Current Surgical Perspective on the Prognosis of Small-Cell Lung Cancer
Abstract
1. Introduction
2. Staging System
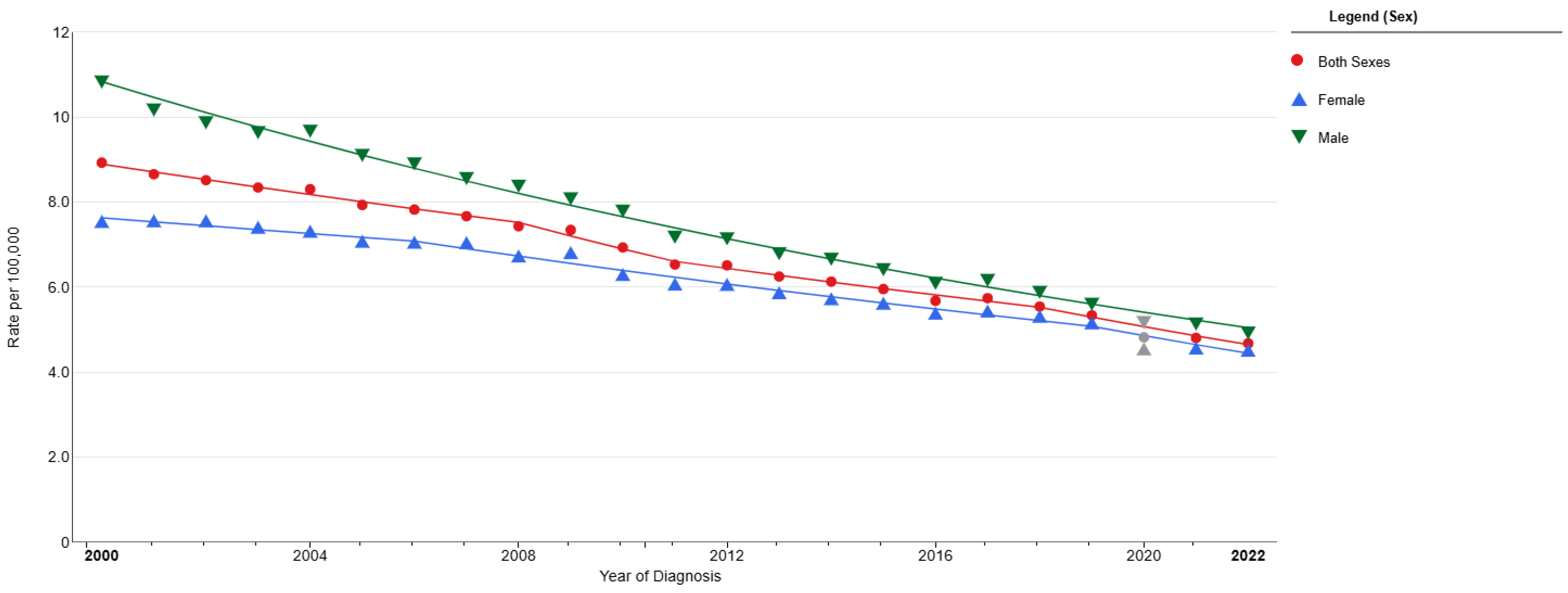
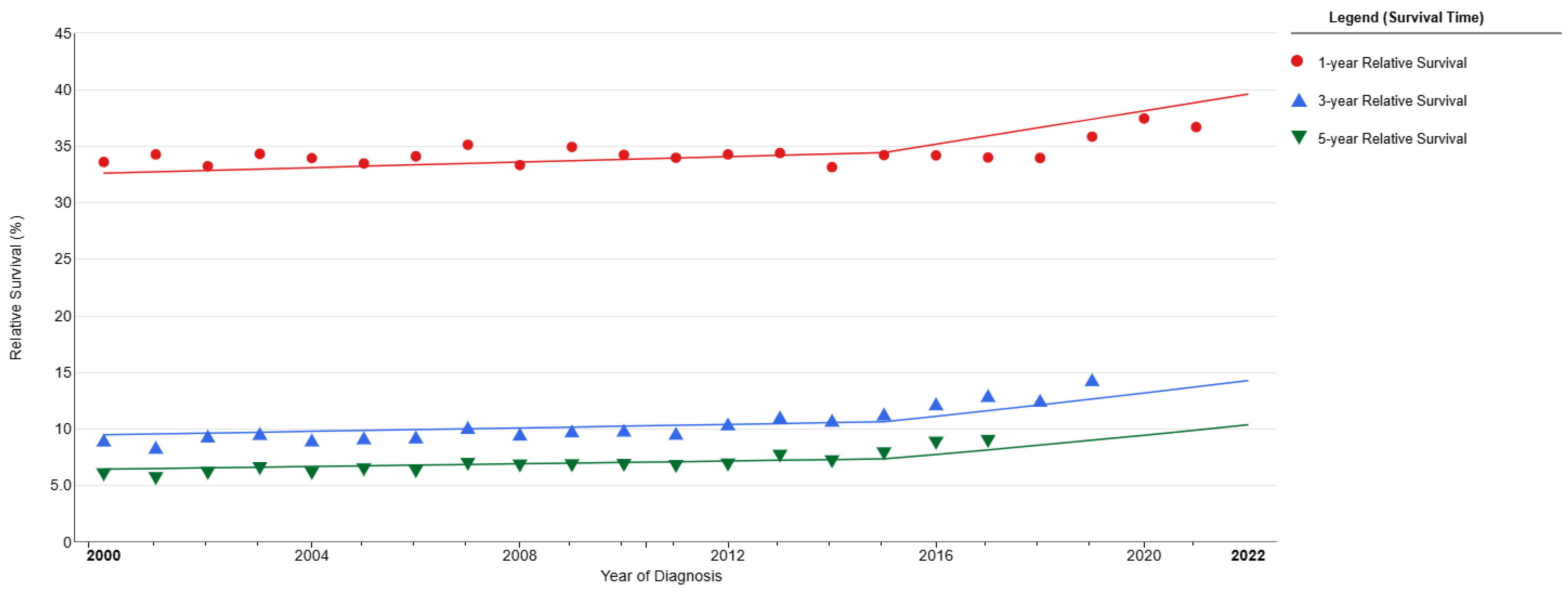
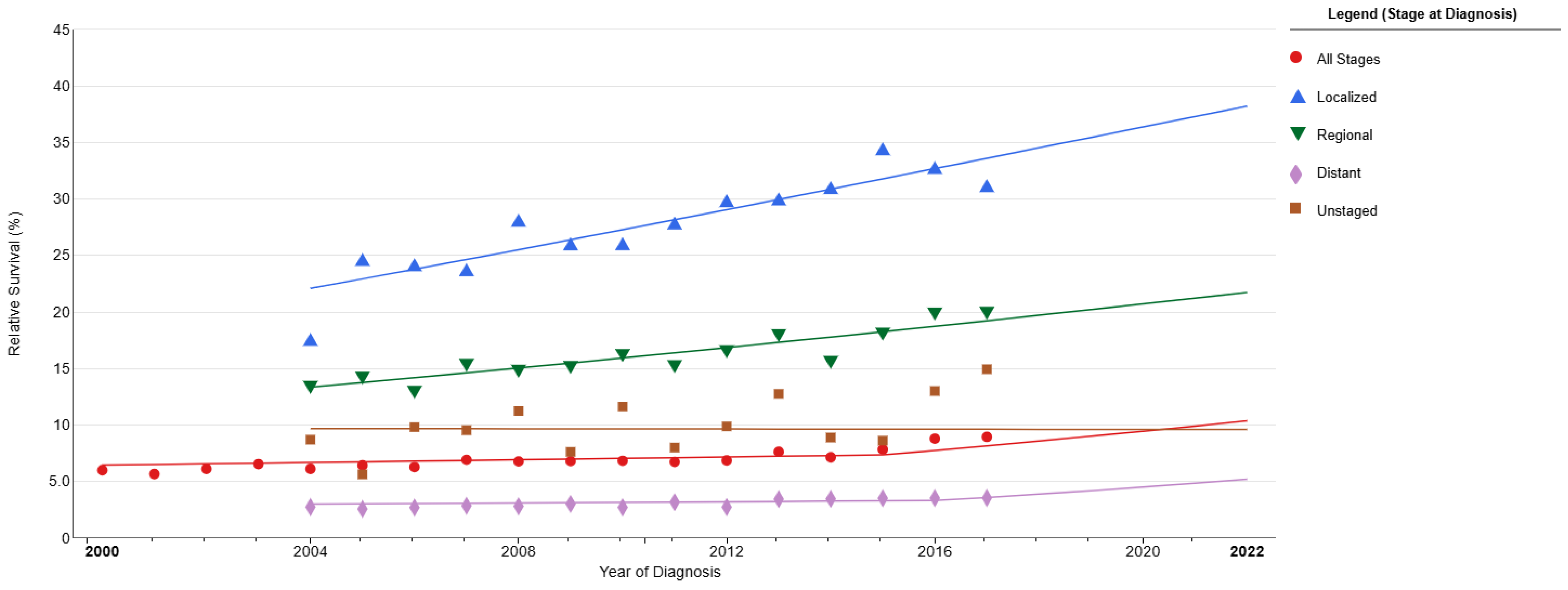
3. Small-Cell Lung Cancer Prognosis and Survival
4. The Role of Surgery in Treatment and the Impact of Surgical Treatment on Survival and Prognosis
4.1. Results Related to Surgical Application
4.2. Surgical Survival-Recurrence Outcomes
4.3. Factors Affecting Prognosis During and After the Surgical Process
4.4. Limitations of Surgical Case Series
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalemkerian, G.P.; Schneider, B.J. Advances in Small Cell Lung