Role of the Ear in Meningitis: A Narrative Review of Neuroimaging
Abstract
1. Introduction
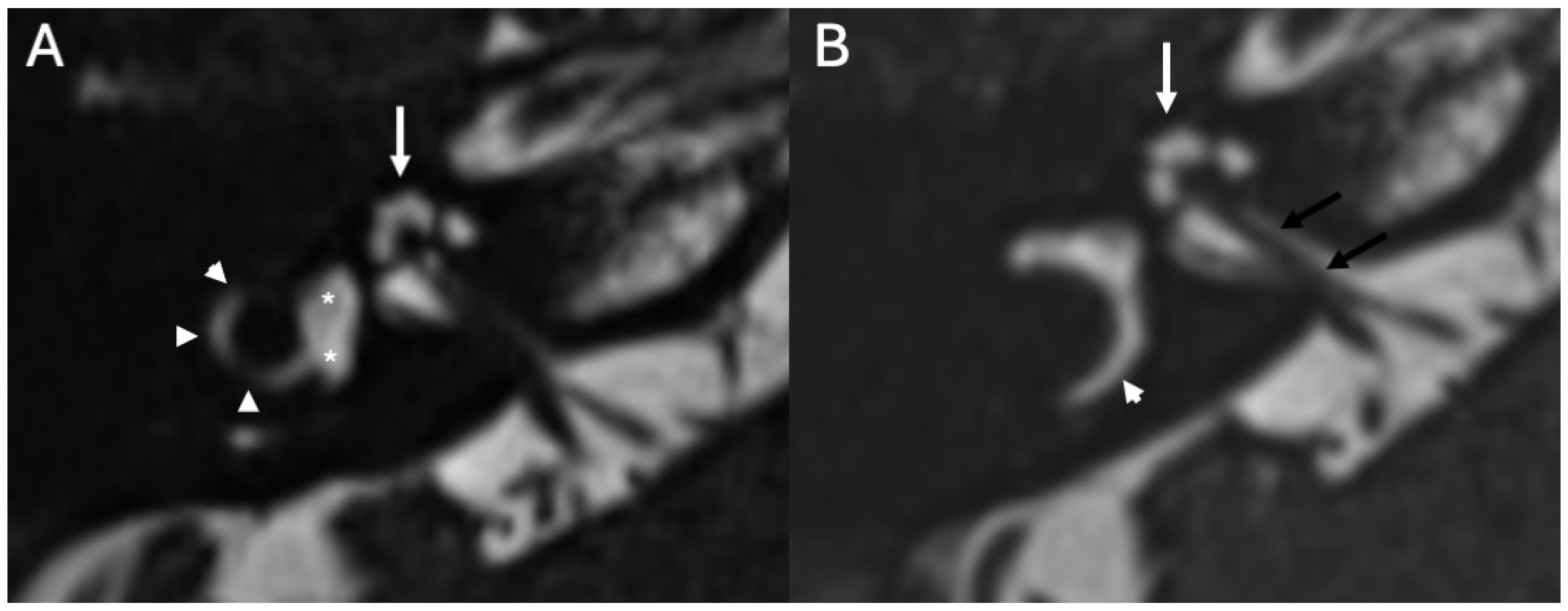
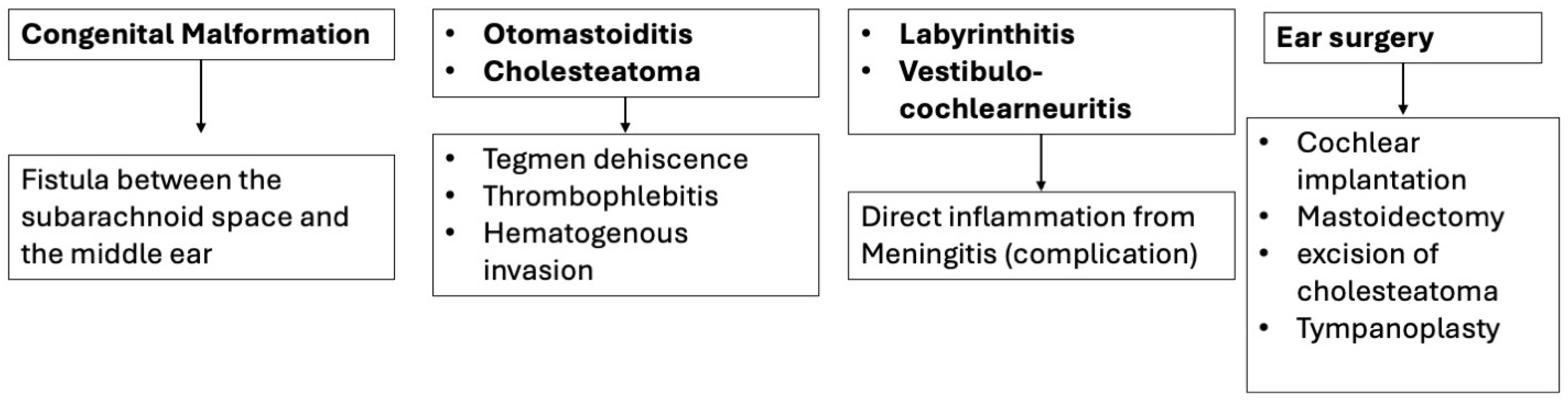
2. Congenital Malformations
3. Infectious Disease
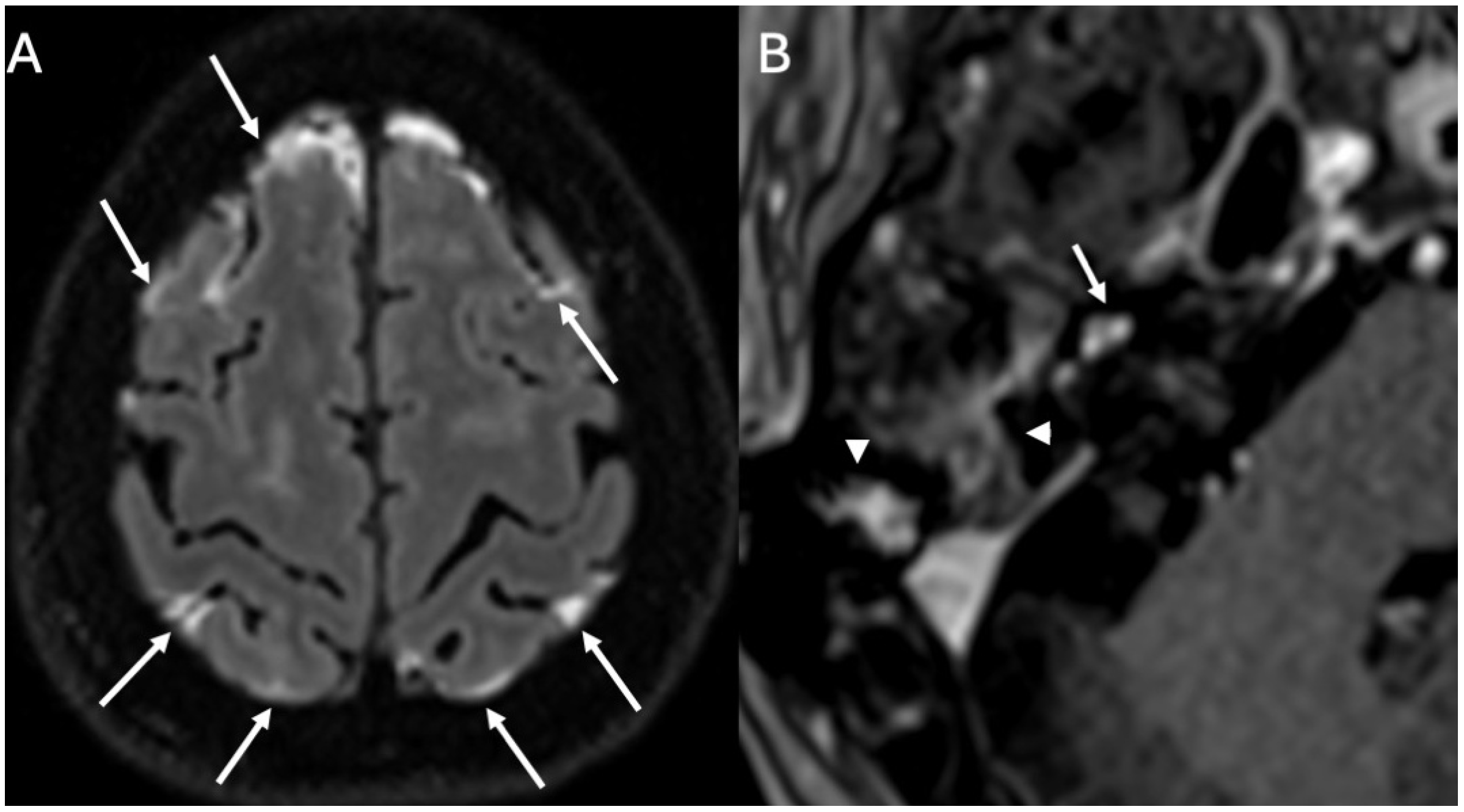
3.1. Otomastoiditis
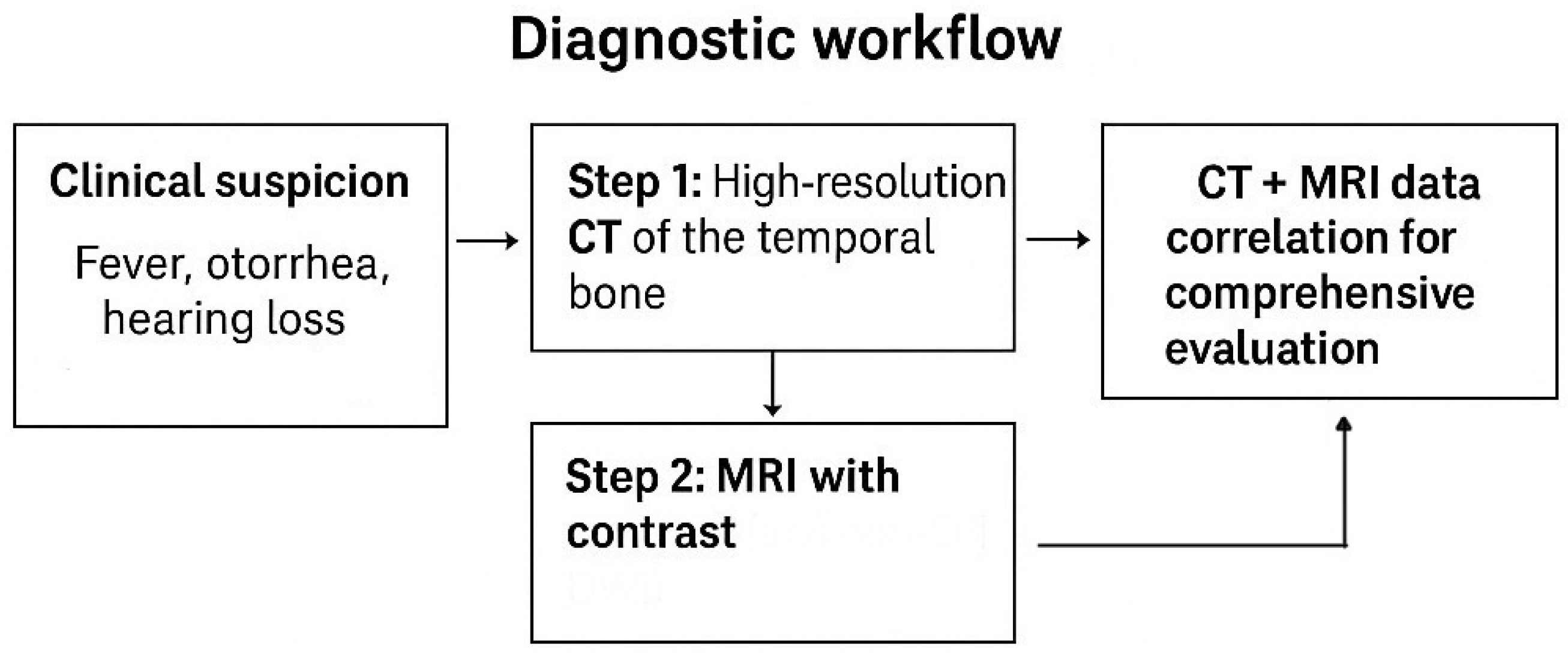
3.1.1. Imaging
3.1.2. Managment
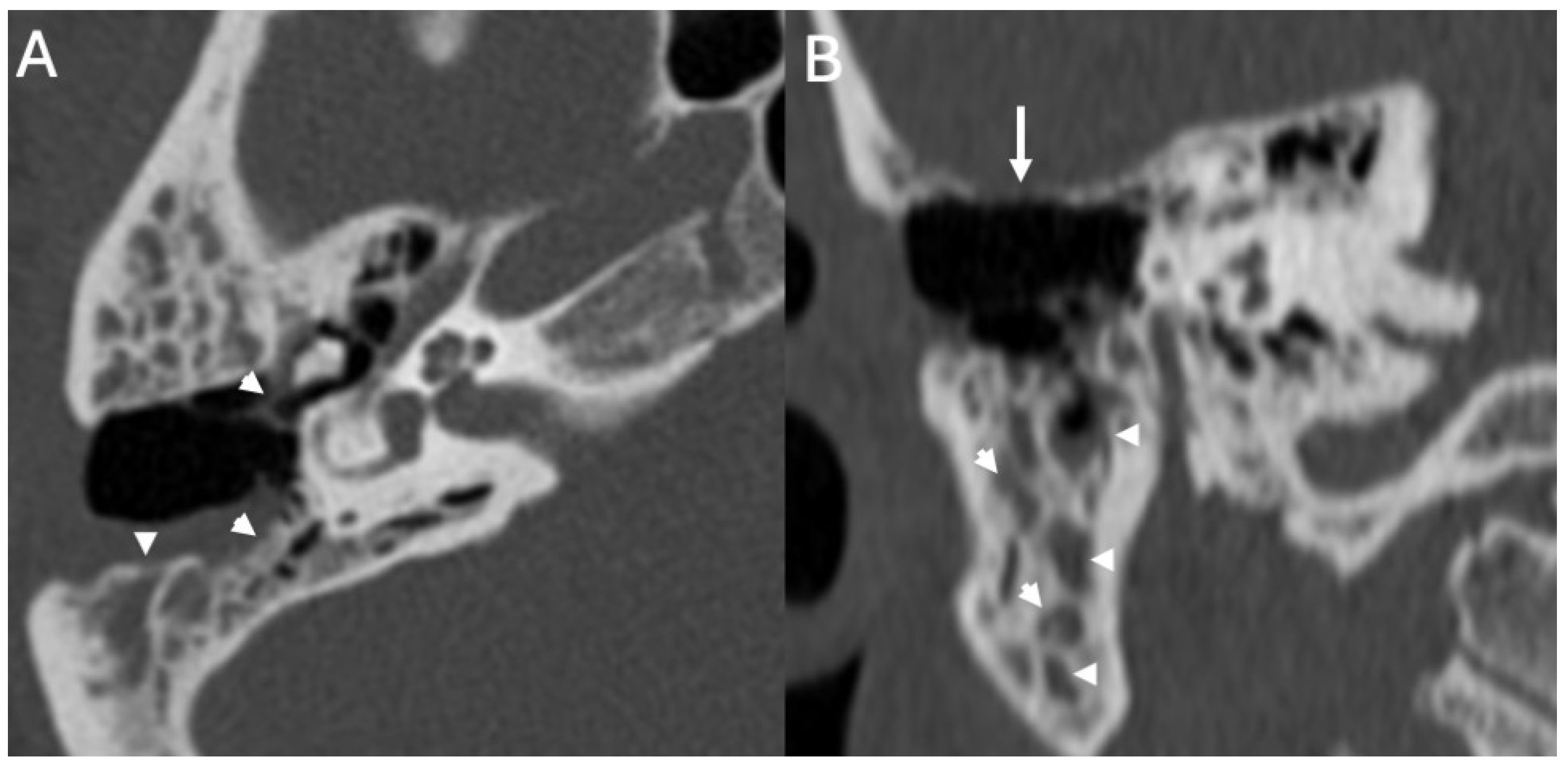
3.2. Bony Dehiscence
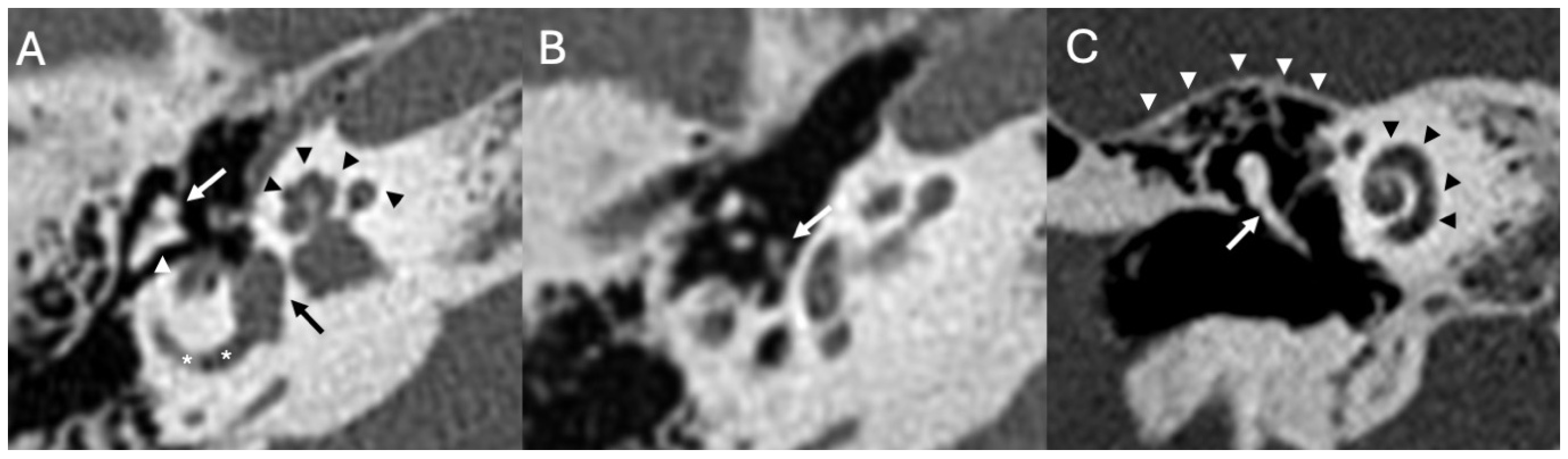
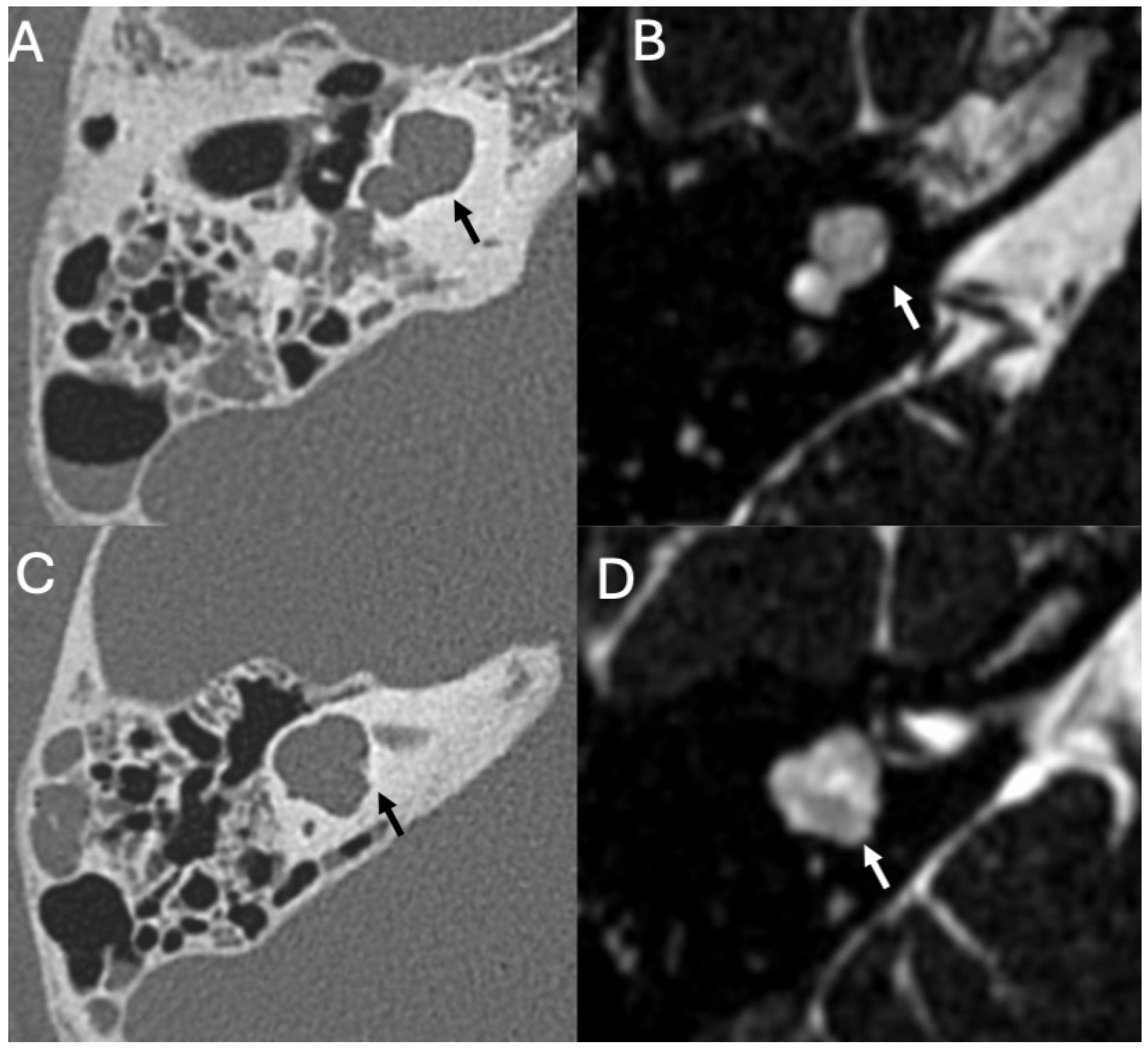
3.3. Cholesteatoma
3.3.1. Imaging
3.3.2. Complications
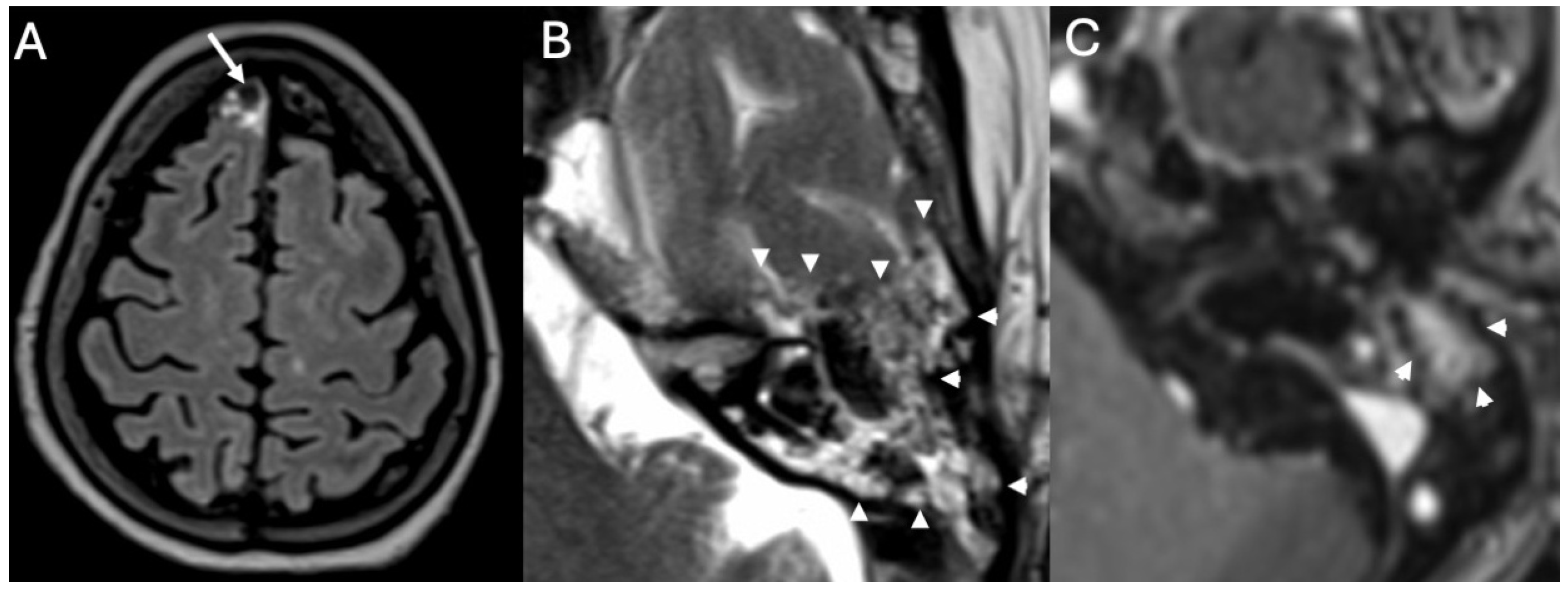
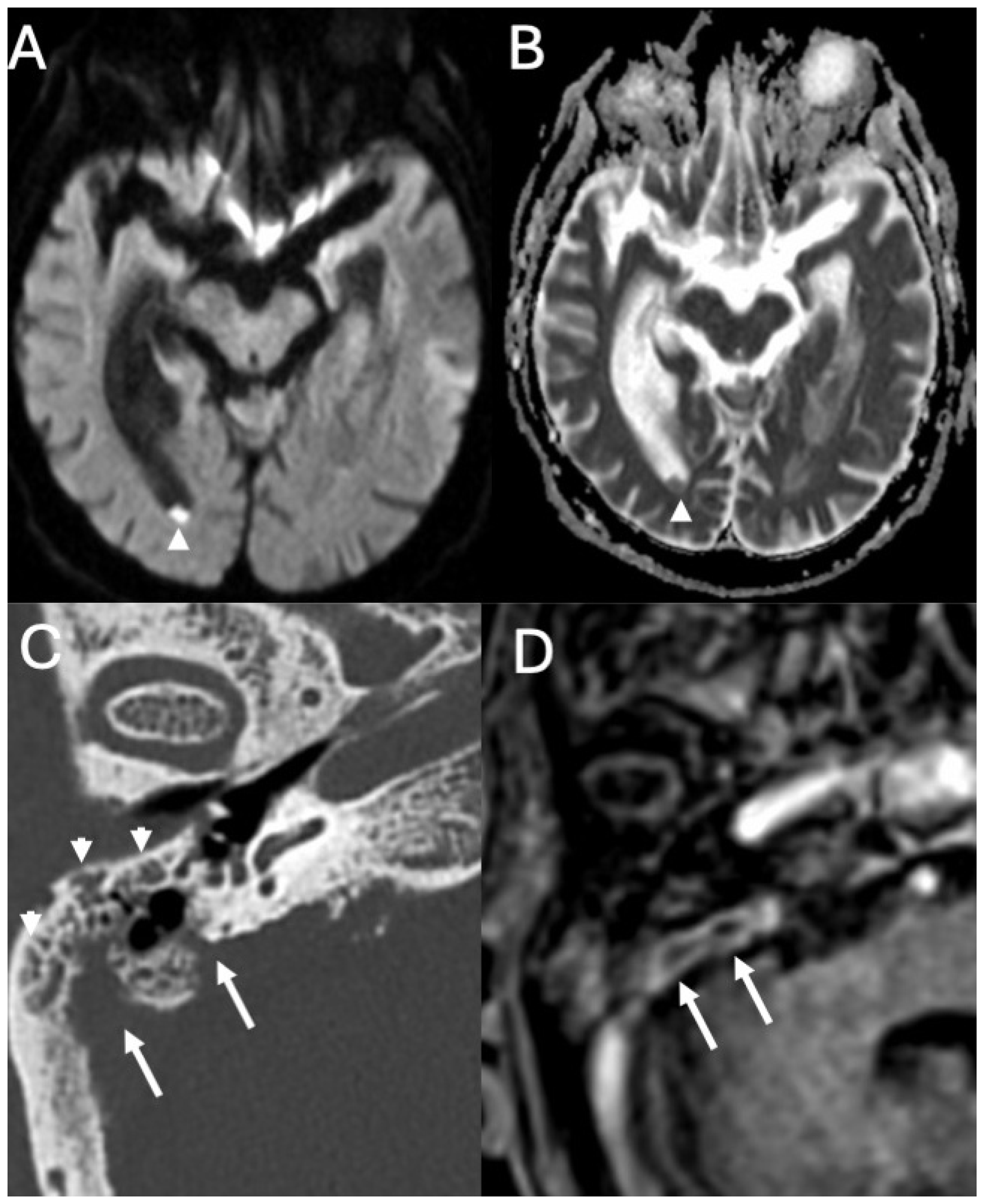
3.4. Labyrinthitis and Vestibulocochlear Neuritis
Imaging
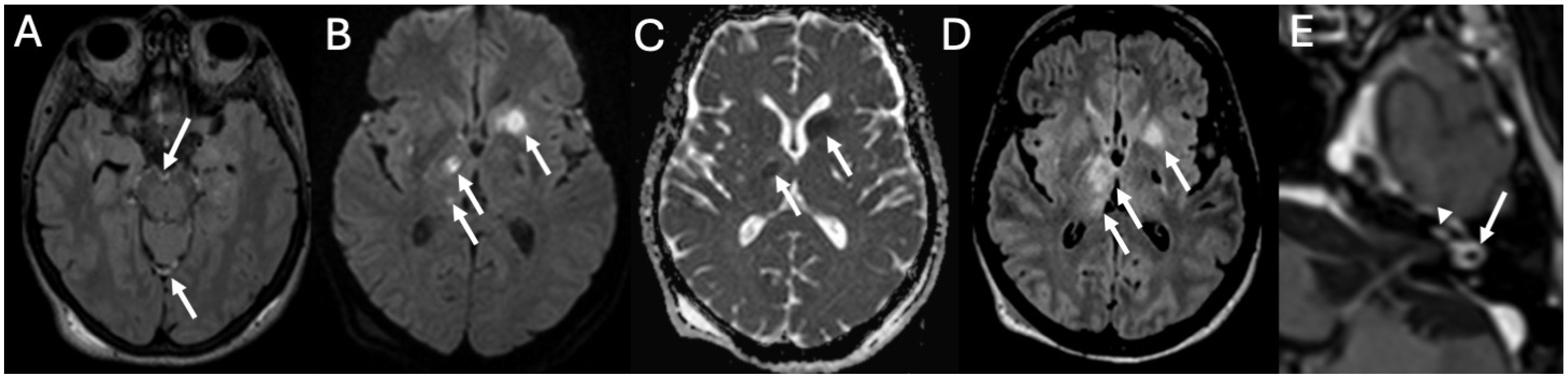
3.5. Cochlear Hemorrhage
4. Iatrogenic Causes
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSF | Cerebrospinal fluid |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| FLAIR | Fluid-attenuated inversion recovery |
| EPI DWI | Diffusion restriction on non-echo-planar |
References
- Mohan, S.; Jain, K.K.; Arabi, M.; Shah, G.V. Imaging of Meningitis and Ventriculitis. Neuroimaging Clin. N. Am. 2012, 22, 557–583. [Google Scholar] [CrossRef]
- Perillo, T.; Capasso, R.; Pinto, A. Neuroimaging of the Most Common Meningitis and Encephalitis of Adults: A Narrative Review. Diagnostics 2024, 14, 1064. [Google Scholar] [CrossRef]
- Kanamalla, U.S.; Ibarra, R.A.; Jinkins, J.R. Imaging of cranial Meningitis and ventriculitis. Neuroimaging Clin. N. Am. 2000, 10, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Van De Beek, D.; De Gans, J.; Spanjaard, L.; Weisfelt, M.; Reitsma, J.B.; Vermeulen, M. Clinical Features and Prognostic Factors in Adults with Bacterial Meningitis. N. Engl. J. Med. 2004, 351, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M. Imaging of Meningitis. Semin. Roentgenol. 2004, 39, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Juliano, A.F.; Ginat, D.T.; Moonis, G. Imaging Review of the Temporal Bone: Part I. Anatomy and Inflammatory and Neoplastic Processes. Radiology 2013, 269, 17–33. [Google Scholar] [CrossRef]
- Lemmerling, M.; De Foer, B. Temporal Bone Imaging; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Arcimowicz, M.; Gotlib, T. Difficult-to-treat rhinosinusitis in IgG4-related disease. Pol. Otorhino. Rev. 2022, 11, 67–74. [Google Scholar] [CrossRef]
