.; Wu, L. Prognostic evaluation of postoperative adjuvant therapy for operable cervical cancer: 10 years’ experience of National Cancer Center in China. Chin. J. Cancer Res. 2017, 29, 510. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 22–36. [Google Scholar] [CrossRef] [PubMed]
- Sevin, B.-U.; Lu, Y.; Bloch, D.A.; Nadji, M.; Koechli, O.R.; Averette, H.E. Surgically defined prognostic parameters in patients with early cervical carcinoma: A multivariate survival tree analysis. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1996, 78, 1438–1446. [Google Scholar] [CrossRef]
- Kamura, T.; Tsukamoto, N.; Tsuruchi, N.; Saito, T.; Matsuyama, T.; Akazawa, K.; Nakano, H. Multivariate analysis of the histopathologic prognostic factors of cervical cancer in patients undergoing radical hysterectomy. Cancer 1992, 69, 181–186. [Google Scholar] [CrossRef]
- Yessaian, A.; Magistris, A.; Burger, R.A.; Monk, B.J. Radical hysterectomy followed by tailored postoperative therapy in the treatment of stage IB2 cervical cancer: Feasibility and indications for adjuvant therapy. Gynecol. Oncol. 2004, 94, 61–66. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.F.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Mezache, L.; Paniccia, B.; Nyinawabera, A.; Nuovo, G.J. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2015, 28, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Albers, A.E.; Qin, J.; Kaufmann, A.M. Prognostic significance of overexpressed p16INK4a in patients with cervical cancer: A meta-analysis. PLoS ONE 2014, 9, e106384. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Maulard, A.; Scherier, S.; Sanson, C.; Zarokian, J.; Zaccarini, F.; Espenel, S.; Pautier, P.; Leary, A.; Genestie, C.; et al. Oncologic results of fertility sparing surgery of cervical cancer: An updated systematic review. Gynecol. Oncol. 2022, 165, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, Y.; Wu, G.; Lu, J.; Sun, L.; Xu, S. The efficacy and safety of local 5-aminolevulinic acid-based photodynamic therapy in the treatment of cervical high-grade squamous intraepithelial lesion: A single center retrospective observational study. Front. Oncol. 2024, 14, 1390982. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.R.; May, D.; Schmoeckel, E.; Horn, L.C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd. 2021, 81, 1145–1153. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.J.; Nussenbaum, B.; Wang, E.W. A practical guide to understanding Kaplan-Meier curves. Otolaryngol. Head Neck Surg. 2010, 143, 331–336. [Google Scholar] [CrossRef]
- ElHafeez, S.A.; D’Arrigo, G.; Leonardis, D.; Fusaro, M.; Tripepi, G.; Roumeliotis, S. Methods to Analyze Time-to-Event Data: The Cox Re-gression Analysis. Oxid. Med. Cell. Longev. 2021, 2021, 1302811. [Google Scholar] [CrossRef]
- Creasman, W.T.; Zaino, R.J.; Major, F.J.; DiSaia, P.J.; Hatch, K.D.; Homesley, H.D. Early invasive carcinoma of the cervix (3 to 5 mm invasion): Risk factors and prognosis: A Gynecologic Oncology Group study. Am. J. Obstet. Gynecol. 1998, 178, 62–65. [Google Scholar] [CrossRef]
- Burghardt, E.; Pickel, H.; Haas, J.; Lahousen, M. Prognostic factors and operative treatment of stages IB to IIB cervical cancer. Am. J. Obstet. Gynecol. 1987, 156, 988–996. [Google Scholar] [CrossRef]
- Hsu, C.-T.; Cheng, Y.-S.; Su, S.-C. Prognosis of uterine cervical cancer with extensive lymph node metastases: Special emphasis on the value of pelvic lymphadenectomy in the surgical treatment of uterine cervical cancer. Am. J. Obstet. Gynecol. 1972, 114, 954–962. [Google Scholar] [CrossRef]
