Spatial Distribution of Senescent Cells and Their Proximity to Immune Subsets in the Human Endometrium During the Implantation Window
Abstract
1. Introduction
2. Materials and Methods
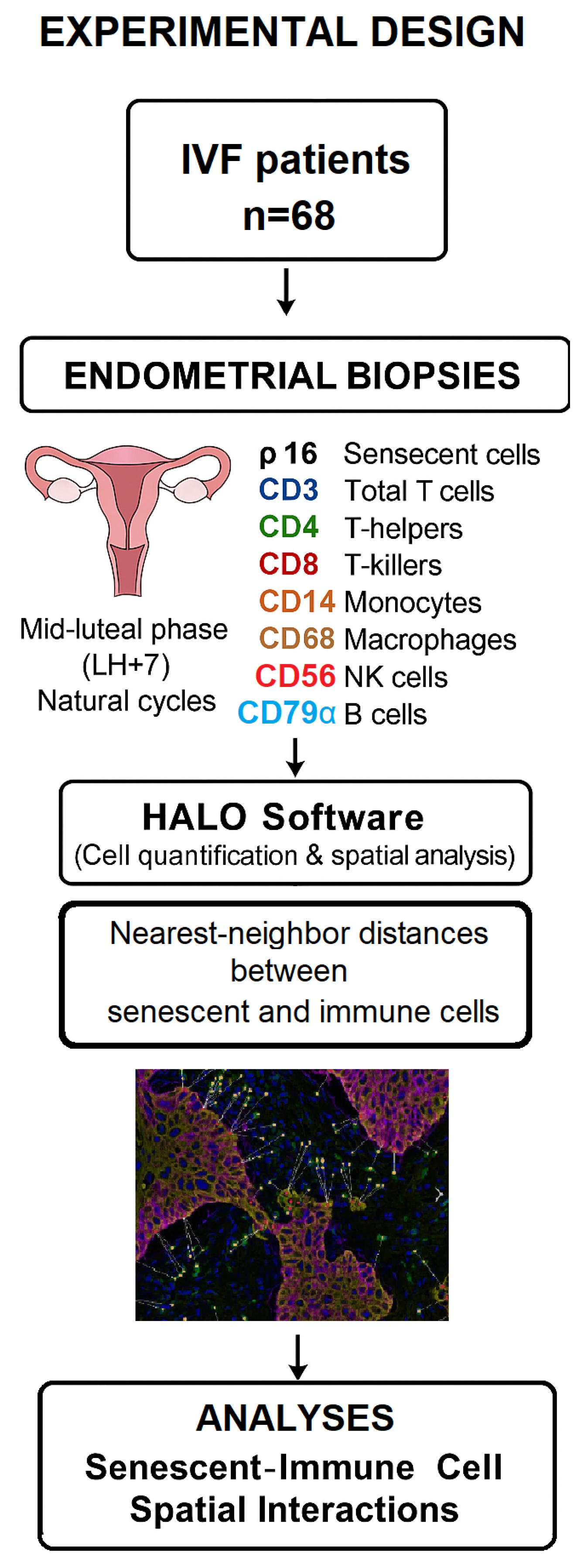
2.1. Study Design and Participants
2.2. Tissue Collection and Processing
2.3. Immunohistochemistry
2.4. Imaging and Digital Spatial Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Abundance of Senescent and Immune Cells in the Endometrium
3.3. Ratios of Senescent to Immune Cells
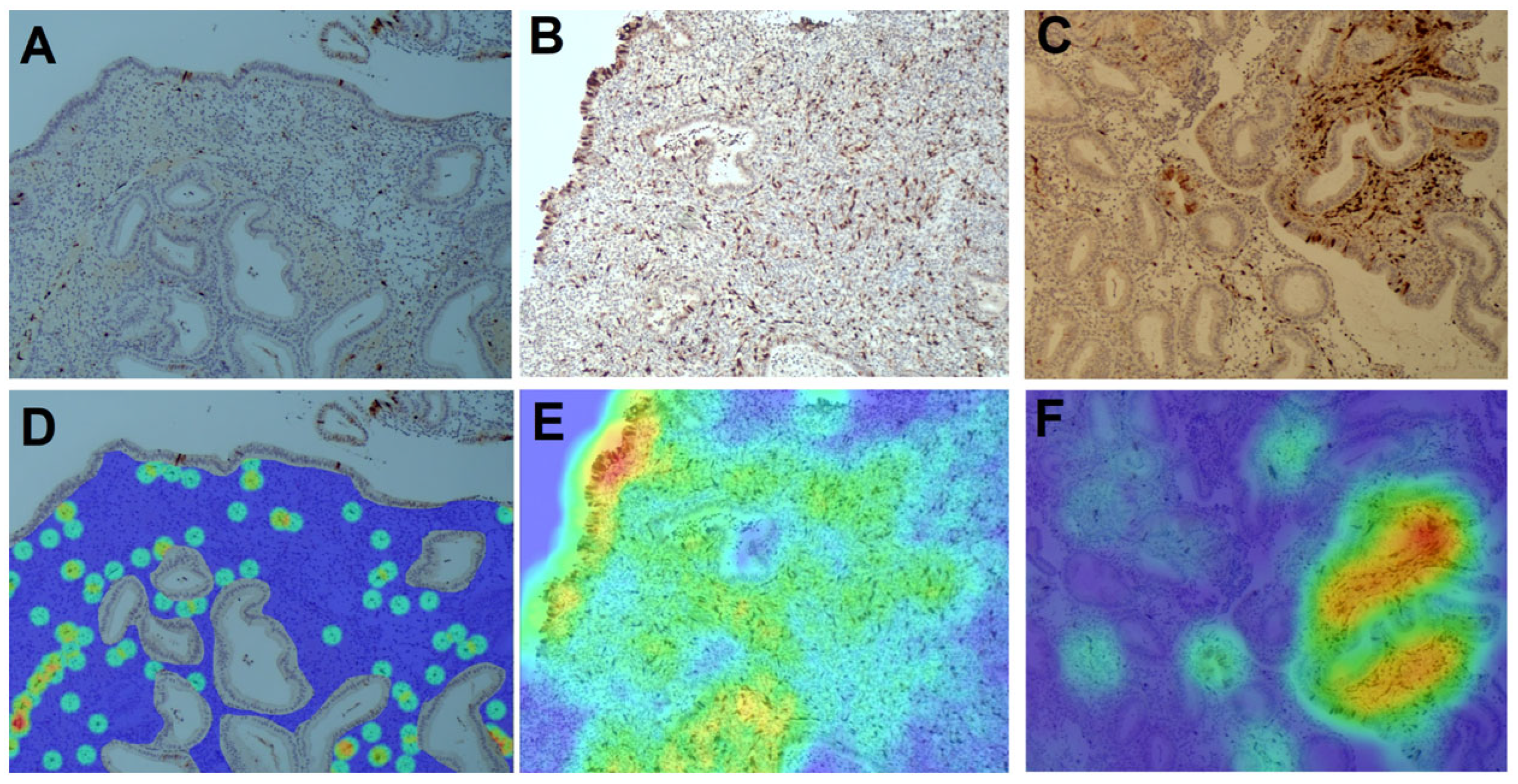
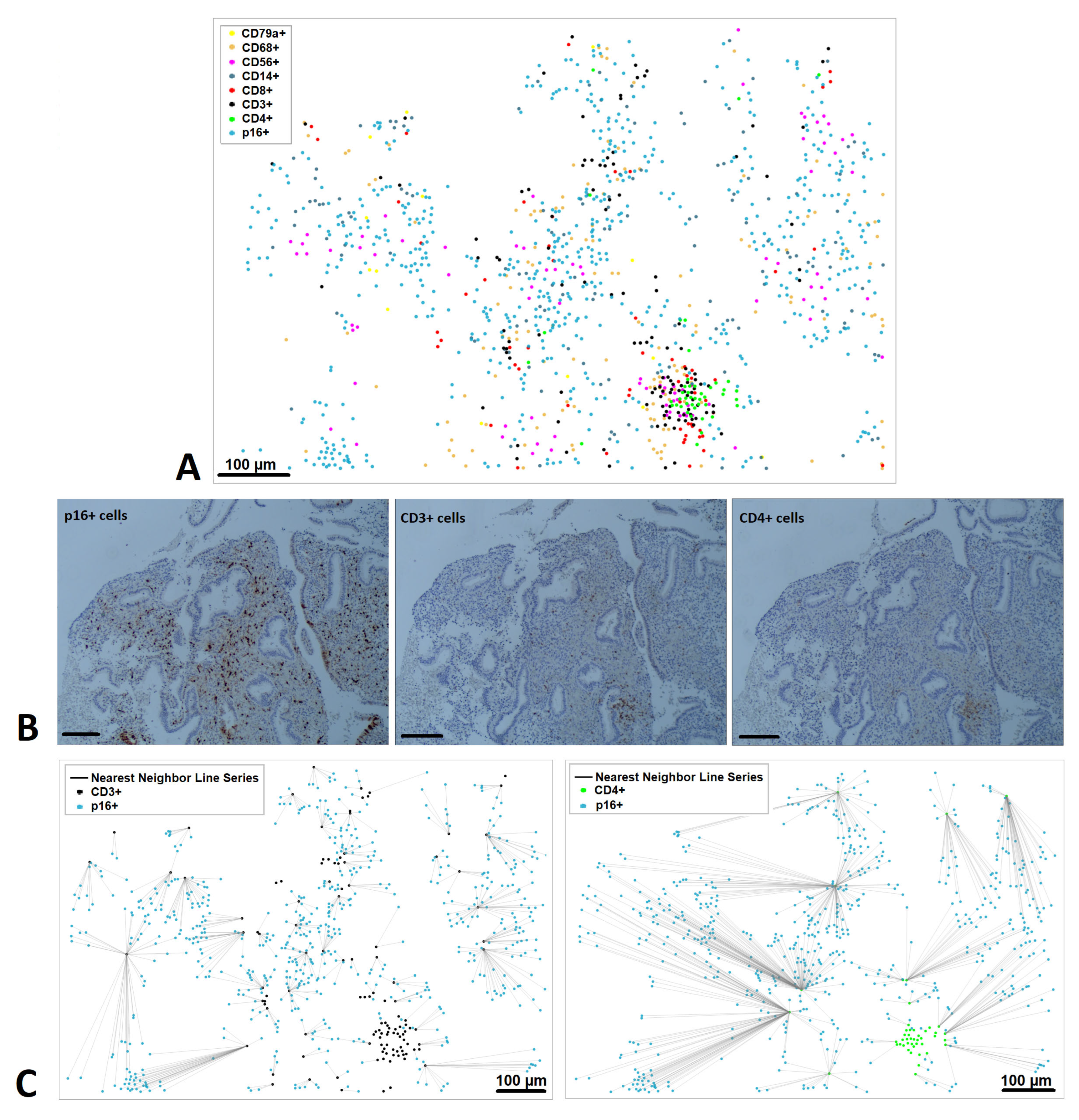
3.4. Distribution of Senescent (p16+) Cells in the Endometrium
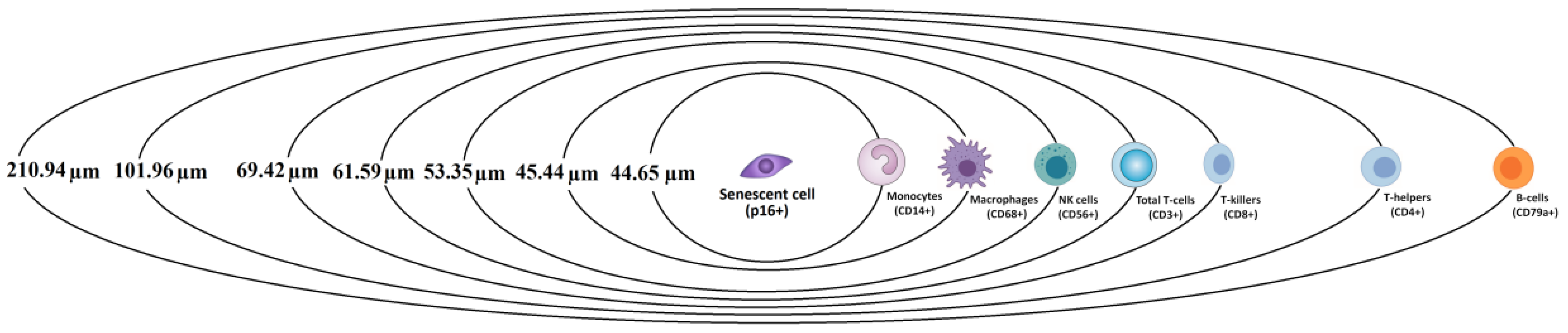
3.5. Spatial Relationships Between Senescent and Immune Cells
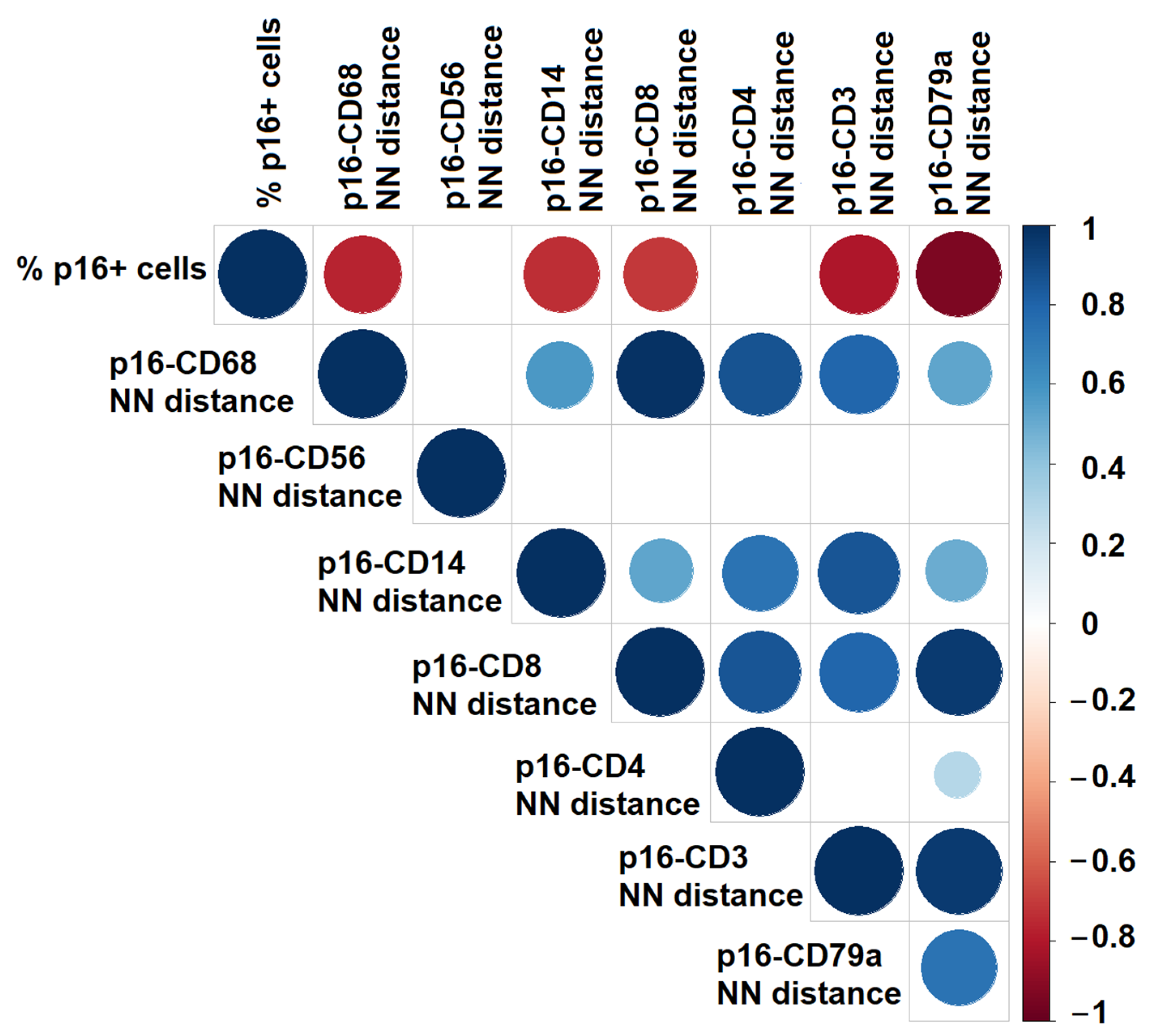
3.6. Correlation Between Senescent Cell Density and Spatial Distances
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 2017, 6, e31274. [Google Scholar] [CrossRef][Green Version]
- Kang, T.W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-da-Costa, M.M.; Brighton, P.J.; Kong, C.S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef]