- Santek, T.; Hofmann, E.; Milewski, C.; Schwager, K.; Prescher, A. Clinical High-Resolution Imaging of the Inner Ear by Using Magnetic Resonance Imaging (MRI) and Cone Beam Computed Tomography (CBCT). J. Pers. Med. 2024, 14, 637. [Google Scholar] [CrossRef]
- Kimitsuki, T.; Inamitsu, M.; Komune, S.; Komiyama, S. Congenital malformation of the inner ear associated with recurrent Meningitis. Eur. Arch. Oto-Rhino-Laryngol. 1999, 256, S11–S14. [Google Scholar] [CrossRef]
- Wani, N.A.; Rawa, A.; Qureshi, U.; Robbani, I.; Kosar, T. Recurrent Meningitis in An Adult Secondary to An Inner Ear Malformation: Imaging Demonstration. Ear Nose Throat J. 2012, 91, E23–E26. [Google Scholar] [CrossRef]
- Zwierz, A.; Masna, K.; Burduk, P. Recurrent Meningitis in Congenital Inner Ear Malformation. Ear Nose Throat J. 2021, 100, 38S–41S. [Google Scholar] [CrossRef] [PubMed]
- Kivekäs, I.; Vasama, J.-P.; Weitz-Tuoretmaa, A.; Hakomäki, J.; Rautiainen, M. Unilateral common cavity deformity: Recurrent Meningitis due to insufficient newborn hearing screening. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Sennaroglu, L. Cochlear Implantation in Inner Ear Malformations—A Review Article. Cochlear Implant. Int. 2010, 11, 4–41. [Google Scholar] [CrossRef] [PubMed]
- Sennaroğlu, L.; Bajin, M.D. Classification and Current Management of Inner Ear Malformations. Balk. Med. J. 2017, 34, 397–411. [Google Scholar] [CrossRef]
- Hamano, Y.; Kase, K.; Tetsuka, A.; Dias, M.; Nakamura, K.; Sasaki, T.; Ito, M. Surgical Indication of Inner Ear Malformation associated with Bacterial Meningitis. Pediatr. Surg. 2018, 3, 5–8. [Google Scholar]
- Saat, R.; Laulajainen-Hongisto, A.H.; Mahmood, G.; Lempinen, L.; Aarnisalo, A.; Markkola, A.; Jero, J. MR Imaging Features of Acute Mastoiditis and Their Clinical Relevance. AJNR Am. J. Neuroradiol. 2015, 36, 361–367. [Google Scholar] [CrossRef]
- Rubini, A.; Ronzani, G.; D’Alessandro, E.; Marchioni, D. Management of Otogenic Meningitis: A Proposal for Practical Guidelines from a Multicenter Experience with a Systematic Review. J. Clin. Med. 2024, 13, 5509. [Google Scholar] [CrossRef]
