Clinical Evaluation of PolyDeep, A Computer-Aided Detection System: A Multicenter Randomized Tandem Colonoscopy Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants of the Study
2.3. Randomization Process
2.4. Clinical Setting
2.5. Endpoints
2.6. Sample Size
2.7. Statistical Analysis
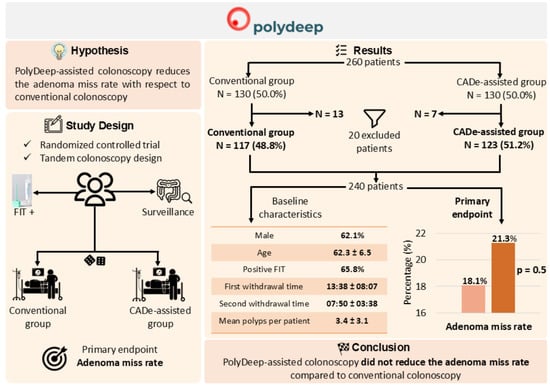
3. Results
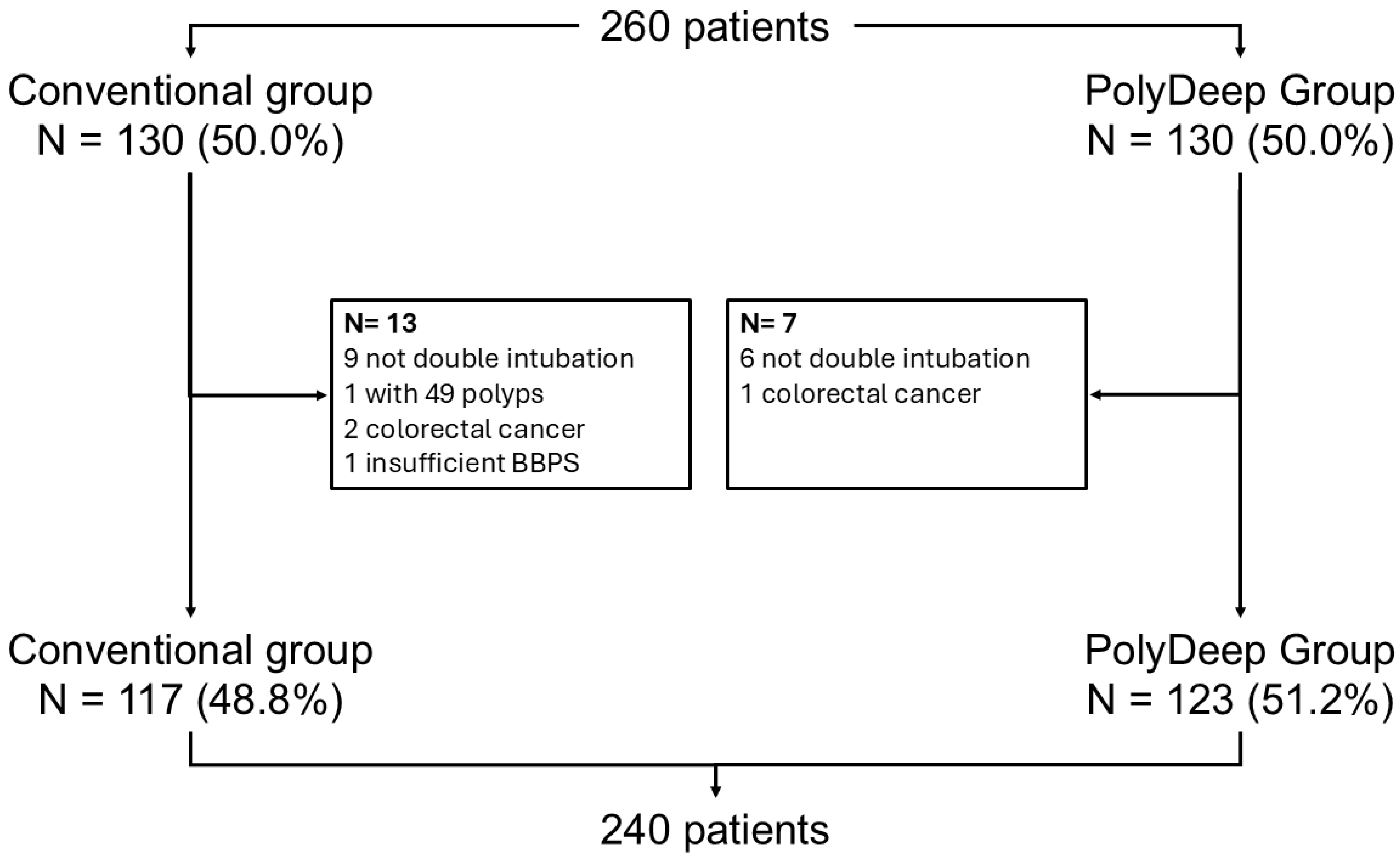
3.1. Population Description
3.2. Diagnostic Performance: Adenoma Miss Rate, Polyp Miss Rate, Serrated Lesion Miss Rate
3.3. Sub-Analysis by Size, Location and Advanced Lesions
3.4. Sub-Analysis by Colonoscopy Indication
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Xia, R.; Si, W.; Zhang, W.; Zhang, Y.; Zhuang, G. Cost Effectiveness of Colorectal Cancer Screening Strategies in Middle- and High-Income Countries: A Systematic Review. J. Gastroenterol. Hepatol. 2025, 40, 584–598. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cha, J.M.; Yoon, J.Y.; Kwak, M.S.; Lee, H.H. Association between Colonoscopy and Colorectal Cancer Occurrence and Mortality in the Older Population: A Population-Based Cohort Study. Endoscopy 2025, 57, 451–459. [Google Scholar] [CrossRef]
- Castells, A.; Quintero, E.; Bujanda, L.; Castán-Cameo, S.; Cubiella, J.; Díaz-Tasende, J.; Lanas, Á.; Ono, A.; Serra-Burriel, M.; Frías-Arrocha, E.; et al. Articles Effect of Invitation to Colonoscopy versus Faecal Immunochemical Test Screening on Colorectal Cancer Mortality (COLONPREV): A Pragmatic, Randomised, Controlled, Non-Inferiority Trial. Lancet 2025, 405, 1231–1239. [Google Scholar] [CrossRef]
- Rasmussen, S.L.; Pedersen, L.; Torp-Pedersen, C.; Rasmussen, M.; Bernstein, I.; Thorlacius-Ussing, O. Post-Colonoscopy Colorectal Cancer and the Association with Endoscopic Findings in the Danish Colorectal Cancer Screening Programme. BMJ Open Gastroenterol. 2025, 12, e001692. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Berzin, T.M.; Glissen Brown, J.R.; Bharadwaj, S.; Becq, A.; Xiao, X.; Liu, P.; Li, L.; Song, Y.; Zhang, D.; et al. Real-Time Automatic Detection System Increases Colonoscopic Polyp and Adenoma Detection Rates: A Prospective Randomised Controlled Study. Gut 2019, 68, 1813–1819. [Google Scholar] [CrossRef]
- Jahn, B.; Bundo, M.; Arvandi, M.; Schaffner, M.; Todorovic, J.; Sroczynski, G.; Knudsen, A.; Fischer, T.; Schiller-Fruehwirth, I.; Öfner, D.; et al. One in Three Adenomas Could Be Missed by White-Light Colonoscopy—Findings from a Systematic Review and Meta-Analysis. BMC Gastroenterol. 2025, 25, 170. [Google Scholar] [CrossRef]