- Parvanov, D.; Ganeva, R.; Arsov, K.; Decheva, I.; Ruseva, M.; Kalinov, K.; Vidolova, N.; Scarpellini, F.; Metodiev, D.; Stamenov, G. Association between endometrial senescent cells and immune cells in women with repeated implantation failure. J. Assist. Reprod. Genet. 2023, 40, 1631–1638. [Google Scholar] [CrossRef]
- Keren, L.; Bosse, M.; Marquez, D.; Angoshtari, R.; Jain, S.; Varma, S.; Yang, S.-R.; Kurian, A.; Van Valen, D.; West, R.; et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 2018, 174, 1373–1387.e19. [Google Scholar] [CrossRef]
- Marx, V. Method of the Year: Spatially resolved transcriptomics. Nat. Methods 2021, 18, 9–14. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Q.; Rajachandran, S.; Grow, E.J.; Evans, M.; Chen, H. Dissecting mammalian reproduction with spatial transcriptomics. Hum. Reprod. Update 2023, 29, 794–810. [Google Scholar] [CrossRef]
- Wang, X.; Hawkins, S.M. Using advanced spatial and single-cell transcriptomics to characterize the human endometrium. Nat. Genet. 2021, 53, 1628–1630. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.-S.; Vomstein, K.; Reiser, E.; Tollinger, S.; Kyvelidou, C.; Feil, K.; Toth, B. NK and T cell subtypes in the endometrium of patients with recurrent pregnancy loss and recurrent implantation failure: Implications for pregnancy success. J. Clin. Med. 2023, 12, 5585. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, R.; Parvanov, D.; Vidolova, N.; Ruseva, M.; Handzhiyska, M.; Arsov, K.; Decheva, I.; Metodiev, D.; Moskova-Doumanova, V.; Stamenov, G. Endometrial immune cell ratios and implantation success in patients with recurrent implantation failure. J. Reprod. Immunol. 2023, 156, 103816. [Google Scholar] [CrossRef]
- Parvanov, D.; Ganeva, R.; Vidolova, N.; Stamenov, G. Decreased number of p16-positive senescent cells in human endometrium as a marker of miscarriage. J. Assist. Reprod. Genet. 2021, 38, 2087–2095. [Google Scholar] [CrossRef]
- Gerner, M.Y.; Kastenmüller, W.; Ifrim, I.; Kabat, J.; Germain, R.N. Histo-cytometry: A method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 2012, 37, 364–376. [Google Scholar] [CrossRef]