Cancer. Hematol. Oncol. Clin. N. Am. 2017, 31, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinmuth, N.; Hoffmann, H. Kleinzelliges Lungenkarzinom [Small Cell Lung Cancer]. Zentralbl. Chir. 2018, 143, 103–116. (In German) [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, W.; Jiang, M.; Wu, L. Research Progress of Immunotherapy and Prognostic Markers in Small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2020, 23, 182–188. (In Chinese) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huber, R.M.; Tufman, A. Update on small cell lung cancer management. Breathe 2012, 8, 314–330. [Google Scholar] [CrossRef]
- Kalemkerian, G.P. Small Cell Lung Cancer. Semin. Respir. Crit. Care Med. 2016, 37, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.F.; Liu, S.V. Small Cell Lung Cancer: Advances in Diagnosis and Management. Semin. Respir. Crit. Care Med. 2020, 41, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Pandjarova, I.; Mercieca, D.; Gijtenbeek, R.G.P.; Pereira, J.O.; Fantin, A.; Castaldo, N.; Keramida, E.; Pannu, K.; Konsoulova, A.; Aujayeb, A. Small cell lung cancer and neuroendocrine tumours. Breathe 2024, 20, 240004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Cancer Institute. Small Cell Lung Cancer Treatment (PDQ®)-Health Professional Version. Available online: https://www.cancer.gov/types/lung/hp/small-cell-lung-treatment-pdq (accessed on 1 September 2025).
- van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, E.B.; Jalal, S.I. Small Cell Lung Cancer. Cancer Treat. Res. 2016, 170, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Savaş, I. Small Cell Lung Cancer. Turk. Klin. J. Thor. Surg-Spec. Top. 2017, 8, 302–306. [Google Scholar]
- Basumallik, N.; Agarwal, M. Small Cell Lung Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Chen, Y.; Yao, L.; Chen, Q.; Hu, Y.; Zhu, X.; Dai, R.; Chen, X.; Zeng, Y.; Zhu, Y.; Song, D.; et al. A retrospective study on the impact of radiotherapy on the survival outcomes of small cell lung cancer patients based on the SEER database. Sci. Rep. 2024, 14, 15552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, H.S.; Lim, J.U.; Yeo, C.D.; Park, C.K.; Lee, S.H.; Kim, S.J.; Korean Association for Lung Cancer, Korea Central Cancer Registry. Characteristics and clinical outcomes of patients with nonsmoking small cell lung cancer in Korea. BMC Pulm. Med. 2022, 22, 200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, J.; Fu, C.; Li, B. Clinical outcomes of extensive-stage small cell lung cancer patients treated with thoracic radiotherapy at different times and fractionations. Radiat. Oncol. 2021, 16, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tartarone, A.; Giordano, P.; Lerose, R.; Rodriquenz, M.G.; Conca, R.; Aieta, M. Progress and challenges in the treatment of small cell lung cancer. Med. Oncol. 2017, 34, 110. [Google Scholar] [CrossRef] [PubMed]
- Kahnert, K.; Kauffmann-Guerrero, D.; Huber, R.M. SCLC-State of the Art and What Does the Future Have in Store? Clin. Lung Cancer 2016, 17, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Nilssen, Y.; Brustugun, O.T.; Fjellbirkeland, L.; Grønberg, B.H.; Haram, P.M.; Helbekkmo, N.; Helland, Å.; Wahl, S.G.F.; Aanerud, M.; Solberg, S. Small Cell Lung Cancer in Norway: Patterns of Care by Health Region and Survival Trends. Clin. Lung Cancer 2024, 25, e221–e228.e3. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program (SEER). Available online: https://seer.cancer.gov/index.html (accessed on 1 September 2025).
- Guney, P.A.; Irmak, I.; Kasapoglu, U.S.; Arinc, S. Neutrophil-to-lymphocyte Ratio as a Predictor of Prognosis in Patients with Small Cell Lung Cancer: A Retrospective Study. South. Clin. Ist. Euras 2021, 32, 260–267. [Google Scholar] [CrossRef]
- Nadir, A.; Kaptanoğlu, M. Prognosıs of Small Cell Lung Cancer. Turk. Klin. J. Surg. Med. Sci. 2006, 2, 44–46. [Google Scholar]
- Käsmann, L.; Bolm, L.; Janssen, S.; Rades, D. Prognostic Factors and Treatment of Early-stage Small-cell Lung Cancer. Anticancer. Res. 2017, 37, 1535–1537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shields, M.D.; Chiang, A.C.; Byers, L.A. Top advances of the year: Small cell lung cancer. Cancer 2025, 131, e35770. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, S.; Perry, M.C. History of small-cell lung cancer. Clin. Lung Cancer 2011, 12, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Megyesfalvi, Z.; Gay, C.M.; Popper, H.; Pirker, R.; Ostoros, G.; Heeke, S.; Lang, C.; Hoetzenecker, K.; Schwendenwein, A.; Boettiger, K.; et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J. Clin. 2023, 73, 620–652. [Google Scholar] [CrossRef] [PubMed]