- Vazquez, E.; Castellote, A.; Piqueras, J.; Mauleon, S.; Creixell, S.; Pumarola, F.; Figueras, C.; Carreño, J.-C.; Lucaya, J. Imaging of Complications of Acute Mastoiditis in Children. RadioGraphics 2003, 23, 359–372. [Google Scholar] [CrossRef]
- Bruschini, L.; Fortunato, S.; Tascini, C.; Ciabotti, A.; Leonildi, A.; Bini, B.; Giuliano, S.; Abbruzzese, A.; Berrettini, S.; Menichetti, F.; et al. Otogenic Meningitis: A Comparison of Diagnostic Performance of Surgery and Radiology. Open Forum Infect. Dis. 2017, 4, ofx069. [Google Scholar] [CrossRef]
- Barry, C.; Rahmani, G.; Bergin, D. Pneumocephalus and Meningitis as Complications of Mastoiditis. Case Rep. Radiol. 2019, 2019, 7876494. [Google Scholar] [CrossRef]
- Barbara, M.; Margani, V.; Voltattorni, A.; Monini, S.; Covelli, E. Concomitant Dehiscences of the Temporal Bone: A Case-Based Study. Ear Nose Throat J. 2022, 101, NP324–NP328. [Google Scholar] [CrossRef]
- Sanna, M.; Paolo, F.; Russo, A.; Falcioni, M. Management of meningoencephalic herniation of the temporal bone: Personal experience and literature review. Laryngoscope 2009, 119, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.M.; Friedland, P.L.; Boeddinghaus, R.; Thompson, A.; Rodrigues, S.J.; Atlas, M. Otitic Meningitis, Superior Semicircular Canal Dehiscence, and Encephalocele: A Case Series. Otol. Neurotol. 2012, 33, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, P.; Kim, J.; Satani, A.A.; Corrales, C.E.; Lacson, R.; Khorasani, R.; Guenette, J.P. Outcomes of Radiologist Recommendations for Temporal Bone CT to Assess Superior Semicircular Canal Dehiscence on Temporal Bone MRI. AJNR Am. J. Neuroradiol. 2025, 46, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-L.; Shiao, A.-S.; Yung, M.; Sakagami, M.; Sudhoff, H.; Wang, C.H.; Hsu, C.-H.; Lien, C.-F. Updates and Knowledge Gaps in Cholesteatoma Research. BioMed Res. Int. 2015, 2015, 854024. [Google Scholar] [CrossRef]
- Baráth, K.; Huber, A.M.; Stämpfli, P.; Varga, Z.; Kollias, S. Neuroradiology of Cholesteatomas. AJNR Am. J. Neuroradiol. 2011, 32, 221–229. [Google Scholar] [CrossRef]
- Mustafa, A.; Kuçi, S.; Behramaj, A. Management of cholesteatoma complications: Our experience in 145 cases. Indian J. Otol. 2014, 20, 45. [Google Scholar] [CrossRef]