- Schapiro, D.; Jackson, H.W.; Raghuraman, S.; Fischer, J.R.; Zanotelli, V.R.T.; Schulz, D.; Giesen, C.; Catena, R.; Varga, Z.; Bodenmiller, B. histoCAT: Analysis of cell phenotypes and interactions in multiplex image cytometry data. Nat. Methods 2017, 14, 873–876. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Chemerinski, A.; Garcia de Paredes, M.; Blackledge, R.; Douglas, N.; Morelli, S. Mechanisms of endometrial aging: Lessons from natural conceptions and ART cycles. Front. Physiol. 2024, 15, 1332946. [Google Scholar] [CrossRef]
- Li, B.; Qi, J.; Cao, Y.; Long, Y.; Wei, Z.; Wang, W.-S.; Hu, S.; Wang, Y.; Zhu, Q.; Hu, X.; et al. From invaginating site to deep lesion: Spatial transcriptomics unravels ectopic endometrial penetration features in adenomyosis. Adv. Sci. 2024, 11, 24011752. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Gu, Z.; Wu, J.; Jia, S.; Shi, J.; Dai, Y.; Wu, Y.; Yan, H.; Zhang, J.; et al. Single-cell and spatial transcriptomic profiling revealed niche interactions sustaining growth of endometriotic lesions. Cell Genom. 