Exploring the Association Between Heart Rate Variability and Intracranial Atherosclerosis in Middle-Aged or over Community-Dwelling Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Baseline Characteristics
2.2. Cardiac Function Assessment
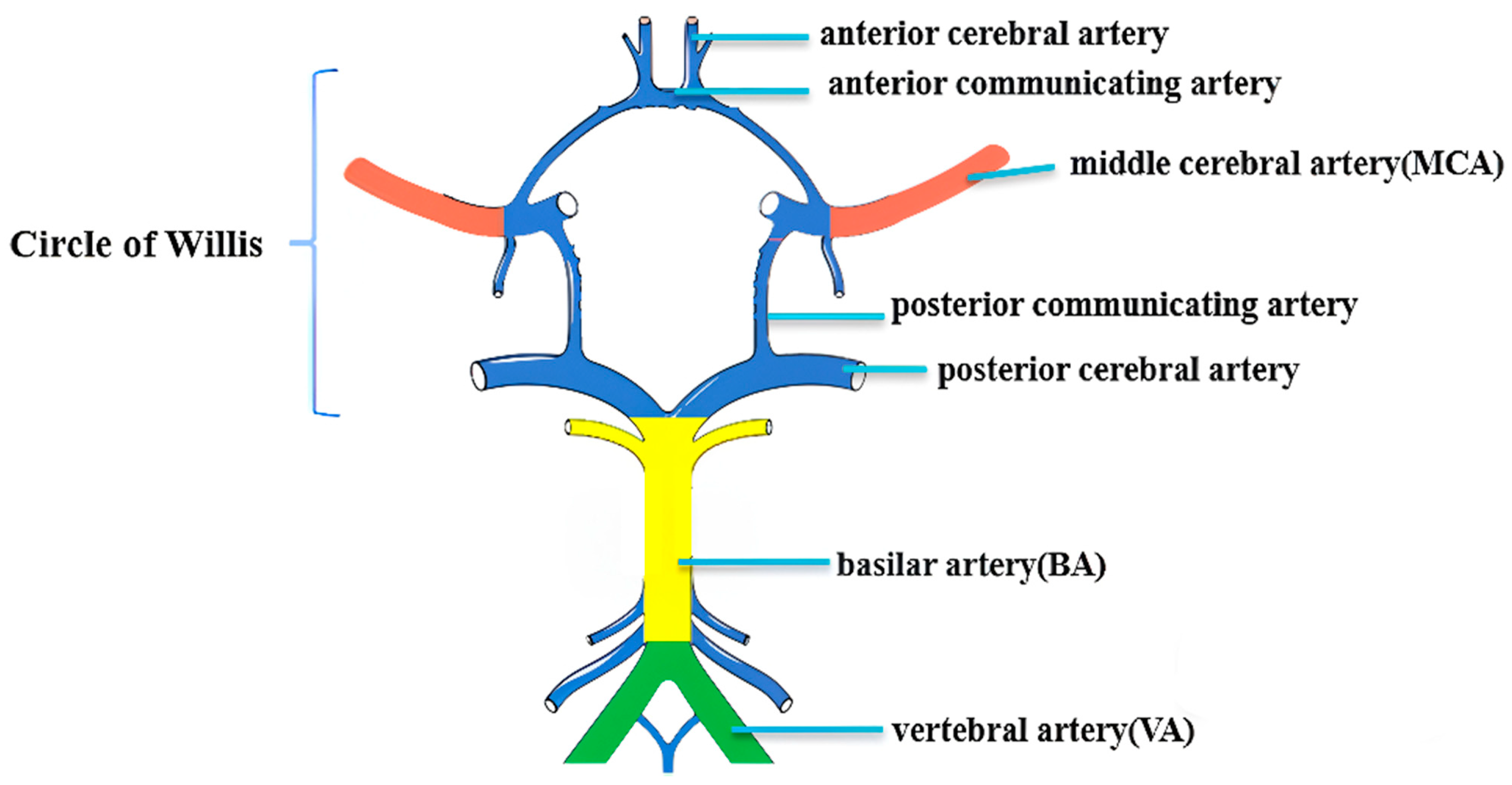
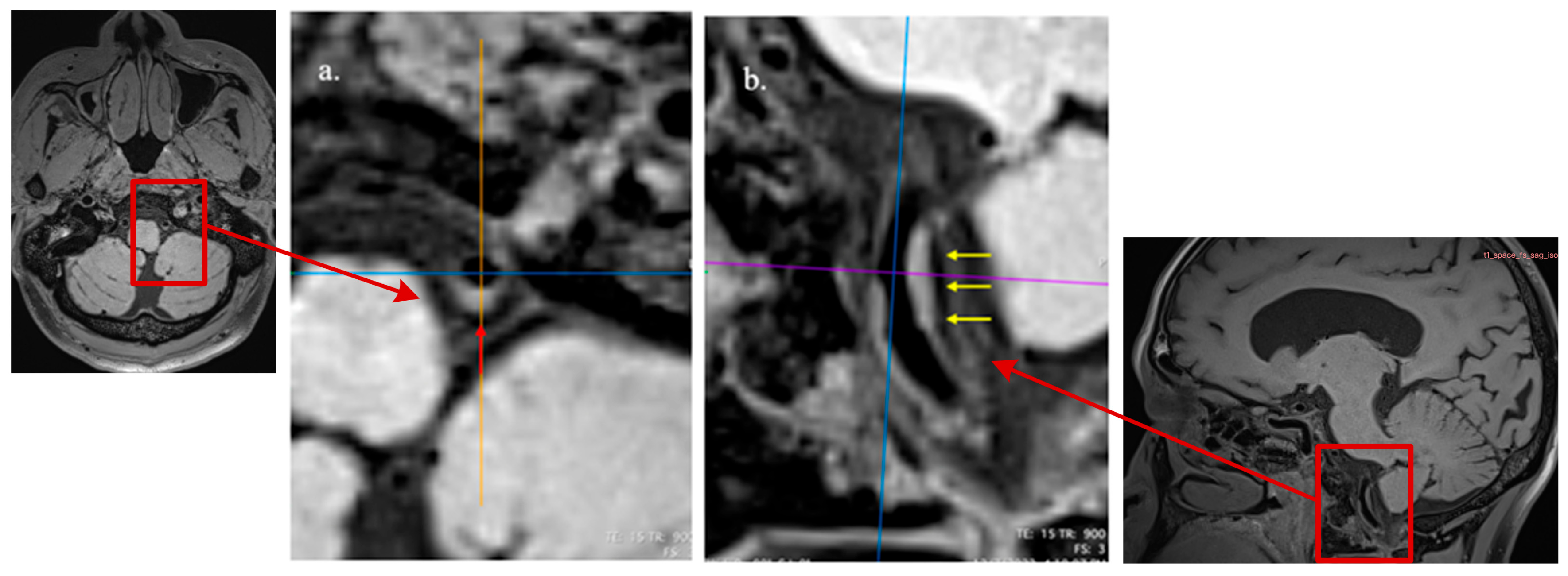
2.3. MRI Imaging Acquisition
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Comparisons of Characteristics of the Electrocardiogram Between Adults with and Without ICAS
3.3. Comparison of Subject Characteristics Across SDNN Tertiles
3.4. Association Between Heart Rate Deviation and the Presence of ICAS
3.5. Inter-Rater Reliability of Assessments on Characteristics of ICAS Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, M.C.; Ribas, G.; Scavasine, V.; Ducci, R.D.-P.; Mendes, D.C.; Zétola, V.d.H.F.; Cabral, N.; Rundek, T. Stroke recurrence in the different subtypes of ischemic stroke. The importance of the intracranial disease. Arq. Neuropsiquiatr. 2018, 76, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Liu, L.; Soo, Y.O.; Pu, Y.; Pan, Y.; Wang, Y.; Zou, X.; Leung, T.W.; Cai, Y.; et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: The Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014, 45, 663–669. [Google Scholar] [CrossRef]
- Suri, M.F.K.; Qiao, Y.; Ma, X.; Guallar, E.; Zhou, J.; Zhang, Y.; Liu, L.; Chu, H.; Qureshi, A.I.; Alonso, A.; et al. Prevalence of intracranial atherosclerotic stenosis using high-resolution magnetic resonance angiography in the general population: The atherosclerosis risk in communities study. Stroke 2016, 47, 1187–1193. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Mera, R.M.; Lama, J.; Zambrano, M.; Del Brutto, V.J. Intracranial arterial stenosis in Ecuadorian Natives/Mestizos. A population-based study in older adults (The Atahualpa Project). Arch. Gerontol. Geriatr. 2015, 61, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, M.; Zhang, D.; Han, F.; Zhou, L.; Yao, M.; Li, M.; Cui, L.; Zhang, S.; Peng, B.; et al. The prevalence and prognosis of asymptomatic intracranial atherosclerosis in a community-based population: Results based on high-resolution magnetic resonance imaging. Eur. J. Neurol. 2023, 30, 3761–3771. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Q.; Wang, X.; Ji, X.; Sang, S.; Shao, S.; Zhao, Y.; Xiang, Y.; Xue, Y.; Li, J.; et al. Prevalence and cardiovascular risk factors of asymptomatic intracranial arterial stenosis: The Kongcun Town Study in Shandong, China. Eur. J. Neurol. 2020, 27, 729–735. [Google Scholar] [CrossRef]
- Bang, O.Y.; Kim, J.W.; Lee, J.H.; Lee, M.A.; Lee, P.H.; Joo, I.S.; Huh, K. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology 2005, 65, 296–298. [Google Scholar] [CrossRef]
- Feldmann, E.; Daneault, N.; Kwan, E.; Ho, K.J.; Pessin, M.S.; Langenberg, P.; Caplan, L.R. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology 1990, 40, 1541–1545. [Google Scholar] [CrossRef]
- Kim, S.J.; Schneider, D.J.; Feldmann, E.; Liebeskind, D.S. Intracranial atherosclerosis: Review of imaging features and advances in diagnostics. Int. J. Stroke 2022, 17, 599–607. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Epidemiology of Cerebrovascular Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Qureshi, A.I.; Caplan, L.R. Intracranial atherosclerosis. Lancet 2014, 383, 984–998. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A.; Kerr, S.; Allan, L.; Steen, I.N.; Ballard, C.; Allen, J.; Murray, A.; Kenny, R.A. Autonomic function is impaired in elderly stroke survivors. Stroke 2005, 36, 1026–1030. [Google Scholar] [CrossRef]
- Esler, M. The sympathetic system and hypertension. Am. J. Hypertens 2000, 13 Pt 2, 99s–105s. [Google Scholar] [CrossRef]
- Hamner, J.; Tan, C.O.; Lee, K.; Cohen, M.A.; Taylor, J.A. Sympathetic control of the cerebral vasculature in humans. Stroke 2010, 41, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zuckerman, J.H.; Iwasaki, K.; Wilson, T.E.; Crandall, C.G.; Levine, B.D. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 2002, 106, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Essibayi, M.A.; Toma, A.; Mowrey, W.; Qin, J.; Hamad, M.; Ryvlin, J.; Holland, R.; Fluss, R.; Altschul, D.; Lin, L.-M.; et al. Heart rate and heart rate variability during diagnostic and interventional neuroendovascular procedures. Interv. Neuroradiol. 2025, 31, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [CrossRef]
- Dekker, J.M.; Crow, R.S.; Folsom, A.R.; Hannan, P.J.; Liao, D.; Swenne, C.A.; Schouten, E.G. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC Study. Atherosclerosis Risk In Communities. Circulation 2000, 102, 1239–1244. [Google Scholar] [CrossRef]
- Nelde, A.; Krumm, L.; Arafat, S.; Hotter, B.; Nolte, C.H.; Scheitz, J.F.; Klammer, M.G.; Krämer, M.; Scheib, F.; Endres, M.; et al. Machine learning using multimodal and autonomic nervous system parameters predicts clinically apparent stroke-associated pneumonia in a development and testing study. J. Neurol. 2024, 271, 899–908. [Google Scholar] [CrossRef]
- Qiu, Q.; Song, W.; Zhou, X.; Yu, Z.; Wang, M.; Hao, H.; Pan, D.; Luo, X. Heart rate variability is associated with cerebral small vessel disease in patients with diabetes. Front. Neurol. 2022, 13, 989064. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, J.; Zhou, G.; Zhang, Y. The Roles of Heart Rate Variability in Cerebral Stroke. Neuropsychiatr. Dis. Treat. 2025, 21, 1057–1065. [Google Scholar] [CrossRef]
- Makikallio, A.M.; Makikallio, T.H.; Korpelainen, J.T.; Sotaniemi, K.A.; Huikuri, H.V.; Myllyla, V.V. Heart rate dynamics predict poststroke mortality. Neurology 2004, 62, 1822–1826. [Google Scholar] [CrossRef]
- Fyfe-Johnson, A.L.; Muller, C.J.; Alonso, A.; Folsom, A.R.; Gottesman, R.F.; Rosamond, W.D.; Whitsel, E.A.; Agarwal, S.K.; MacLehose, R.F. Heart rate variability and incident stroke: The atherosclerosis risk in communities study. Stroke 2016, 47, 1452–1458. [Google Scholar] [CrossRef]
- Pramanda, A.N.; Farabi, F.; Prameswari, H.S.; Achmad, C.; Tiksnadi, B.B. Myocardial-alternation index (MMI) is correlated with soluble suppression of tumorigenecity-2 (sST2) in patients with ischemic cardiomyopathy. Egypt. Heart J. 2025, 77, 39. [Google Scholar] [CrossRef] [PubMed]
- Tiksnadi, B.B.; Putra, A.P.; Ikhsani, R.; Tarsidin, N.F. Myocardial micro-alternation index (MMI) does not associate with the ischemic response in coronary artery disease patients. Eur. J. Prev. Cardiol. 2024, 31 (Suppl. S1), zwae175.078. [Google Scholar] [CrossRef]
- Watanabe, E.; Kiyono, K.; Hayano, J.; Yamamoto, Y.; Inamasu, J.; Yamamoto, M.; Ichikawa, T.; Sobue, Y.; Harada, M.; Ozaki, Y. Multiscale Entropy of the Heart Rate Variability for the Prediction of an Ischemic Stroke in Patients with Permanent Atrial Fibrillation. PLoS ONE 2015, 10, e0137144. [Google Scholar] [CrossRef]
- Kellett, J.; Rasool, S.; McLoughlin, B. Prediction of mortality 1 year after hospital admission. QJM Int. J. Med. 2012, 105, 847–853. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.-J.; Zheng, L.; Du, H.; Chu, W.C.-W.; Leung, T.W.-H.; Chen, X.-Y. Vertebrobasilar Junction Angle Over 90°: A Potential Imaging Marker Associated With Vertebrobasilar Atherosclerosis. Front. Neurosci. 2021, 15, 789852. [Google Scholar] [CrossRef]
- Wang, M.; Wu, F.; Yang, Y.; Miao, H.; Fan, Z.; Ji, X.; Li, D.; Guo, X.; Yang, Q. Quantitative assessment of symptomatic intracranial atherosclerosis and lenticulostriate arteries in recent stroke patients using whole-brain high-resolution cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2018, 20, 35. [Google Scholar] [CrossRef]
- Yang, W.-J.; Abrigo, J.; Soo, Y.O.-Y.; Wong, S.; Wong, K.-S.; Leung, T.W.-H.; Chu, W.C.-W.; Chen, X.-Y. Regression of Plaque Enhancement Within Symptomatic Middle Cerebral Artery Atherosclerosis: A High-Resolution MRI Study. Front. Neurol. 2020, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, J.; Yang, W.; Lam, H.-C.C.; Wong, K.L.; Chu, W.; Leung, T.W.H.; Chen, X. Patterns and Implications of Intracranial Atherosclerosis in Anterior and Posterior Circulation Identified by High-Resolution Vessel Wall Imaging. Cerebrovasc. Dis. 2023, 53, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, N.; Yang, W.; Abrigo, J.M.; Chu, W.C.W.; van der Kolk, A.G.; Siero, J.C.; Wong, K.S.; Hendrikse, J.; Chen, X.Y. Magnetic Resonance Imaging of Plaque Morphology, Burden, and Distribution in Patients with Symptomatic Middle Cerebral Artery Stenosis. Stroke 2016, 47, 1797–1802. [Google Scholar] [CrossRef]
- Dieleman, N.; Yang, W.; van der Kolk, A.G.; Abrigo, J.; Lee, K.L.; Chu, W.C.W.; Zwanenburg, J.J.M.; Siero, J.C.W.; Wong, K.S.; Hendrikse, J.; et al. Qualitative Evaluation of a High-Resolution 3D Multi-Sequence Intracranial Vessel Wall Protocol at 3 Tesla MRI. PLoS ONE 2016, 11, e0160781. [Google Scholar] [CrossRef]
- Yang, W.-J.; Wasserman, B.A.; Zheng, L.; Huang, Z.-Q.; Li, J.; Abrigo, J.; Wong, S.S.-M.; Ying, M.T.-C.; Chu, W.C.-W.; Wong, L.K.-S.; et al. Understanding the Clinical Implications of Intracranial Arterial Calcification Using Brain CT and Vessel Wall Imaging. Front. Neurol. 2021, 12, 619233. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Yang, W.-J.; Sze-To, C.-Y.; Leung, T.W.-H.; Chen, X.-Y. Plaque Wall Distribution Pattern of the Atherosclerotic Middle Cerebral Artery Associates With the Circle of Willis Completeness. Front. Neurol. 2020, 11, 599459. [Google Scholar] [CrossRef]
- Yang, W.J.; Fisher, M.; Zheng, L.; Niu, C.B.; Paganini-Hill, A.; Zhao, H.L.; Xu, Y.; Wong, K.S.; Ng, H.K.; Chen, X.Y. Histological Characteristics of Intracranial Atherosclerosis in a Chinese Population: A Postmortem Study. Front. Neurol. 2017, 8, 488. [Google Scholar] [CrossRef]
- Qiao, Y.; Anwar, Z.; Intrapiromkul, J.; Liu, L.; Zeiler, S.R.; Leigh, R.; Zhang, Y.; Guallar, E.; Wasserman, B.A. Patterns and Implications of Intracranial Arterial Remodeling in Stroke Patients. Stroke 2016, 47, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Fan, P.; Li, Z.; Mossa-Basha, M.; Ju, Y.; Zhao, X.; Kong, Q.; Pei, X.; Zhang, X.; Sui, B.; et al. Evaluating Middle Cerebral Artery Plaque Characteristics and Lenticulostriate Artery Morphology Associated With Subcortical Infarctions at 7T MRI. J. Magn. Reson Imaging 2024, 59, 1045–1055. [Google Scholar] [CrossRef]
- Tao, L.; Li, X.Q.; Hou, X.W.; Yang, B.Q.; Xia, C.; Ntaios, G.; Chen, H.S. Intracranial Atherosclerotic Plaque as a Potential Cause of Embolic Stroke of Undetermined Source. J. Am. Coll. Cardiol. 2021, 77, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Alkan, O.; Kizilkilic, O.; Yildirim, T.; Atalay, H. Intracranial cerebral artery stenosis with associated coronary artery and extracranial carotid artery stenosis in Turkish patients. Eur. J. Radiol. 2009, 71, 450–455. [Google Scholar] [CrossRef]
- Samuels, O.B.; Joseph, G.J.; Lynn, M.J.; Smith, H.A.; Chimowitz, M.I. A standardized method for measuring intracranial arterial stenosis. AJNR Am. J. Neuroradiol. 2000, 21, 643–646. [Google Scholar] [PubMed]
- Yasaka, M.; Yamaguchi, T.; Shichiri, M. Distribution of atherosclerosis and risk factors in atherothrombotic occlusion. Stroke 1993, 24, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Nakanishi, K.; Jin, Z.; Homma, S.; Elkind, M.S.; Rundek, T.; Lee, S.C.; Tugcu, A.; Yoshita, M.; DeCarli, C.; Wright, C.B.; et al. Association Between Heart Rate and Subclinical Cerebrovascular Disease in the Elderly. Stroke 2018, 49, 319–324. [Google Scholar] [CrossRef]
- Korpelainen, J.T.; A Sotaniemi, K.; Suominen, K.; Tolonen, U.; Myllylä, V.V. Cardiovascular autonomic reflexes in brain infarction. Stroke 1994, 25, 787–792. [Google Scholar] [CrossRef]
- Lees, T.; Shad-Kaneez, F.; Simpson, A.M.; Nassif, N.T.; Lin, Y.; Lal, S. Heart rate variability as a biomarker for predicting stroke, post-stroke complications and functionality. Biomark. Insights 2018, 13, 1177271918786931. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; Blankstein, R.; Al-Mallah, M.H.; Lima, J.A.; Bluemke, D.A.; Hundley, W.G.; Polak, J.F.; Blumenthal, R.S.; Nasir, K.; Blaha, M.J. Association of resting heart rate with carotid and aortic arterial stiffness: Multi-ethnic study of atherosclerosis. Hypertension 2013, 62, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Arenillas, J.F. Intracranial atherosclerosis and inflammation: Lessons from the East and the West. Brain Circ. 2015, 1, 47–52. [Google Scholar] [CrossRef]
- Koichubekov, B.; Sorokina, M.; Laryushina, Y.; Turgunova, L.; Korshukov, I. Nonlinear analyses of heart rate variability in hypertension. Ann. Cardiol. Angeiol. 2018, 67, 174–179. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Driver, M.P.; Huang, T.Y.; Magraner, J.; Botting, P.G.; Wang, M.; Chen, P.-S.; Bello, N.A.; Ouyang, D.; Theurer, J.; et al. Blood pressure variability supersedes heart rate variability as a real-world measure of dementia risk. Sci. Rep. 2024, 14, 1838. [Google Scholar] [CrossRef]
- Takahashi, M.K.N.; Paradela, R.S.; Grinberg, L.T.; Leite, R.E.P.; Farias-Itao, D.S.; Paes, V.R.; Braga, M.E.; Naslavsky, M.S.; Zatz, M.; Jacob-Filho, W.; et al. Hypertension may associate with cerebral small vessel disease and infarcts through the pathway of intracranial atherosclerosis. Neurobiol. Aging 2025, 145, 84–95. [Google Scholar] [CrossRef]
- Kinlay, S.; Libby, P.; Ganz, P. Endothelial function and coronary artery disease. Curr. Opin. Lipidol. 2001, 12, 383–389. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109 (Suppl. S1), III27–III32. [Google Scholar] [CrossRef]
- Drexler, H. Factors involved in the maintenance of endothelial function. Am. J. Cardiol. 1998, 82, 3s–4s. [Google Scholar] [CrossRef] [PubMed]
- Kinlay, S.; Behrendt, D.; Wainstein, M.; Beltrame, J.; Fang, J.C.; Creager, M.A.; Selwyn, A.P.; Ganz, P. Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation 2001, 104, 1114–1148. [Google Scholar] [CrossRef]
- Chen, L.H.; Spagnolo-Allende, A.; Yang, D.; Qiao, Y.; Gutierrez, J. Epidemiology, Pathophysiology, and Imaging of Atherosclerotic Intracranial Disease. Stroke 2024, 55, 311–323. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Lee, J.; Kim, Y.-J.; Kwon, S.U.; Lee, D.; Jung, S.-C.; Kang, D.-W.; Kim, J.S. Isolated MCA disease in patients without significant atherosclerotic risk factors: A high-resolution magnetic resonance imaging study. Stroke 2015, 46, 697–703. [Google Scholar] [CrossRef]
- Ohara, T.; Toyoda, K.; Otsubo, R.; Nagatsuka, K.; Kubota, Y.; Yasaka, M.; Naritomi, H.; Minematsu, K. Eccentric stenosis of the carotid artery associated with ipsilateral cerebrovascular events. AJNR Am. J. Neuroradiol. 2008, 29, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Swartz, R.H.; Bhuta, S.S.; Farb, R.I.; Agid, R.; Willinsky, R.A.; Terbrugge, K.G.; Butany, J.; Wasserman, B.A.; Johnstone, D.M.; Silver, F.L.; et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009, 72, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Korpelainen, J.T.; Sotaniemi, K.A.; Huikuri, H.V.; Myllylä, V.V. Abnormal heart rate variability as a manifestation of autonomic dysfunction in hemispheric brain infarction. Stroke 1996, 27, 2059–2063. [Google Scholar] [CrossRef]
- Esina, E.; Zuikova, A.; Dobrynina, I.; Lyutov, V.; Tsygan, V. ECG Dispersion Mapping in Preclinical Diagnosis of Cardiovascular Diseases. Sovrem Tekhnologii Med. 2021, 12, 87–92. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total (n = 272) | Adults Without ICAS (n = 120) | Adults with ICAS (n = 152) | p-Value |
|---|---|---|---|---|
| Age, y, mean ± SD | 63.4 ± 6.8 | 62.4 ± 6.7 | 64.1 ± 6.8 | 0.041 |
| Sex, male, n (%) | 118 (43.4) | 46 (38.3) | 72 (47.4) | 0.135 |
| SBP, mmHg, (mean ± SD) | 129.9 ± 17.1 | 126.4 ± 17.5 | 132.7 ± 16.3 | 0.003 |
| DBP, mmHg, (mean ± SD) | 79.9 ± 10.5 | 77.8 ± 10.7 | 81.5 ± 10.1 | 0.005 |
| Body mass index ≥25 kg/m2, n (%) | 87 (32.0) | 27 (22.5) | 60 (39.5) | 0.003 |
| Hypertension | 80 (29.5) | 21 (17.5) | 59(38.8) | <0.001 |
| Diabetes mellitus | 33 (12.2) | 13 (10.8) | 20 (13.2) | 0.560 |
| Coronary artery disease | 17 (6.3) | 4 (3.3) | 13 (8.6) | 0.077 |
| Hyperlipidemia | 104 (38.4) | 32 (26.7) | 72 (47.4) | <0.001 |
| Statins | 84 (30.9) | 25 (21.0) | 59 (38.8) | 0.002 |
| Antiplatelet | 13 (4.8) | 4 (3.4) | 9 (5.9) | 0.328 |
| Anticoagulant | 31 (11.4) | 11 (9.2) | 20 (13.2) | 0.315 |
| Antihypertensives | 73 (26.8) | 18 (15.1) | 55 (36.2) | <0.001 |
| Antidiabetics | 16 (5.9) | 6 (5.0) | 10 (6.6) | 0.594 |
| Smoker | 14 (5.2) | 5 (4.2) | 9 (5.9) | 0.516 |
| Alcohol Drinking | 20 (7.4) | 9 (7.5) | 11 (7.2) | 0.934 |
| Characteristics | Total (n = 272) | Adults Without ICAS (n = 120) | Adults with ICAS (n = 152) | p-Value |
|---|---|---|---|---|
| Abnormal, n (%) | 102 (37.5) | 45 (37.5) | 57 (37.7) | 0.967 |
| Heart rate, bmp | 72.2 ± 10.4 | 71.9 ± 11.2 | 72.4 ± 9.8 | 0.740 |
| MMI, mean ± SD | 19.1 ± 8.6 | 19.8 ± 10.4 | 18.4 ± 6.9 | 0.193 |
| Atrial deviation, n (%) | 175 (64.3) | 69 (57.5) | 106 (70.2) | 0.030 |
| Ventricular deviation, n (%) | 235 (86.3) | 103 (85.8) | 132 (87.4) | 0.703 |
| Power HF, ms2 median (IQR) | 154.9 (67.4, 319.8) | 161.5 (75.0, 317.8) | 154.6 (64.2, 320.6) | 0.345 |
| Power LF, ms2 median (IQR) | 100.5 (47.0, 235.2) | 96.3 (52.7, 194.8) | 107.7 (43.7, 270.3) | 0.326 |
| LF/HF < 1, n (%) | 168 (61.7) | 71 (59.2) | 97 (64.2) | 0.393 |
| SDNN, abnormal, n (%) | 94 (34.5) | 34 (28.3) | 60 (39.7) | 0.040 |
| Characteristics | Group 1 (n = 90) | Group 2 (n = 91) | Group 3 (n = 91) | p-Value |
|---|---|---|---|---|
| Age, y, mean ± SD | 65.0 ± 5.9 | 63.3 ± 6.6 | 62.0 ± 7.4 ac | 0.010 |
| Sex, male, n (%) | 40 (44.4) | 38 (41.8) | 40 (44.4) | 0.915 |
| SBP, mmHg, mean ± SD | 128.8 ± 17.7 | 131.5 ± 16.9 | 128.9 ± 16.4 | 0.487 |
| DBP, mmHg, mean ± SD | 79.1 ± 9.4 | 80.9 ± 11.1 | 79.3 ± 10.7 | 0.432 |
| Body mass index ≥25 kg/m2, n (%) | 26 (28.9) | 31 (34.1) | 30 (33.3) | 0.723 |
| Hypertension | 31 (34.4) | 32 (35.2) bc | 17 (18.9) ac | 0.026 |
| Diabetes mellitus | 14 (15.6) | 11 (12.1) | 8 (8.9) | 0.392 |
| Coronary artery disease | 7 (7.8) | 5 (5.5) | 5 (5.6) | 0.771 |
| Hyperlipidemia | 36 (34.6) | 33 (36.3) | 35 (38.9) | 0.868 |
| The presence of ICAS | 50 (55.5) | 44 (48.3) ab | 45 (49.4) ac | 0.032 |
| Plaque burden%, mean ± SD | 47.4 ± 3.7 | 38.5 ± 3.8 | 44.8 ±3.9 | 0.243 |
| Luminal stenosis, mean ± SD | 23.0 ± 19.8 | 17.1 ± 19.9 | 17.4 ± 20.1 | 0.121 |
| Irregular surface, n (%) | 21 (23.3) | 22 (24.2) | 21 (23.3) | 0.933 |
| Diffuse lesion, n (%) | 35 (38.9) | 38 (41.8) | 36 (40.0) | 0.757 |
| Eccentric lesion, n (%) | 40 (44.4) | 38 (41.8) | 41 (45.6) | 0.869 |
| Positive remodeling, n (%) | 33 (36.7) | 28 (30.8) | 33 (36.7) | 0.629 |
| Intracranial Large Artery Lesion, Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| Characteristics | ||
| SDNN deviation | 1.40 (1.01–1.94) | 0.044 |
| Atrial hypoxia | 1.74 (1.05–2.87) | 0.031 |
| Model 1 Adjusted for confounding factors (age, gender) | ||
| SDNN deviation | 1.57 (1.12–2.21) | 0.009 |
| Atrial hypoxia | 1.72 (1.03–2.85) | 0.038 |
| Model 2 Adjusted for confounding factors (age, WMH burden, gender, hypertension, BMI, hyperlipidemia) | ||
| SDNN deviation | 1.55 (1.10–2.18) | 0.012 |
| Atrial hypoxia | 1.85 (1.10–3.14) | 0.022 |
| Rater 1 | Rater 2 | Inter-Observer Agreement Coefficient (95% CI) | |
|---|---|---|---|
| Presence of ICAS (MCA, BA, VA); n, % | 127 (55.9) | 123 (54.2) | 0.86 (0.793–0.926) |
| Presence of ICAS (MCA, BA, VA, ACA, PCA, ICA); n, % | 152 (66.9%) | 144 (63.4) | 0.83 (0.753–0.906) |
| Plaque eccentricity; n, % | 102 (45%) | 86 (37.9%) | 0.77 (0.692–0.847) |
| Plaque irregular surface; n, % | 51 (22.5) | 45 (20.0%) | 0.82 (0.727–0.912) |
| Plaque diffuse; n, % | 94 (41.4%) | 78 (34.4%) | 0.71 (0.631–0.797) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Lai, L.; Luo, J.; Ying, M.T.C. Exploring the Association Between Heart Rate Variability and Intracranial Atherosclerosis in Middle-Aged or over Community-Dwelling Adults. Diagnostics 2025, 15, 2731. https://doi.org/10.3390/diagnostics15212731
Cheng Y, Lai L, Luo J, Ying MTC. Exploring the Association Between Heart Rate Variability and Intracranial Atherosclerosis in Middle-Aged or over Community-Dwelling Adults. Diagnostics. 2025; 15(21):2731. https://doi.org/10.3390/diagnostics15212731
Chicago/Turabian StyleCheng, Yangyang, Lihua Lai, Jieqi Luo, and Michael Tin Cheung Ying. 2025. "Exploring the Association Between Heart Rate Variability and Intracranial Atherosclerosis in Middle-Aged or over Community-Dwelling Adults" Diagnostics 15, no. 21: 2731. https://doi.org/10.3390/diagnostics15212731
APA StyleCheng, Y., Lai, L., Luo, J., & Ying, M. T. C. (2025). Exploring the Association Between Heart Rate Variability and Intracranial Atherosclerosis in Middle-Aged or over Community-Dwelling Adults. Diagnostics, 15(21), 2731. https://doi.org/10.3390/diagnostics15212731