- Dubey, S.P.; Larawin, V.; Molumi, C.P. Intracranial spread of chronic middle ear suppuration. Am. J. Otolaryngol. 2010, 31, 73–77. [Google Scholar] [CrossRef]
- Sun, J.; Sun, J. Intracranial complications of chronic otitis media. Eur. Arch. Otorhinolaryngol. 2014, 271, 2923–2926. [Google Scholar] [CrossRef]
- Lee, J.A.; Fuller, S.R.; Nguyen, S.A.; Meyer, T.A. Factors affecting complications and comorbidities in children with cholesteatoma. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110080. [Google Scholar] [CrossRef]
- Taxak, P.; Ram, C. Labyrinthitis and Labyrinthitis Ossificans—A case report and review of the literature. Radiol. Case 2020, 14, 1–6. [Google Scholar] [CrossRef]
- Kharrat, G.; Ferchichi, S.; Jouini, Z. Pseudomonas Labyrinthitis Complicating Acute Otitis Media: Case Report. Ear Nose Throat J. 2024, 01455613241299675. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, V.; Bhagat, S.; Gupta, A.; Kalra, P. A case report with imaging features of labyrinthitis ossificans in an adult male. J. Clin. Images Med. Case Rep. 2023, 4, 2427. [Google Scholar] [CrossRef]
- Kim, K.; Park, S.; Lee, S.; Park, E.; Kim, B.; Kim, B.; Kim, J. Four-hour-delayed 3D—FLAIR MRIS in patients with acute unilateral peripheral vestibulopathy. Ann. Clin. Transl. Neurol. 2024, 11, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Perillo, T.; Capasso, R.; Pinto, A. Cochlear hemorrhage in a patient with pneumococcal Meningitis. Acta Neurol. Belg. 2024, 125, 1475–1476. [Google Scholar] [CrossRef]
- Engelen-Lee, J.-Y.; Brouwer, M.C.; Aronica, E.; Van De Beek, D. Pneumococcal Meningitis: Clinical-pathological correlations (meningene-path). Acta Neuropathol. Commun. 2016, 4, 26. [Google Scholar] [CrossRef]
- Gowrishankar, S.V.; Fleet, A.; Tomasoni, M.; Durham, R.; Umeria, R.; Merchant, S.A.; Shah, S.F.; Muzaffar, J.; Mohammed, H.; Kuhn, I.; et al. The Risk of Meningitis After Cochlear Implantation: A Systematic Review and Meta-Analysis. Otolaryngol. Head Neck Surg. 2023, 169, 467–481. [Google Scholar] [CrossRef]
- Reefhuis, J.; Honein, M.A.; Whitney, C.G.; Chamany, S.; Mann, E.A.; Biernath, K.R.; Broder, K.; Manning, S.; Avashia, S.; Victor, M.; et al. Risk of Bacterial Meningitis in Children with Cochlear Implants. N. Engl. J. Med. 2003, 349, 435–445. [Google Scholar] [CrossRef]
- Howitz, M.F.; Homøe, P. The risk of acquiring bacterial Meningitis following surgery in Denmark, 1996–2009: A nationwide retrospective cohort study with emphasis on ear, nose and throat (ENT) and neurosurgery. Epidemiol. Infect. 2014, 142, 1300–1309. [Google Scholar] [CrossRef]
- Nielsen, T.R.; Thomsen, J. Meningitis following stapedotomy: A rare and early complication. J. Laryngol. Otol. 2000, 114, 781–783. [Google Scholar] [CrossRef]
- Emilio, A.; De Luca, P.; Max, T.; Agnese, S.; Pasquale, V.; Massimo, R.; Giuseppe, C.; Filippo, R.; Antonio, S.F.; Alfonso, S. How reliable is artificial intelligence in the diagnosis of cholesteatoma on CT images? Am. J. Otolaryngol. 2025, 46, 104519. [Google Scholar] [CrossRef] [PubMed]
- Petrus, L.V.; Lo, W.W. Spontaneous CSF otorrhea caused by abnormal development of the facial nerve canal. AJNR Am. J. Neuroradiol. 1999, 20, 275–277. [Google Scholar] [PubMed]
- Wang, K.-W.; Chang, W.-N.; Chang, H.-W.; Wang, H.C.; Lu, C.H. Clinical relevance of hydrocephalus in bacterial Meningitis in adults. Surg. Neurol. 2005, 64, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Phelps, P.D. Congenital cerebrospinal fluid flstulae of the petrous temporal bone. Clin. Otolaryngol. 1986, 11, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.; Roopa, S.; Madhu, N.; Saini, J.; Yadav, R.; Veerendrakumar, M.; Nagaraja, D. Cystic cochleovestibular anomaly presenting with congenital deafness and recurrent bacterial Meningitis in childhood. Ann. Indian Acad. Neurol. 2013, 16, 272. [Google Scholar] [CrossRef]
- Phelps, P.D.; King, A.; Michaels, L. Cochlear dysplasia and Meningitis. Am. J. Otol. 1994, 15, 551–557. [Google Scholar]
- Mafee, M.F.; Aimi, K.; Kahen, H.L.; Valvassori, G.E.; Capek, V. Chronic otomastoiditis: A conceptual understanding of CT findings. Radiology 1986, 160, 193–200. [Google Scholar] [CrossRef]
- Chieng, J.S.L. Imaging of Otomastoiditis: Acute and Chronic. In Temporal Bone Imaging Made Easy; Pulickal, G.G., Tan, T.Y., Chawla, A., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 79–86. [Google Scholar]
- Larem, A.; Abu Rajab Altamimi, Z.; Aljariri, A.A.; Haidar, H.; Elsotouhy, A.; Alsaadi, A.; Alqahtani, A. Reliability of HIGH-RESOLUTION CT scan in diagnosis of ossicular tympanosclerosis. Laryngoscope Investig. Otolaryngol. 2021, 6, 540–548. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.W.; Kim, S.H. Spontaneous Pneumocephalus Caused by Pneumococcal Meningitis. J. Korean Neurosurg. Soc. 2013, 53, 249. [Google Scholar] [CrossRef]
- Castle, J.T. Cholesteatoma Pearls: Practical Points and Update. Head Neck Pathol. 2018, 12, 419–429. [Google Scholar] [CrossRef]
- Kloth, C.; Beck, A.; Sollmann, N.; Beer, M.; Horger, M.; Thaiss, W.M. Imaging of Pathologies of the Temporal Bone and Middle Ear: Inflammatory Diseases, Their Mimics and Potential Complications—Pictorial Review. Tomography 2023, 9, 2190–2210. [Google Scholar] [CrossRef]
- Reardon, T.; Turnow, M.; Elston, S.; Brown, N.J.; Koller, G.M.; Sharma, S.; Kortz, M.W.; Mohyeldin, A.; Fraser, J.F. Surgical management of petrous apex cholesteatomas in the pediatric population: A systematic review. Surg. Neurol. Int. 2022, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Čukman, M. Rhinogenic Meningitis Caused by Congenital Petrous Apex Cholesteatoma: Simultaneous Surgical Treatment through Transotic and Transsphenoidal Approach. Acta Clin. Croat. 2022, 61, 96. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Hatano, G.; Fukada, N.; Kawasaki, T.; Aoki, H.; Yagi, T. Brain abscess secondary to the middle ear cholesteatoma: A report of two cases. Auris Nasus Larynx 2004, 31, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Danner, C.J. Complications of Chronic Otitis Media and Cholesteatoma. Otolaryngol. Clin. N. Am. 2006, 39, 1237–1255. [Google Scholar] [CrossRef]
- Vashishth, A.; Singh Nagar, T.R.; Mandal, S.; Venkatachalam, V.P. Extensive intratemporal cholesteatomas: Presentation, complications and surgical outcomes. Eur. Arch. Otorhinolaryngol. 2015, 272, 289–295. [Google Scholar] [CrossRef]
- Cucu, A.I.; Patrascu, R.E.; Cosman, M.; Costea, C.F.; Vonica, P.; Blaj, L.A.; Hartie, V.; Istrate, A.C.; Prutianu, I.; Boisteanu, O.; et al. Cerebellar Abscess Secondary to Cholesteatomatous Otomastoiditis—An Old Enemy in New Times. Diagnostics 2023, 13, 3566. [Google Scholar] [CrossRef]
- Hegemann, S.C.A.; Wenzel, A. Diagnosis and Treatment of Vestibular Neuritis/Neuronitis or Peripheral Vestibulopathy (PVP)? Open Questions and Possible Answers. Otol. Neurotol. 2017, 38, 626–631. [Google Scholar] [CrossRef]
- Angeli, S.; Balkany, T. Post-cochlear implant Meningitis. Oper. Tech. Otolaryngol. Head Neck Surg. 2003, 14, 293–296. [Google Scholar] [CrossRef]
- Alanazi, G.A.; Alrashidi, A.S.; Alqarni, K.S.; Al Khozym, S.A.; Alenzi, S. Meningitis post-cochlear implant and role of vaccination. Saudi Med. J. 2022, 43, 1300–1308. [Google Scholar] [CrossRef]
- Harbert, F.; Thomas, G.K.; Rovit, R.L. Otitic Meningitis Following Mastoidectomy. J. Laryngol. Otol. 1966, 80, 325–329. [Google Scholar] [CrossRef]
- Lairmont, A.A.; Nicholson, W.L.; Turner, J.S. Pseudomonas aeruginosa Meningitis following stapedectomy. Laryngoscope 1975, 85, 1076–1085. [Google Scholar] [CrossRef]




| Topic/Section | Main Reference(s) | Study Design/Type | Key Findings/Contribution |
|---|---|---|---|
| Congenital Inner Ear Malformations | Kimitsuki et al., 1999 [10]; Wani et al., 2012 [11]; Zwierz et al., 2021 [12]; Kivekäs et al., 2015 [13]; Sennaroglu 2010 [14]; Sennaroğlu & Bajin 2017 [15]; Hamano et al., 2018 [16] | Case reports, case series, and reviews | Recurrent meningitis often results from CSF fistulas associated with cochlear dysplasia or incomplete partition anomalies; CT/MRI key for diagnosis and surgical planning. |
| Otomastoiditis and Otogenic Meningitis | Saat et al., 2015 [17]; Rubini et al., 2024 [18]; Vazquez et al., 2003 [19]; Bruschini et al., 2017 [20]; Barry et al., 2019 [21] | Retrospective studies, imaging reviews, and case reports | HRCT and MRI identify middle ear/mastoid infection and intracranial spread; meningitis occurs in up to 35–46% of untreated cases. |
| Bony Dehiscence/Temporal Bone Defects | Barbara et al., 2022 [22]; Sanna et al., 2009 [23]; Lim et al., 2012 [24]; Rabiei et al., 2025 [25] | Case-based studies and reviews | Tegmen tympani or semicircular canal defects create a route for meningitis and meningoencephalocele; HRCT coronal reconstructions preferred. |
| Cholesteatoma and Otogenic Complications | Kuo et al., 2015 [26]; Baráth et al., 2011 [27]; Mustafa et al., 2014 [28]; Dubey et al., 2010 [29]; Sun et al., 2014 [30]; Lee et al., 2020 [31] | Reviews and retrospective studies | Cholesteatoma causes erosion and CSF leak, leading to meningitis (12–30% incidence); MRI improves detection; pediatric risk emphasized. |