- Van Rijn, J.C.; Reitsma, J.B.; Stoker, J.; Bossuyt, P.M.; Van Deventer, S.J.; Dekker, E. Polyp Miss Rate Determined by Tandem Colonoscopy: A Systematic Review. Am. J. Gastroenterol. 2006, 101, 343–350. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Pan, P.; Xia, T.; Chang, X.; Yang, X.; Guo, L.; Meng, Q.; Yang, F.; Qian, W.; et al. Magnitude, Risk Factors, and Factors Associated with Adenoma Miss Rate of Tandem Colonoscopy: A Systematic Review and Meta-Analysis. Gastroenterology 2019, 156, 1661–1674.e11. [Google Scholar] [CrossRef]
- Misawa, M.; Kudo, S.E. Current Status of Artificial Intelligence Use in Colonoscopy. Digestion 2025, 106, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Aung, Y.Y.M.; Wong, D.C.S.; Ting, D.S.W. The Promise of Artificial Intelligence: A Review of the Opportunities and Challenges of Artificial Intelligence in Healthcare. Br. Med. Bull 2021, 139, 4–15. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, Q.; Zhou, B.; Chen, D. Convolutional Neural Networks for Computer-Aided Detection or Diagnosis in Medical Image Analysis: An Overview. Math. Biosci. Eng. 2019, 16, 6536–6561. [Google Scholar] [CrossRef]
- Shah, S.; Park, N.; Chehade, N.E.H.; Chahine, A.; Monachese, M.; Tiritilli, A.; Moosvi, Z.; Ortizo, R.; Samarasena, J. Effect of Computer-Aided Colonoscopy on Adenoma Miss Rates and Polyp Detection: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2023, 38, 162–176. [Google Scholar] [CrossRef]
- Glissen Brown, J.R.; Mansour, N.M.; Wang, P.; Chuchuca, M.A.; Minchenberg, S.B.; Chandnani, M.; Liu, L.; Gross, S.A.; Sengupta, N.; Berzin, T.M. Deep Learning Computer-Aided Polyp Detection Reduces Adenoma Miss Rate: A United States Multi-Center Randomized Tandem Colonoscopy Study (CADeT-CS Trial). Clin. Gastroenterol. Hepatol. 2022, 20, 1499–1507.e4. [Google Scholar] [CrossRef]
- Hiratsuka, Y.; Hisabe, T.; Ohtsu, K.; Yasaka, T.; Takeda, K.; Miyaoka, M.; Ono, Y.; Kanemitsu, T.; Imamura, K.; Takeda, T.; et al. Evaluation of Artificial Intelligence: Computer-Aided Detection of Colorectal Polyps. J. Anus Rectum Colon 2025, 9, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, G.; Cocomazzi, F.; Gentile, M.; Loconte, I.; Mileti, A.; Paolillo, R.; Marra, A.; Castellana, S.; Mazza, T.; Di Leo, A.; et al. Real-Time, Computer-Aided, Detection-Assisted Colonoscopy Eliminates Differences in Adenoma Detection Rate between Trainee and Experienced Endoscopists. Endosc. Int. Open 2022, 10, E616–E621. [Google Scholar] [CrossRef]