2025, 1, 100737. [Google Scholar] [CrossRef]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- Ruscetti, M.; Morris, J.P.; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.-J.; Fennell, M.; et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell 2020, 181, 424–441.e21. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Zhang, X.-X.; Zhao, T.-T.; Wu, X.-H. Using proteomics and single-cell sequencing to analyze the pathogenesis of recurrent implantation failure associated with uterine natural killer cells. Arch. Gynecol. Obstet. 2025, 312, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ji, M.; Fu, X.; Zhu, L.; Duan, T.; Ni, W. Age-matched analysis of endometrial immune profile between frozen embryo transfer success and recurrent implantation failure cases with predictive model. J. Reprod. Immunol. 2025, 170, 104539. [Google Scholar] [CrossRef]
- Cai, J.Y.; Tang, Y.Y.; Deng, X.H.; Li, Y.J.; Liang, G.; Meng, Y.Q.; Zhou, H. Recurrent implantation failure may be identified by a combined biomarker panel. Front. Endocrinol. 2022, 13, 865807. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Garifallou, J.; Kim, S.-J.; Simoni, M.K.; Huh, D.D.; Gordon, S.M.; Mainigi, M. Uterine macrophages and NK cells exhibit population and gene-level changes after implantation but maintain pro-invasive properties. Front. Immunol. 2024, 15, 1364036. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Y.; Cheng, Y.; Wang, J.; Zhang, B.; Zhai, Y.; Zhu, K.; Liu, Y.; Shang, Y.; Xiao, X.; et al. Time-series single-cell transcriptomic profiling of luteal-phase endometrium uncovers dynamic characteristics and its dysregulation in recurrent implantation failures. Nat. Commun. 2025, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Marečková, M.; Garcia-Alonso, L.; Moullet, M.; Lorenzi, V.; Petryszak, R.; Sancho-Serra, C.; Oszlanczi, A.; Icoresi Mazzeo, C.; Wong, F.C.K.; Kelava, I.; et al. An integrated single-cell reference atlas of the human endometrium. Nat. Genet. 2024, 56, 1925–1937. [Google Scholar] [CrossRef]
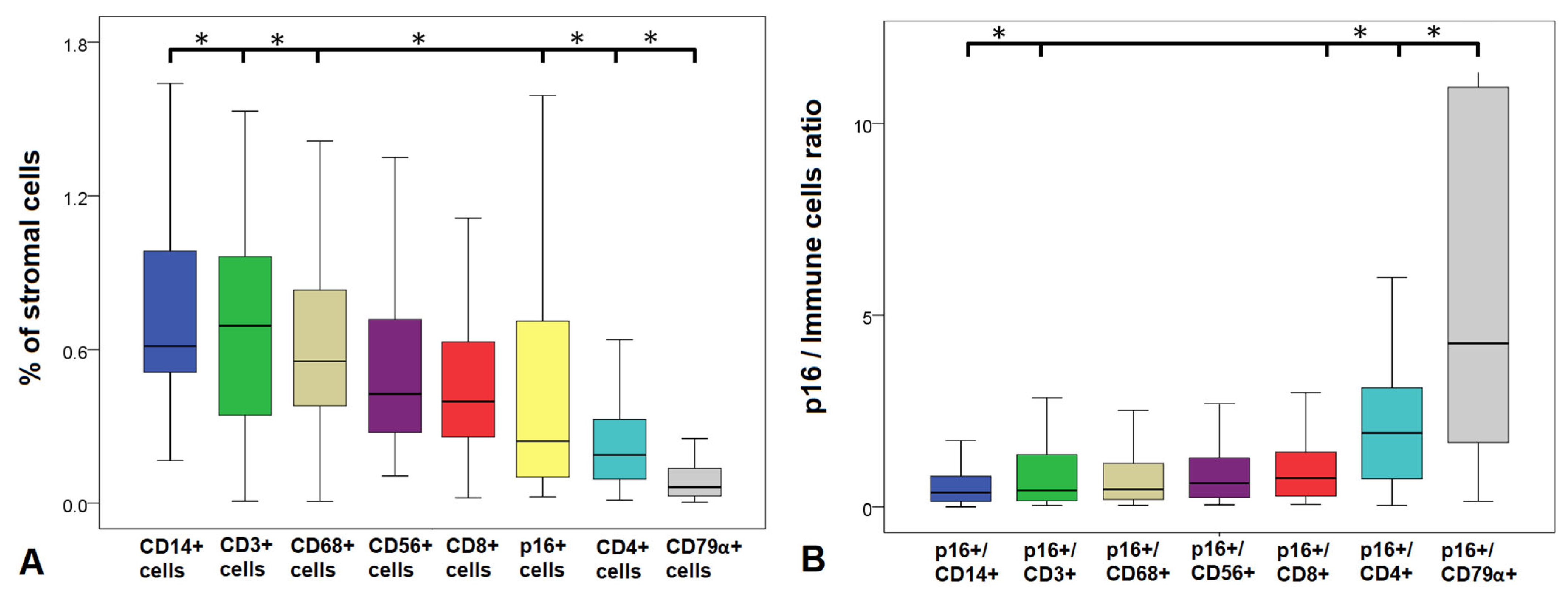
| Cell Population | Median (Min–Max) | Senescent/Immune Cell Ratios | Median (Min–Max) |