- Matera, R.; Chiang, A. What Is New in Small Cell Lung Cancer. Hematol. Oncol. Clin. N. Am. 2023, 37, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Ernani, V.; Ganti, A.K. Surgery for limited-stage small cell lung cancer: Ready for prime-time? J. Thorac. Dis. 2017, 9, 3576–3578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Saxena, A.; Giaccone, G. Advancements in small cell lung cancer. Semin. Cancer Biol. 2023, 93, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Guan, X.; Bao, G.; Yao, Y.; Zhong, X. Molecular subtyping of small cell lung cancer. Semin. Cancer Biol. 2022, 86 Pt 2, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H. Accurate treatment of small cell lung cancer: Current progress, new challenges and expectations. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188798. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, H.C.; Ergen, Ş.A.; Tiken, E.E. Limited stage small cell lung cancer: Treatment results and prognostic factors. Türk Onkol. Derg. 2015, 30, 188–194. [Google Scholar] [CrossRef]
- Bogart, J.A.; Waqar, S.N.; Mix, M.D. Radiation and Systemic Therapy for Limited-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 661–670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Jiang, R.; Garces, Y.I.; Jatoi, A.; Stoddard, S.M.; Sun, Z.; Marks, R.S.; Liu, Y.; Yang, P. Prognostic factors for limited-stage small cell lung cancer: A study of 284 patients. Lung Cancer 2010, 67, 221–226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Y.; Yang, L.; Liu, L.; Wei, J.; Teng, F.; Zhang, J.; Zhu, Y.; Xing, P.; Li, J. Comparative study of clinicopathological characteristicsand prognosis between combined and pure small cell lung cancer (SCLC) aftersurgical resection. Thorac. Cancer 2020, 11, 2782–2792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, K.; Wang, Y.; Qi, J.; Zhao, L.; Wang, P. Analysis ofPrognostic Factors and Clinical Characteristics for Patients with Limited StageSmall Cell Lung Cancer with Pleural Effusion. Zhongguo Fei Ai Za Zhi 2018, 21, 16–23, In Chinese. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winston, W.T.; Magaffor, I. Small CellLung Cancer (SCLC). Medscape. 2024. Available online: https://emedicine.medscape.com/article/280104-overview?form=fpf (accessed on 1 September 2025).
- Oruç, A.F.; Karabulut Gul, S.; Tepetam, H. Küçükhücreli akciğer kanserinde tedavi sonuçları ve etki eden prognostik faktörler. CBU-SBED Haziran 2022, 9, 251–255. [Google Scholar] [CrossRef]
- Hamilton, G.; Hochmair, M.J.; Stickler, S. Overcoming resistance in small-cell lung cancer. Expert. Rev. Respir. Med. 2024, 18, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Society. Survival Statistics for Small Cell Lung Cancer. 2020. Available online: https://cancer.ca/en/cancer-information/cancer-types/lung/statistics (accessed on 1 September 2025).
- Kim, S.Y.; Park, H.S.; Chiang, A.C. Small Cell Lung Cancer: A Review. JAMA 2025, 333, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Chan, D.Y.; Speicher, P.J.; Gulack, B.C.; Wang, X.; Hartwig, M.G.; Onaitis, M.W.; Tong, B.C.; D’Amico, T.A.; Berry, M.F.; et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 1057–1064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugisaka, J.; Fujimoto, D.; Tamiya, M.; Hata, A.; Matsumoto, H.; Yokoyama, T.; Taniguchi, Y.; Uchida, J.; Sato, Y.; Kijima, T.; et al. Long-term outcome of chemoimmunotherapy for extensive-stage small-cell lung cancer according to key clinical trial eligibility: 3-year outcomes from a prospective cohort study. Lung Cancer 2025, 199, 108056. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.J.; Leonetti, A.; Airò, G.; Tiseo, M.; Rolfo, C.; Giovannetti, E.; Vahabi, M. Small cell lung cancer: Novel treatments beyond immunotherapy. Semin. Cancer Biol. 2022, 86 Pt 2, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Ersöz Köse, E. Evaluation of Surgical Treatment Results and Survival in Lung Cancer. In Akciğer Kanseri ve Cerrahi Tedavisi, 1st ed.; Atinkaya Baytemir, C., Eren, T.S.̧., Eds.; Türkiye Klinikleri: Ankara, Turkey, 2025; pp. 52–55. [Google Scholar]
- Al Zreibi, C.; Gibault, L.; Fabre, E.; LePimpec-Barthes, F. Chirurgie du cancer pulmonaire à petites cellules [Surgeryfor small-cell lung cancer]. Rev. Mal. Respir. 2021, 38, 840–847. (In French) [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, C.; Gao, J.; Wang, J.; Fu, F.; Wang, Y.; Wang, Q.; Zhang, M.; Zhang, S.; Fan, F.; et al. Integrative spatial analysis reveals tumor heterogeneity and immune colony niche related to clinical outcomes in small cell lung cancer. Cancer Cell 2025, 43, 519–536.e5. [Google Scholar] [CrossRef]
- Vrána, D. Advances in the therapy of small cell lung cancer. Klin. Onkol. 2021, 34 (Suppl. 1), 66–70. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Giffin, M.J.; Bailis, J.M.; Smit, M.D.; Carbone, D.P.; He, K. DLL3: An emerging target in small cell lung cancer. J. Hematol. Oncol. 2019, 12, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, J.; Shen, L.; Ren, Z.; Liu, Y.; Liang, C. Long-term results of postoperative unsuspected small cell lung cancer on real-world data. BMC Cancer 2022, 22, 1256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.; Dong, Y.; Zhou, Y.; Chen, G.; Hong, X.; Zhang, Q. Peripheral Tumor Location Predicts a Favorable Prognosis in Patients with Resected Small Cell Lung Cancer. Int. J. Clin. Pract. 2022, 2022, 4183326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dingemans, A.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, Z.; Li, D.; Deng, C.; Zhang, J.; Bai, J.; Li, Y.; Chen, H.; Zhang, Y. Excellent survival of pathological N0 small cell lung cancer patients following surgery. Eur. J. Med. Res. 2023, 28, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, J.T.; Shen, L.L.; Liang, C.Y.; Liu, X.; Zhang, T.; Ma, Y.F.; Liu, Y. Survival analysis of unexpected small cell lung cancer followingsurgery. Zhonghua Zhong Liu Za Zhi 2022, 44, 550–554. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Chan, D.Y.; Shah, S.A.; Yerokun, B.A.; Wang, X.F.; D’Amico, T.A.; Berry, M.F.; Harpole, D.H., Jr. Long-term Survival After Surgery Compared With Concurrent Chemoradiation for Node-negative Small Cell Lung Cancer. Ann. Surg. 2018, 268, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Bott, M.; Park, B.J.; Vallières, E.; Wilshire, C.L.; Yasufuku, K.; Spicer, J.D.; Jones, D.R.; Sepesi, B.; Small Cell Lung Cancer Working Group. Predictors of survival following surgical resection of limited-stage small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2021, 161, 760–771.e2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, L.; Shen, L.; Wang, K.; Lu, S. Propensity score matched analysis for the role of surgery in stage III small cell lung cancer based on the eighth edition of the TNM classification: A population study of the US SEER database and a Chinese hospital. Lung Cancer 2021, 162, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Motas, N.; Manolache, V.; Scarci, M.; Nimigean, V.; Nimigean, V.R.; Simion, L.; Mizea, M.C.; Trifanescu, O.G.; Galateanu, B.; Gherghe, M.; et