- Yamaguchi, D.; Shimoda, R.; Miyahara, K.; Yukimoto, T.; Sakata, Y.; Takamori, A.; Mizuta, Y.; Fujimura, Y.; Inoue, S.; Tomonaga, M.; et al. Impact of an Artificial Intelligence-Aided Endoscopic Diagnosis System on Improving Endoscopy Quality for Trainees in Colonoscopy: Prospective, Randomized, Multicenter Study. Dig. Endosc. 2024, 36, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Du, F.; Song, W.; Xia, Y.; Yue, X.; Yang, D.; Cui, B.; Liu, Y.; Han, P. Artificial Intelligence for Colorectal Neoplasia Detection during Colonoscopy: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. EClinicalMedicine 2023, 66, 102341. [Google Scholar] [CrossRef]
- Wang, P.; Liu, P.; Glissen Brown, J.R.; Berzin, T.M.; Zhou, G.; Lei, S.; Liu, X.; Li, L.; Xiao, X. Lower Adenoma Miss Rate of Computer-Aided Detection-Assisted Colonoscopy vs Routine White-Light Colonoscopy in a Prospective Tandem Study. Gastroenterology 2020, 159, 1252–1261.e5. [Google Scholar] [CrossRef]
- Maida, M.; Marasco, G.; Maas, M.H.J.; Ramai, D.; Spadaccini, M.; Sinagra, E.; Facciorusso, A.; Siersema, P.D.; Hassan, C. Effectiveness of Artificial Intelligence Assisted Colonoscopy on Adenoma and Polyp Miss Rate: A Meta-Analysis of Tandem RCTs. Dig. Liver Dis. 2025, 57, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.M.; Parker, C.H.; Liu, L.W.; Farahvash, A.; Jeyalingam, T. Impact of Study Design on Adenoma Detection in the Evaluation of Artificial Intelligence-Aided Colonoscopy: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2024, 99, 676–687.e16. [Google Scholar] [CrossRef]
- Maas, M.H.J.; Rath, T.; Spada, C.; Soons, E.; Forbes, N.; Kashin, S.; Cesaro, P.; Eickhoff, A.; Vanbiervliet, G.; Salvi, D.; et al. A Computer-Aided Detection System in the Everyday Setting of Diagnostic, Screening and Surveillance Colonoscopy: An International, Randomized Trial. Endoscopy 2024, 56, 843–850. [Google Scholar] [CrossRef]
- Bretthauer, M.; Ahmed, J.; Antonelli, G.; Beaumont, H.; Beg, S.; Benson, A.; Bisschops, R.; De Cristofaro, E.; Gibbons, E.; Häfner, M.; et al. Use of Computer-Assisted Detection (CADe) Colonoscopy in Colorectal Cancer Screening and Surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2025, 57, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Rodríguez, A.; Domínguez-Carbajales, R.; Campos-Tato, F.; Herrero, J.; Puga, M.; Remedios, D.; Rivas, L.; Sánchez, E.; Iglesias, Á.; Cubiella, J.; et al. Real-Time Polyp Detection Model Using Convolutional Neural Networks. Neural Comput. Appl. 2022, 34, 10375–10396. [Google Scholar] [CrossRef]
- Nogueira Rodríguez, A.; Daniel González, D.; Hugo López Fernández, P.D. Deep Learning Techniques for Computer-Aided Diagnosis in Colorectal Cancer; University of Vigo: Vigo, Spain, 2022. [Google Scholar]
- Nogueira-Rodríguez, A.; Glez-Peña, D.; Reboiro-Jato, M.; López-Fernández, H. Negative Samples for Improving Object Detection—A Case Study in AI-Assisted Colonoscopy for Polyp Detection. Diagnostics 2023, 13, 966. [Google Scholar] [CrossRef]
- Nogueira-Rodríguez, A.; Reboiro-Jato, M.; Glez-Peña, D.; López-Fernández, H. Performance of Convolutional Neural Networks for Polyp Localization on Public Colonoscopy Image Datasets. Diagnostics 2022, 12, 898. [Google Scholar] [CrossRef]
- Davila-Piñón, P.; Nogueira-Rodríguez, A.; Díez-Martín, A.I.; Codesido, L.; Herrero, J.; Puga, M.; Rivas, L.; Sánchez, E.; Fdez-Riverola, F.; Glez-Peña, D.; et al. Optical Diagnosis in Still Images of Colorectal Polyps: Comparison between Expert Endoscopists and PolyDeep, a Computer-Aided Diagnosis System. Front Oncol. 2024, 14, 1393815. [Google Scholar] [CrossRef]
- Davila-Piñón, P.; Pedrido, T.; Díez-Martín, A.I.; Herrero, J.; Puga, M.; Rivas, L.; Sánchez, E.; Zarraquiños, S.; Pin, N.; Vega, P.; et al. PolyDeep Advance 1: Clinical Validation of a Computer-Aided Detection System for Colorectal Polyp Detection with a Second Observer Design. Diagnostics 2025, 15, 458. [Google Scholar] [CrossRef] [PubMed]
- Makar, J.; Abdelmalak, J.; Con, D.; Hafeez, B.; Garg, M. Use of Artificial Intelligence Improves Colonoscopy Performance in Adenoma Detection: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2024, 101, 68–81.E8. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sanjuan, C.; de-Castro, L.; Cubiella, J.; Díez-Redondo, P.; Suárez, A.; Pellisé, M.; Fernández, N.; Zarraquiños, S.; Núñez-Rodríguez, H.; Álvarez-García, V.; et al. Role of Artificial Intelligence in Colonoscopy Detection of Advanced Neoplasias: A Randomized Trial. Ann. Intern. Med. 2023, 176, 1145–1152. [Google Scholar] [CrossRef]
- Cubiella, J.; González, A.; Almazán, R.; Rodríguez-Camacho, E.; Fontenla Rodiles, J.; Domínguez Ferreiro, C.; Tejido Sandoval, C.; Sánchez Gómez, C.; de Vicente Bielza, N.; Lorenzo, I.P.-R.; et al. pT1 Colorectal Cancer Detected in a Colorectal Cancer Mass Screening Program: Treatment and Factors Associated with Residual and Extraluminal Disease. Cancers 2020, 12, 2530. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.L.; Zhang, Z. The YOLO Framework: A Comprehensive Review of Evolution, Applications, and Benchmarks in Object Detection. Computers 2024, 13, 336. [Google Scholar] [CrossRef]
- Lalinia, M.; Sahafi, A. Colorectal Polyp Detection in Colonoscopy Images Using YOLO-V8 Network. Signal Image Video Process. 2024, 18, 2047–2058. [Google Scholar] [CrossRef]
| Conventional Group 1 (N = 117) | PolyDeep Group 2 (N = 123) | p 3 | |
|---|---|---|---|
| Age (years) 4 | 63.0 ± 6.8 | 61.6 ± 6.2 | 0.1 |
| Sex (male) 4 | 69 (59.0%) | 80 (65.0%) | 0.4 |
| Indication (FIT) | 75 (64.1%) | 83 (67.5%) | 0.7 |
| Boston Bowel cleansing | 7.59 ± 1.28 | 7.42 ± 1.31 | 0.3 |
| First withdrawal time (minutes: seconds) | 13.34 ± 8.39 | 13.41 ± 07.37 | 0.9 |
| Second withdrawal time (minutes: seconds) | 7.58 ± 3.17 | 7.42 ± 3.57 | 0.6 |
| Detection of lesions (yes) | 90 (76.9%) | 94 (76.4%) | 0.7 |
| Number of polyps | 3.4 ± 3.3 | 3.4 ± 2.9 | 1.0 |
| Polyp size (millimetres) | 4.5 ± 4.7 | 4.9 ± 4.7 | 0.4 |
| Conventional Group 1 | PolyDeep Group 2 | ||||
|---|---|---|---|---|---|
| 1st Withdrawal | 2nd Withdrawal 3 | 1st Withdrawal | 2nd Withdrawal 3 | p 4 | |
| Adenoma | 172 (81.9%) | 38 (18.1%) | 185 (78.7%) | 50 (21.3%) | 0.5 |
| Polyp 5 | 239 (79.7%) | 61 (20.3%) | 244 (78.2%) | 68 (21.8%) | 0.7 |
| Serrated lesion | 67 (74.4%) | 23 (25.6%) | 59 (76.6%) | 18 (23.4%) | 0.9 |
| Other polyp | 12 (75.0%) | 4 (25.0%) | 16 (84.2%) | 3 (15.8%) | - |
| Not histology | 12 (66.7%) | 6 (33.3%) | 6 (66.6%) | 3 (33.3%) | - |
| Advanced adenoma 6 | 40 (95.2%) | 2 (4.8%) | 37 (94.9%) | 2 (5.1%) | 1.0 |
| Advanced serrated lesion 7 | 9 (64.3%) | 5 (35.7%) | 19 (86.4%) | 3 (13.6%) | 0.2 |
| Advanced polyp 8 | 47 (88.7%) | 6 (11.3%) | 51 (92.7%) | 4 (7.3%) | 0.5 |
| Proximal polyp 9 | 141 (81.5%) | 32 (18.5%) | 134 (80.7%) | 32 (19.3%) | 0.8 |
| Distal polyp 10 | 98 (77.2%) | 29 (22.8%) | 110 (75.3%) | 36 (24.7%) | 0.8 |
| <5 mm polyp | 161 (75.9%) | 51 (24.1%) | 149 (74.5%) | 51 (25.5%) | 0.8 |
| <10 mm polyp | 203 (77.2%) | 60 (22.8%) | 209 (76.6%) | 64 (23.4%) | 0.9 |
| ≥5 mm polyp | 78 (88.6%) | 10 (11.4%) | 95 (84.8%) | 17 (15.2%) | 0.6 |
| Screening 1 | p 4 | Surveillance 1 | p 4 | |||
|---|---|---|---|---|---|---|
| Conventional Group 2 | PolyDeep Group 3 | Conventional Group 2 | PolyDeep Group 3 | |||
| Adenoma miss rate | (14.9%) 5 | (20.4%) | 0.2 | (25.8%) | (24.1%) | 1.0 |
| Polyp miss rate 6 | (18.6%) | (21.6%) | 0.5 | (23.8%) | (22.4%) | 1.0 |
| Serrated lesion miss rate | (29.4%) | (25.0%) | 0.7 | (20.5%) | (15.4%) | 1.0 |
| Advanced polyp miss rate 7 | (6.8%) | (8.2%) | 1.0 | (33.3%) | (0.0%) | 0.2 |
| Proximal polyp miss rate 8 | (18.3%) | (17.2%) | 0.9 | (18.8%) | (26.3%) | 0.5 |
| Distal polyp miss rate 9 | (18.9%) | (26.5%) | 0.3 | (34.4%) | (17.2%) | 0.2 |
| <5 mm polyp miss rate | (21.3%) | (25.0%) | 0.6 | (28.2%) | (26.9%) | 1.0 |
| ≥5 mm polyp miss rate | (13.9%) | (16.5%) | 0.8 | (0.0%) | (6.7%) | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davila-Piñón, P.; Díez Martín, A.I.; Nogueira-Rodríguez, A.; Domínguez-Carbajales, R.; Fdez-Riverola, F.; Zarraquiños, S.; de Castro, L.; Herrero, J.; Fernández, N.; Vega, P.; et al. Clinical Evaluation of PolyDeep, A Computer-Aided Detection System: A Multicenter Randomized Tandem Colonoscopy Trial. Diagnostics 2025, 15, 2751. https://doi.org/10.3390/diagnostics15212751
Davila-Piñón P, Díez Martín AI, Nogueira-Rodríguez A, Domínguez-Carbajales R, Fdez-Riverola F, Zarraquiños S, de Castro L, Herrero J, Fernández N, Vega P, et al. Clinical Evaluation of PolyDeep, A Computer-Aided Detection System: A Multicenter Randomized Tandem Colonoscopy Trial. Diagnostics. 2025; 15(21):2751. https://doi.org/10.3390/diagnostics15212751
Chicago/Turabian StyleDavila-Piñón, Pedro, Astrid Irene Díez Martín, Alba Nogueira-Rodríguez, Ruben Domínguez-Carbajales, Florentino Fdez-Riverola, Sara Zarraquiños, Luisa de Castro, Jesús Herrero, Nereida Fernández, Pablo Vega, and et al. 2025. "Clinical Evaluation of PolyDeep, A Computer-Aided Detection System: A Multicenter Randomized Tandem Colonoscopy Trial" Diagnostics 15, no. 21: 2751. https://doi.org/10.3390/diagnostics15212751
APA StyleDavila-Piñón, P., Díez Martín, A. I., Nogueira-Rodríguez, A., Domínguez-Carbajales, R., Fdez-Riverola, F., Zarraquiños, S., de Castro, L., Herrero, J., Fernández, N., Vega, P., Remedios, D., Martínez, A., Puga, M., Alonso, S., Pin, N., García-Morales, N., Rivas, L., Ledo, A., Macenlle, R., ... Cubiella, J. (2025). Clinical Evaluation of PolyDeep, A Computer-Aided Detection System: A Multicenter Randomized Tandem Colonoscopy Trial. Diagnostics, 15(21), 2751. https://doi.org/10.3390/diagnostics15212751