|---|---|---|---|
| p16+ cells (Senescent cells), % | 0.29 (0–12.65) | ||
| CD3+ cells (T-cells), % | 0.65 (0.01–20.88) | p16+/CD3+ | 0.49 (0–100.00) |
| CD4+ cells (T-helpers), % | 0.19 (0.00–4.05) | p16+/CD4+ | 1.93 (0.04–25.51) |
| CD8+ cells (T-killers), % | 0.38 (0.02–9.85) | p16+/CD8+ | 0.81 (0–5.98) |
| CD56+ cells (NK cells), % | 0.44 (0.11–13.15) | p16+/CD14+ | 0.39 (0–8.56) |
| CD14+ cells (Monocytes), % | 0.69 (0.04–17.14) | p16+/CD68+ | 0.52 (0–118.43) |
| CD68+ cells (Macrophages), % | 0.56 (0.01–14.44) | p16+/CD56+ | 0.72 (0–11.00) |
| CD79α+ cells (B-cells), % | 0.06 (0.01–5.57) | p16+/CD79α+ | 4.74 (0–119.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvanov, D.; Ganeva, R.; Ruseva, M.; Handzhiyska, M.; Safir, J.; Jelezarsky, L.; Vidolova, N.; Metodiev, D.; Stamenov, G.; Hadjidekova, S. Spatial Distribution of Senescent Cells and Their Proximity to Immune Subsets in the Human Endometrium During the Implantation Window. Diagnostics 2025, 15, 2679. https://doi.org/10.3390/diagnostics15212679
Parvanov D, Ganeva R, Ruseva M, Handzhiyska M, Safir J, Jelezarsky L, