Integrated Exhaled VOC and Clinical Biomarker Profiling for Predicting Bronchodilator Responsiveness in Asthma and COPD Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Collection of Clinical Data
2.3. Collection of Exhaled Breath and Measurement of VOCs
2.4. Data Processing
2.5. VOCs Annotation
2.6. Statistical Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Identification of VOCs as Predictors of Asthma and COPD
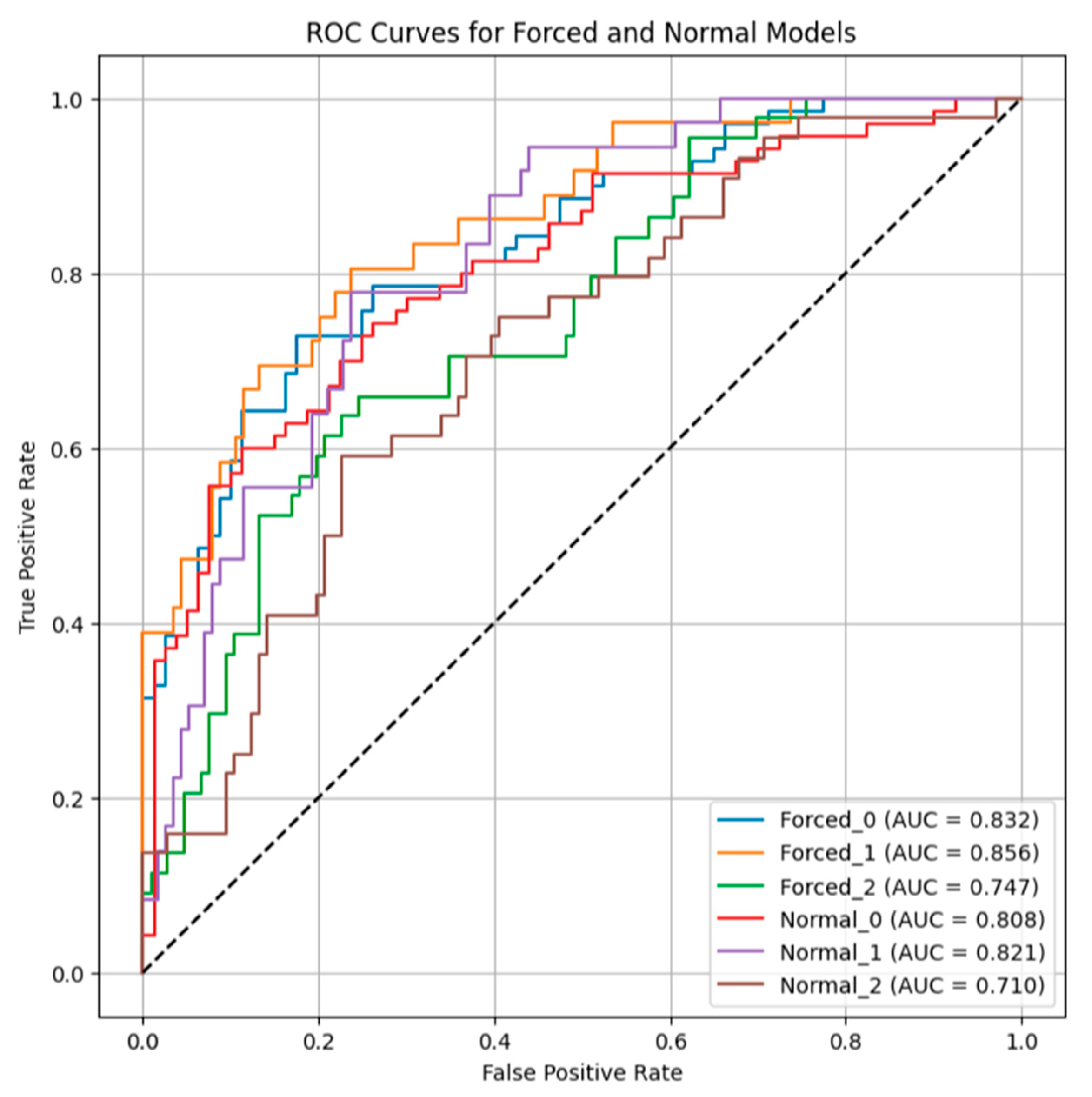
3.3. Characterization of VOC Signatures Associated with Bronchodilator Responsiveness in Normal and Forced Expired Breath Samples
4. Discussion
4.1. Profile Differences and Chemical Origins of VOCs in Asthma and COPD
4.2. Bronchodilator Response and Predictive VOC Signatures
4.3. Limitations and Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BA | Bronchial asthma |
| COPD | Chronic obstructive pulmonary disease |
| BDR | Bronchodilator responsiveness |
| PTR-TOF-MS | Proton-transfer reaction time-of-flight mass spectrometry |
| eVOCs | Exhaled volatile organic compounds |
| ATS | the American Thoracic Society |
| ERS | the European Respiratory Society |
| GLI | the Global Lung Function Initiative |
| AUC | Area under the curve |
| BMI | Body mass index |
| FEV1 | Forced expiratory volume in 1 s |
| FEF75 | Forced expiratory flow when 75% of FVC has been exhaled |
| mMRC | Modified Medical Research Council |
References
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention; GINA: Fontana, WI, USA, 2023; Available online: https://ginasthma.org/ (accessed on 1 January 2024).
- Barnes, P.J. Asthma and COPD: Basic Mechanisms and Clinical Management, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 report: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2023, 207, 819–837. [Google Scholar] [CrossRef]
- Postma, D.S.; Rabe, K.F. The asthma–COPD overlap syndrome. N. Engl. J. Med. 2015, 373, 1241–1249. [Google Scholar] [CrossRef]
- Barjaktarevic, I.; Kaner, R.; Buhr, R.G.; Cooper, C.B. Bronchodilator responsiveness or reversibility in asthma and COPD—A need for clarity. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3511–3513. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Celli, B.; Decramer, M.; Liu, D.; Burkhart, D.; Cassino, C.; Kesten, S. Bronchodilator responsiveness in patients with COPD. Eur. Respir. J. 2008, 31, 742–750. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed]
- van de Kant, K.D.; van der Sande, L.J.; Jöbsis, Q.; van Schayck, O.C.; Dompeling, E. Clinical use of exhaled volatile organic compounds in pulmonary diseases: A systematic review. Respir. Res. 2012, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Basanta, M.; Ibrahim, B.; Dockry, R.; Douce, D.; Morris, M.; Singh, D.; Woodcock, A.; Fowler, S.J. Exhaled volatile organic compounds for phenotyping chronic obstructive pulmonary disease: A cross-sectional study. Respir. Res. 2012, 13, 72. [Google Scholar] [CrossRef]
- Caldeira, M.; Barros, A.S.; Bilelo, M.J.; Parada, A.; Câmara, J.S.; Rocha, S.M. Profiling allergic asthma volatile metabolic patterns using a headspace–solid phase microextraction/gas chromatography based methodology. J. Chromatogr. A 2011, 1218, 3771–3780. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Chen, Q.; Pan, Z.; Chen, J.; Sun, M.; Wang, J.; Li, Y.; Ye, Q. Development and validation of a screening model for lung cancer using machine learning: A large-scale, multi-center study of biomarkers in breath. Front. Oncol. 2022, 12, 975563. [Google Scholar] [CrossRef]
- Gmachowska, K.; Podlecka, D.; Bonikowski, R.; Majak, P.; Kapka, K.; Jerzyńska, J. Exhaled volatile organic compounds (VOCs) for prediction of asthma exacerbation in children. Int. J. Occup. Med. Environ. Health 2024, 37, 351–359. [Google Scholar] [CrossRef]
- van der Schee, M.P.; Paff, T.; Brinkman, P.; van Aalderen, W.M.C.; Haarman, E.G.; Sterk, P.J. Breathomics in lung disease. Chest 2015, 147, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; Schot, R.; Mertens, B.J.; Le Cessie, S.; Gauw, S.A.; Spanevello, A.; Resta, O.; Willard, N.P.; Vink, T.J.; Rabe, K.F.; et al. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 2007, 120, 856–862. [Google Scholar] [CrossRef]
- Olaguibel, J.M.; Quirce, S.; Juliá, B.; Fernández, C.; Fortuna, A.M.; Molina, J.; Plaza, V. Measurement of asthma control according to Global Initiative for Asthma guidelines: A comparison with the Asthma Control Questionnaire. Respir. Res. 2012, 13, 50. [Google Scholar] [CrossRef]
- Sundh, J.; Janson, C.; Lisspers, K.; Montgomery, S.; Ställberg, B. Clinical COPD Questionnaire score (CCQ) and mortality. Int. J. Chron. Obstruct. Pulmon. Dis. 2012, 7, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.M.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Wanger, J. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Aguilar, M.; Ramírez-García, S.; Ilizaliturri-Hernández, C.; Gómez-Gómez, A.; Van-Brussel, E.; Díaz-Barriga, F.; Medellín-Garibay, S.; Flores-Ramírez, R. Ultrafast gas chromatography coupled to electronic nose to identify volatile biomarkers in exhaled breath from chronic obstructive pulmonary disease patients: A pilot study. Biomed. Chromatogr. 2019, 33, e4684. [Google Scholar] [CrossRef]
- Bos, L.D.; Sterk, P.J.; Schultz, M.J. Volatile metabolites of pathogens: A systematic review. PLoS Pathog. 2013, 9, e1003311. [Google Scholar] [CrossRef] [PubMed]
- Kushch, I.; Schwarz, K.; Schwentner, L.; Baumann, B.; Dzien, A.; Schmid, A.; Unterkofler, K.; Gastl, G.; Spaněl, P.; Smith, D.; et al. Compounds enhanced in a mass spectrometric profile of smokers’ exhaled breath versus non-smokers as determined in a pilot study using PTR-MS. J. Breath Res. 2008, 2, 026002. [Google Scholar] [CrossRef]
- Shahbazi Khamas, S.; Alizadeh Bahmani, A.H.; Vijverberg, S.J.H.; Brinkman, P.; Maitland-van der Zee, A.H. Exhaled volatile organic compounds associated with risk factors for obstructive pulmonary diseases: A systematic review. ERJ Open Res. 2023, 9, 00143-2023. [Google Scholar] [CrossRef]
- Fens, N.; de Nijs, S.B.; Peters, S.; Dekker, T.; Knobel, H.H.; Vink, T.J.; Willard, N.P.; Zwinderman, A.H.; Krouwels, F.H.; Janssen, H.G.; et al. Exhaled air molecular profiling in relation to inflammatory subtype and activity in COPD. Eur. Respir. J. 2011, 38, 1301–1309. [Google Scholar] [CrossRef]
- Mustafina, M.; Silantyev, A.; Krasovskiy, S.; Chernyak, A.; Naumenko, Z.; Suvorov, A.; Gognieva, D.; Abdullaev, M.; Bektimirova, A.; Bykova, A.; et al. Exhaled breath analysis in adult patients with cystic fibrosis by real-time proton mass spectrometry. Clin. Chim. Acta 2024, 560, 119733. [Google Scholar] [CrossRef]
- Mustafina, M.; Silantyev, A.; Krasovskiy, S.; Chernyak, A.; Naumenko, Z.; Suvorov, A.; Gognieva, D.; Abdullaev, M.; Suvorova, O.; Schmidt, A.; et al. Identification of exhaled metabolites correlated with respiratory function and clinical features in adult patients with cystic fibrosis by real-time proton mass spectrometry. Biomolecules 2024, 14, 1189. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lozano Sinues, P.; Meier, L.; Berchtold, C.; Ivanov, M.; Sievi, N.; Camen, G.; Kohler, M.; Zenobi, R. Breath analysis in real time by mass spectrometry in chronic obstructive pulmonary disease. Respiration 2014, 87, 301–310. [Google Scholar] [CrossRef]
- Beasley, R.; Hughes, R.; Agusti, A.; Calverley, P.; Chipps, B.; Del Olmo, R.; Papi, A.; Price, D.; Reddel, H.; Müllerová, H.; et al. Prevalence, diagnostic utility and associated characteristics of bronchodilator responsiveness. Am. J. Respir. Crit. Care Med. 2024, 209, 390–401. [Google Scholar] [CrossRef]
- Scarlata, S.; Finamore, P.; Santangelo, S.; Giannunzio, G.; Pennazza, G.; Grasso, S.; Santonico, M.; Incalzi, R.A. Cluster analysis on breath print of newly diagnosed COPD patients: Effects of therapy. J. Breath Res. 2018, 12, 036022. [Google Scholar] [CrossRef]
- Brinkman, P.; Ahmed, W.M.; Gómez, C.; Knobel, H.H.; Weda, H.; Vink, T.J.; Nijsen, T.M.; Wheelock, C.E.; Dahlen, S.E.; Montuschi, P.; et al. Exhaled volatile organic compounds as markers for medication use in asthma. Eur. Respir. J. 2020, 55, 1900544. [Google Scholar] [CrossRef] [PubMed]
- Mustafina, M.K.; Krasovsky, S.A.; Silantyev, A.S.; Suvorov, A.Y.; Kopylov, P.Y. Effect provided by inhaled salbutamol on exhaled breath signatures in adult patients with cystic fibrosis. Bull. Contemp. Clin. Med. 2024, 17, 49–55. [Google Scholar] [CrossRef]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.; Wenzel, S.E.; Wilson, A.M.; Small, M.B.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef]
- Oldham, M.J.; Wagner, K.A.; Gilman, I.G.; Beach, J.B.; Liu, J.; Rostami, A.A.; Sarkar, M.A. Development/verification of methods for measurement of exhaled breath and environmental e-vapor product aerosol. Regul. Toxicol. Pharmacol. 2017, 85, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, H.; Shi, J.; Chai, S.; Yan, L.; Meng, D.; Cai, Z.; Guan, J.; Xin, Y.; Zhang, X.; et al. Exhaled breath analysis for the discrimination of asthma and chronic obstructive pulmonary disease. J. Breath Res. 2024, 18, 046002. [Google Scholar] [CrossRef]
- Zhu, J.; Bean, H.D.; Jiménez-Díaz, J.; Hill, J.E. Secondary electrospray ionization–mass spectrometry (SESI-MS) breathprinting of multiple bacterial lung pathogens: A mouse model study. J. Appl. Physiol. 2013, 114, 1544–1549. [Google Scholar] [CrossRef]

| BA | COPD | Controls | p-Value | |||
|---|---|---|---|---|---|---|
| BA vs. COPD | BA vs. Controls | COPD vs. Controls | ||||
| Number of subjects | 160 | 128 | 254 | |||
| Gender, % male | 56 (35.0%) | 111 (86.7%) | 98 (38.6%) | 0.001 | 0.547 | 0.001 |
| Age at enrollment, years | 58.413 ± 17.055 | 66.609 ± 10.345 | 42.386 ± 18.100 | <0.001 | <0.001 | <0.001 |
| BMI kg·m2 | 29.294 ± 8.460 | 25.861 ± 5.777 | 26.154 ± 5.949 | 0.001 | <0.001 | 0.967 |
| Smoking status | ||||||
| Never | 85 (53%) | 25 (19.5%) | 135 (53.1%) | <0.001 | <0.001 | <0.001 |
| Former | 54 (33.9%) | 43 (33.6%) | 105 (41.3) | 0.002 | 0.001 | <0.001 |
| Current | 21 (13.1%) | 60 (46.9%) | 14 (5.6%) | 0.001 | 0.007 | 0.001 |
| mMRC, scores | 2.054 ± 0.917 | 2.585 ± 0.917 | 0 | 0.001 | <0.001 | <0.001 |
| FVC % pred | 78.074 ± 18.664 | 72.647 ± 22.309 | 99.339 ± 11.641 | 0.021 | <0.001 | <0.001 |
| FEV1% pred | 61.942 ± 17.079 | 49.675 ± 21.627 | 99.614 ± 11.358 | <0.001 | <0.001 | <0.001 |
| FEV1/FVC, % | 62.434 ± 9.992 | 51.060 ± 11.670 | 81.820 ± 6.197 | <0.001 | <0.001 | <0.001 |
| FEF75%pred | 61.835 ± 27.019 | 52.499 ± 27.000 | 124.607 ± 53.110 | <0.001 | <0.001 | <0.001 |
| FVC post BD % pred | 84.945 ± 17.911 | 77.550 ± 22.873 | NA | 0.006 | NA | NA |
| FEV1 post BD % pred | 72.298 ± 19.310 | 55.648 ± 24.040 | NA | <0.001 | NA | NA |
| FEV1/FVC post BD, % | 66.305 ± 11.792 | 52.713 ± 13.632 | NA | <0.001 | NA | NA |
| FEF75 post BD %pred | 78.502 ± 33.912 | 60.980 ± 28.523 | NA | <0.001 | NA | NA |
| BDR, % | 16.570 ± 14.040 | 10.583 ± 10.852 | NA | <0.001 | NA | NA |
| BDR ≥ 10% | 101 (70.1%) | 50 (45.5%) | NA | 0.001 | NA | NA |
| FeNO, ppb | 30.310 ± 23.050 | 24.015 ± 21.476 | NA | <0.001 | NA | NA |
| Blood eosinophils (×109/L) | 0.502 ± 0.428 | 0.407 ± 0.377 | NA | 0.001 | NA | NA |
| Total IgE, IU/mL | 180.321 ± 144.691 | 111.000 ± 124.532 | NA | <0.001 | NA | NA |
| ACQ | 2.7 ± 1.1 | NA | NA | NA | NA | NA |
| CCQ | NA | 3.8 ± 4.3 | NA | NA | NA | NA |
| GOLD class I/II/III/IV (%) | NA | 10.9/32.8/37.5/18.8 | NA | |||
| m/z | Feature Importances | |
|---|---|---|
| Forced Expiratory Maneuver | Normal Quiet Breathing | |
| 44.991 * | 0.01397864 | 0.01173620 |
| 45.992 | 0.00943758 | 0.00058650 |
| 49.005 | 0.00643448 | 0.00270099 |
| 51.039 | 0.00576903 | 0.00316140 |
| 53.037 | 0.00494756 | 0.01042149 |
| 69.073 | 0.00188930 | 0.00162506 |
| 71.055 | 0.01875065 | 0.00823190 |
| 79.054 | 0.04068980 | 0.03198569 |
| 83.086 | 0.00348485 | 0.00807589 |
| 95.054 | 0.01917045 | 0.01707253 |
| 132.050 | 0.00844352 | 0.00627082 |
| Parameters | BDR ≥ 10% | BDR < 10% | p-Value |
|---|---|---|---|
| Number of subjects | 151 | 103 | |
| BA | 101 (66.9%) | 48 (46.7%) | 0.320 |
| COPD | 55 (53.3%) | 50 (33.1%) | 0.450 |
| Sex, % male | 86 (57.0%) | 65 (63.1%) | 0.818 |
| Age at enrollment, years | 61.358 ± 15.966 | 62.553 ± 14.593 | 0.730 |
| BMI kg·m2 | 28.884 ± 8.601 | 26.549 ± 5.967 | 0.043 |
| Smoking status | |||
| Never | 51 (33.7%) | 32 (31.0%) | 0.065 |
| Former | 65 (43.0%) | 35 (34.0%) | 0.077 |
| Current | 35 (23.3%) | 36 (35.0%) | 0.065 |
| mMRC, scores | 2.183 ± 0.871 | 2.429 ± 1.037 | 0.075 |
| FVC % pred | 74.128 ± 18.769 | 77.765 ± 22.571 | 0.179 |
| FEV1% pred | 56.932 ± 18.179 | 57.783 ± 23.173 | 0.621 |
| FEV1/FVC, % | 59.398 ± 11.822 | 56.346 ± 12.464 | 0.161 |
| FEF75%pred | 59.830 ± 25.017 | 56.327 ± 28.002 | 0.079 |
| FEF25–75% pred | 77.149 ± 41.083 | 76.606 ± 35.248 | 0.121 |
| FVC post-BD % pred | 83.653 ± 18.520 | 79.251 ± 22.871 | 0.090 |
| FEV1 post-BD % pred | 68.857 ± 20.905 | 59.923 ± 24.903 | 0.006 |
| FEV1/FVC post-BD, % | 63.068 ± 13.593 | 56.632 ± 14.600 | 0.001 |
| FEF25–75 post-BD % pred | 54.228 ± 40.606 | 40.471 ± 22.021 | 0.001 |
| FEF75 post-BD %pred | 77.103 ± 32.589 | 62.415 ± 31.470 | <0.000 |
| BDR, % | 21.464 ± 11.270 | 3.002 ± 5.721 | <0.000 |
| FeNO, ppb | 38.773 ± 23.976 | 13.878 ± 11.161 | <0.000 |
| Blood eosinophils (×109/L) | 0.654 ± 0.417 | 0.202 ± 0.220 | <0.000 |
| Total IgE, IU/mL | 156.080 ± 121.275 | 102.248 ± 27.141 | <0.000 |
| VOCs in normal quiet breathing * | |||
| 51.039 | 0.070 ± 0.050 | 0.063 ± 0.043 | 0.393 |
| 77.059 ** | 0.028 ± 0.015 | 0.031 ± 0.037 | 0.450 |
| 79.054 | 0.025 ± 0.031 | 0.029 ± 0.026 | 0.042 |
| 101.039 | 0.020 ± 0.009 | 0.019 ± 0.011 | 0.024 |
| VOCs in forced expiratory maneuver * | |||
| 51.039 | 0.062 ± 0.044 | 0.057 ± 0.039 | 0.502 |
| 77.059 ** | 0.027 ± 0.013 | 0.032 ± 0.056 | 0.399 |
| 79.054 | 0.026 ± 0.030 | 0.028 ± 0.025 | 0.052 |
| 101.039 | 0.022 ± 0.013 | 0.019 ± 0.011 | 0.003 |
| Clinical Predictors and VOCs * | Feature Importances | |
|---|---|---|
| Forced Expiratory Maneuver | Normal Quiet Breathing | |
| Blood eosinophils, % | 0.01574812 | 0.02867968 |
| FEV1, l | 0.01776611 | 0.04279317 |
| FEV25–75 post-BD, l | 0.01593777 | 0.04099168 |
| FEV25–75 post-BD, % pred. | 0.03839435 | 0.03620051 |
| FEV75 post BD, l | 0.01200586 | 0.01911791 |
| Total IgE, IU/mL | 0.05262020 | 0.05668990 |
| FeNO, ppb | 0.01156907 | 0.03228240 |
| 51.039 | 0.02341965 | 0.01837680 |
| 77.059 ** | 0.04816510 | 0.03026989 |
| 79.054 | 0.05094414 | 0.04666028 |
| 101.039 | 0.01853833 | 0.01798187 |
| m/z * | Putative Chemical Identity | Biological Relevance/Origin | Association in This Study | Supporting Literature |
|---|---|---|---|---|
| 44.991 | Formic acid fragment/Acetaldehyde | Product of lipid peroxidation and oxidative stress; can be influenced by diet. | Elevated in asthma and COPD vs. controls. | [21] |
| 51.039 | Aryl ion of aromatic compounds | Positive effect on mucociliary clearance, respectively, a compensatory increase in this metabolite in case of impaired clearance | Significant predictor of BDR | [35] |
| 53.037 | Not confidently identified (Potential alkyne or diene fragment) | Unknown endogenous pathway. | Decreased in asthma vs. COPD. | - |
| 71.055 | 2-Pentanone, Fragments of C5-compounds | Associated with metabolic activity of common respiratory pathogens (e.g., Pseudomonas, Haemophilus); product of lipid peroxidation. | Highest in COPD vs. asthma and controls. | [22] |
| 77.059 | Protonated Propylene Glycol | Common excipient in inhalers; can also be a product of oxidative stress. | Significant predictor of BDR; highest in COPD. | [34] |
| 79.054 | Benzene/Pyridine fragment | By-product of smoking; associated with aromatic hydrocarbon exposure and oxidative stress. | Key predictor for asthma and BDR; decreased in BA vs. COPD. | [23] |
| 95.054 | Phenol/Hydroxybenzyl ion (toluene derivative) | Marker of oxidative stress; associated with bacterial infection and inflammation. | Increased in asthma vs. COPD. | [25,27] |
| 101.039 | Not confidently identified (e.g., C6H12O2) | In the literature, associated with bacterial pathogens (e.g., Streptococcus pneumoniae). | Elevated in patients with positive BDR. | [36] |
| 118.071 | Indole/Methyl Indole derivatives | Metabolites associated with bacterial activity (e.g., Pseudomonas aeruginosa); linked to exacerbation severity. | Additional discriminative marker for COPD. | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafina, M.; Silantyev, A.; Suvorov, A.; Chernyak, A.; Suvorova, O.; Shmidt, A.; Gordeeva, A.; Vergun, M.; Gognieva, D.; Avdeev, S.; et al. Integrated Exhaled VOC and Clinical Biomarker Profiling for Predicting Bronchodilator Responsiveness in Asthma and COPD Patients. Diagnostics 2025, 15, 2738. https://doi.org/10.3390/diagnostics15212738
Mustafina M, Silantyev A, Suvorov A, Chernyak A, Suvorova O, Shmidt A, Gordeeva A, Vergun M, Gognieva D, Avdeev S, et al. Integrated Exhaled VOC and Clinical Biomarker Profiling for Predicting Bronchodilator Responsiveness in Asthma and COPD Patients. Diagnostics. 2025; 15(21):2738. https://doi.org/10.3390/diagnostics15212738
Chicago/Turabian StyleMustafina, Malika, Artemiy Silantyev, Aleksander Suvorov, Alexander Chernyak, Olga Suvorova, Anna Shmidt, Anastasia Gordeeva, Maria Vergun, Daria Gognieva, Sergey Avdeev, and et al. 2025. "Integrated Exhaled VOC and Clinical Biomarker Profiling for Predicting Bronchodilator Responsiveness in Asthma and COPD Patients" Diagnostics 15, no. 21: 2738. https://doi.org/10.3390/diagnostics15212738
APA StyleMustafina, M., Silantyev, A., Suvorov, A., Chernyak, A., Suvorova, O., Shmidt, A., Gordeeva, A., Vergun, M., Gognieva, D., Avdeev, S., Betelin, V., & Kopylov, P. (2025). Integrated Exhaled VOC and Clinical Biomarker Profiling for Predicting Bronchodilator Responsiveness in Asthma and COPD Patients. Diagnostics, 15(21), 2738. https://doi.org/10.3390/diagnostics15212738