| Labyrinthitis/Vestibulocochlear Neuritis | Taxak & Ram 2020 [32]; Kharrat et al., 2024 [33]; Singh et al., 2023 [34]; Kim et al., 2024 [35] | Case reports and small series | Meningitis can cause secondary labyrinthitis with cochlear enhancement on MRI; progression to ossification possible if chronic. |
| Cochlear Hemorrhage (Rare Complication) | Perillo et al., 2024 [36]; Engelen-Lee et al., 2016 [37] | Case report; pathologic correlation study | First documented case of cochlear hemorrhage secondary to pneumococcal meningitis; likely due to microvascular injury. |
| Iatrogenic Causes (Ear Surgery, Cochlear Implants) | Gowrishankar et al., 2023 [38]; Reefhuis et al., 2003 [39]; Howitz & Homøe 2014 [40]; Nielsen & Thomsen 2000 [41] | Systematic review, retrospective cohort, case reports | Post-cochlear-implant meningitis risk = 0.07%; increased in children and malformations; mastoidectomy and stapedotomy occasionally associated. |
| Artificial Intelligence in Otology | Emilio et al., 2025 [42] | Diagnostic AI validation study | Deep learning on CT shows high accuracy for automatic cholesteatoma detection, supporting imaging standardization. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perillo, T.; Capasso, R.; Califano, T.; Cataldo, F.; Fulgione, L.; Scaravilli, A.; Vitale, G.; Pinto, A. Role of the Ear in Meningitis: A Narrative Review of Neuroimaging. Diagnostics 2025, 15, 2733. https://doi.org/10.3390/diagnostics15212733
Perillo T, Capasso R, Califano T, Cataldo F, Fulgione L, Scaravilli A, Vitale G, Pinto A. Role of the Ear in Meningitis: A Narrative Review of Neuroimaging. Diagnostics. 2025; 15(21):2733. https://doi.org/10.3390/diagnostics15212733
Chicago/Turabian StylePerillo, Teresa, Raffaella Capasso, Teresa Califano, Federica Cataldo, Ludovica Fulgione, Alessandra Scaravilli, Giuseppe Vitale, and Antonio Pinto. 2025. "Role of the Ear in Meningitis: A Narrative Review of Neuroimaging" Diagnostics 15, no. 21: 2733. https://doi.org/10.3390/diagnostics15212733
APA StylePerillo, T., Capasso, R., Califano, T., Cataldo, F., Fulgione, L., Scaravilli, A., Vitale, G., & Pinto, A. (2025). Role of the Ear in Meningitis: A Narrative Review of Neuroimaging. Diagnostics, 15(21), 2733. https://doi.org/10.3390/diagnostics15212733