- Ho, C.-M.; Chien, T.-Y.; Huang, S.-H.; Wu, C.-J.; Shih, B.-Y.; Chang, S.-C. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol. Oncol. 2004, 93, 458–464. [Google Scholar] [CrossRef]
- Obrzut, B.; Semczuk, A.; Naróg, M.; Obrzut, M.; Król, P. Prognostic Parameters for Patients with Cervical Cancer FIGO Stages IA2-IIB: A Long-Term Follow-Up. Oncology 2017, 93, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; George, E.M.; Tanyi, J.L. Controversies in the Staging of Patients with Locally Advanced Cervical Cancer. Diagnostics 2023, 13, 1747. [Google Scholar] [CrossRef] [PubMed]
- Landoni, F.; Maneo, A.; Cormio, G.; Perego, P.; Milani, R.; Caruso, O.; Mangioni, C. Class II versus class III radical hysterectomy in stage IB-IIA cervical cancer: A prospective randomized study. Gynecol. Oncol. 2001, 80, 3–12. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, S.-G.; Sun, J.-Y.; Liao, X.-L.; Li, F.-Y.; Lin, H.-X.; Yang, L.-C.; He, Z.-Y. Incorporation of the number of positive lymph nodes leads to better prognostic discrimination of node-positive early stage cervical cancer. Oncotarget 2017, 8, 26057. [Google Scholar] [CrossRef]
- Balaya, V.; Guani, B.; Magaud, L.; Bonsang-Kitzis, H.; Ngô, C.; Mathevet, P.; Lécuru, F.; On Behalf of the Senticol Group. Validation of the 2018 FIGO Classification for Cervical Cancer: Lymphovascular Space Invasion Should Be Considered in IB1 Stage. Cancers 2020, 12, 3554. [Google Scholar] [CrossRef]
- Pol, F.J.; Zusterzeel, P.L.; van Ham, M.A.; Kuijpers, D.A.; Bulten, J.; Massuger, L.F. Satellite lymphovascular space invasion: An independent risk factor in early stage cervical cancer. Gynecol. Oncol. 2015, 138, 579–584. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef] [PubMed]
- McCann, G.A.; Taege, S.K.; Boutsicaris, C.E.; Phillips, G.S.; Eisenhauer, E.L.; Fowler, J.M.; O’Malley, D.M.; Copeland, L.J.; Cohn, D.E.; Salani, R. The impact of close surgical margins after radical hysterectomy for early-stage cervical cancer. Gynecol. Oncol. 2013, 128, 44–48. [Google Scholar] [CrossRef]
- Hou, W.; Ma, Y.; Sun, S.; Gao, Y.; Ling, J.; Shi, R. Predictive factors for postoperative recurrence in early cervical cancer patients: A meta-analysis. Front. Surg. 2025, 12, 1588558. [Google Scholar] [CrossRef]
- Takeda, N.; Sakuragi, N.; Takeda, M.; Okamoto, K.; Kuwabara, M.; Negishi, H.; Yamamoto, R.; Yamada, H.; Fujimoto, S. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet. Gynecol. Scand. 2002, 81, 1144–1151. [Google Scholar] [CrossRef]
- Santoro, A.; Inzani, F.; Angelico, G.; Arciuolo, D.; Bragantini, E.; Travaglino, A.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Sfregola, S.; et al. Recent Advances in Cervical Cancer Management: A Review on Novel Prognostic Factors in Primary and Recurrent Tumors. Cancers 2023, 15, 1137. [Google Scholar] [CrossRef]
- Abrar, S.S.; Isa, S.A.M.; Hairon, S.M.; Yaacob, N.M.; Ismail, M.P. Prognostic Factors for Cervical Cancer in Asian Populations: A Scoping Review of Research From 2013 to 2023. Cureus 2024, 16, e71359. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.L.; Shintaku, P.I.; Moatamed, N.A. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn. Pathol. 2017, 12, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gong, Y.; Gou, F.; Qin, Q.; Tian, W.; Zhao, W.; Zi, D. Construction and validation of prognostic models for young cervical cancer patients: Age stratification based on restricted cubic splines. Sci. Rep. 2024, 14, 29808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).