al. Salvage Surgery for Small-Cell Lung Cancer-A Literature Review. Cancers 2023, 15, 2241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wakeam, E.; Acuna, S.A.; Leighl, N.B.; Giuliani, M.E.; Finlayson, S.R.G.; Varghese, T.K.; Darling, G.E. Surgery Versus Chemotherapy and Radiotherapy For Early and Locally Advanced Small Cell Lung Cancer: APropensity-Matched Analysis of Survival. Lung Cancer 2017, 109, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kallakury, B.; Chahine, J.J.; Hartmann, D.; Zhang, Y.; Chen, Y.; Zhang, H.; Zhang, B.; Wang, C.; Giaccone, G. Surgical Resection of SCLC: Prognostic Factors and the Tumor Microenvironment. J. Thorac. Oncol. 2019, 14, 914–923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balduyck, B.; Hendriks, J.; Sardari Nia, P.; Lauwers, P.; Van Schil, P. Quality of life after lung cancer surgery: A review. Minerva Chir. 2009, 64, 655–663. [Google Scholar] [PubMed]
- Li, W.W.; Lee, T.W.; Yim, A.P. Quality of life after lung cancer resection. Thorac. Surg. Clin. 2004, 14, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Jiang, C.; Zhang, H.; Fan, P.; Yu, H.; Zhang, H.; Fei, K.; Zhang, P. The number of lymph nodes examined is associated with survival outcomes and nodal upstaging in patients with stage I small cell lung cancer. Surg. Oncol. 2021, 37, 101513. [Google Scholar] [CrossRef] [PubMed]
- Rucker, A.J.; Raman, V.; Jawitz, O.K.; Voigt, S.L.; Tong, B.C.; D’Amico, T.A.; Harpole, D.H. Effect of Lymph Node Assessment on Outcomes in Surgery for Limited Stage Small Cell Lung Cancer. Ann. Thorac. Surg. 2020, 110, 1854–1860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stinchcombe, T.E. Current Treatments for Surgically Resectable, Limited-Stage, and Extensive-Stage Small Cell Lung Cancer. Oncologist 2017, 22, 1510–1517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barnes, H.; See, K.; Barnett, S.; Manser, R. Surgery for limited-stage small-cell lung cancer. Cochrane Database Syst. Rev. 2017, 4, CD011917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chai, Y.; Ma, Y.; Feng, W.; Lu, H.; Jin, L. Effect of surgery on survival in patients with stage III N2 small cell lung cancer: Propensity score matching analysis and nomogram development and validation. World J. Surg. Oncol. 2021, 19, 258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, Q.; Li, J.; Tan, F.; Sun, N.; Mao, Y.; Gao, Y.; Xue, Q.; Gao, S.; Zhao, J.; He, J. Development and Validation of a Nomogram Prognostic Model for Resected Limited-Stage Small Cell Lung Cancer Patients. Ann. Surg. Oncol. 2021, 28, 4893–4904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Pang, Z.; Chen, X.; Yan, T.; Liu, J.; Du, J. Development and validation of a prognostic model of resectable small-cell lung cancer: A large population-based cohort study and external validation. J. Transl. Med. 2020, 18, 237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| n | Stage | Surgical Method | Survival | Surgical Prognosis: Poor or Good | |
|---|---|---|---|---|---|
| Guo et al. (2020) [35] | 297 | Limited 286 (96.3%) Extend 11 (3.7%) | Lobectomy 236 (79.5%) Pneumonectomy 20 (6.7%) Sublobar 19 (6.4%) | OS 5 years: 63.8% DFS 5 years: 51.8% | (−) sublobar resection (+) Lobectomy |
| Fu et al. (2023) [53] | 196 | Stage 1—71 (36.22%) Stage 2—45 (22.96%) Stage 3—58 (29.59%) Unknown—19 (9.69%) | Lobectomy 144 (73.5%) Pneumonectomy 10 (5.1%) Sleeve 3 (1.54%) Bilobectomy 20 (10.2%) Wedge 9 (4.5%) | OS 5 years: 49% | (−) Smoking, advanced age, T-N advanced stage |
| Guo et al. (2022) [54] | 120 | Stage 1—39 (32.5%) Stage 2—37 (30.83%) Stage 3—42 (35%) Stage 4—2 (1.67%) | Lobectomy: 87 (71.3%) Pneumonectomy 5 (4.17%) Bilobectomy 6 (5%) Sublobar 9 (11.67%) | OS 5 years: 46% DFS 5 years: 30.63% | (−) Advanced age, advanced T-N-M stages, sublobar resection, pneumonectomy, vascular thrombus |
| Yang et al. (2018) [55] | 681 | T1 410 (60.2%) T2 195 (28.6%) T3 13 (1.9%) T4 12 (1.8%) N0 461 (79.4%) N1 90 (15.5%) N2 29 (5.0%) N3 < 10 | Lobectomy 458 (67.3%) Pneumonectomy 23 (3.4%) Segmentectomy: 22 (3.2%) Wedge: 178 (26.1%) | OS 5 years: 48.1% | |
| Zhou et al. (2021) [56] | 164 | Stage 1—82 (50%) Stage 2—43 (26.2 2%) Stage 3—39(23.78%) | Lobectomy 101 (61.59%) > Lobectomy 17 (10.3 7%) Sublobar 46 (28.05%) | OS (month) 26 (1986–1989) 37 (1990–1999) 60 (2000–2010) 59 (2010–2019) | (−) Coronary artery disease, nodal disease (+) Lobectomy, adjuvant chemotherapy |
| Gao et al. (2021) [57] | 418 | Stage 3 | Lobectomy: 224 (53.59%) Pneumonectomy 31 (7.41%) Sublobar: 147 (35.17%) Unknown: 16 (3.83%) | OS 5 years: 20.90% (19 months) Lobectomy: 28.8% Pneumonectomy 8.7% Sublobar 12.5% Unknown 13.5% | (−) Advanced age, male gender, T-N advanced stage, sublobar resection, pneumonectomy (+) lobectomy, chemotherapy, radiotherapy |
| Yang et al. (2016) [42] | 954 | Lobectomy: 666 (69.8%) Pneumonectomy 18 (1.9%) Segmentectomy: 26 (2.7%) Other 45 (4.7%) Wedge: 199 (20.9%) | OS: 5 years 47.4% (55.6 months) | (−) Advanced age, tumor size (+) Lobectomy | |
| Motas et al. * (2023) [58] | 17 | Stage 1—11 (64.71%) Stage 2—2 (11.76%) Stage 3—3 (17.65%) | Lobectomy: 10 (58.82%) Pneumonectom 2 (11.76%) Segmentectomy 1 (5.8 8%) Bilobectomy: 2 (11.76%) Wedge: 2 (11.76%) | OS: 86 months 2 years 92%, 3 years 80%, 5 years 66% | |
| Wakeam et al. (2017) [59] | 2089 | Stage 1—1310 (60.27%) Stage 2—335 (16.37%) Stage 3—401 (19.60%) | Lobectomy: 741 (35.5%) Pneumonectomy: 87 (4.2%) Sublobar: 1261 (60.4%) | OS: Stage 1—38.6 months, Stage 2—23.4 months, Stage 3a—21.7 months | (−) Lymph node metastasis (+) Lobectomy, R0 surgery, chemotherapy-radiotherapy |
| Zhao et al. (2019) [60] | 205 | Stage 1—79 (38.5%) Stage 2—42 (20.5%) Stage 3—61 (29.8%) Stage 4—9 (4.4%) NA 14 (6.8 %) | Lobectomy: 151 (73.7%) Pneumonectomy: 20 (9.8%) Wedge: 34 (16.6%) | OS: 69 months. 1-3-5 years survival rates: 84.8%-60%-51.1%. 5-year survival: Stage 1—63.8%, Stage 2—65.5%, Stage 3—34.9%, Stage 4—0%. | (−) smoking, lymph node metastasis, PD-L1 positivity (+) R0 resection, T and B cell tumour |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sezer, H.F. Current Surgical Perspective on the Prognosis of Small-Cell Lung Cancer. Diagnostics 2025, 15, 2704. https://doi.org/10.3390/diagnostics15212704
Sezer HF. Current Surgical Perspective on the Prognosis of Small-Cell Lung Cancer. Diagnostics. 2025; 15(21):2704. https://doi.org/10.3390/diagnostics15212704
Chicago/Turabian StyleSezer, Hüseyin Fatih. 2025. "Current Surgical Perspective on the Prognosis of Small-Cell Lung Cancer" Diagnostics 15, no. 21: 2704. https://doi.org/10.3390/diagnostics15212704
APA StyleSezer, H. F. (2025). Current Surgical Perspective on the Prognosis of Small-Cell Lung Cancer. Diagnostics, 15(21), 2704. https://doi.org/10.3390/diagnostics15212704





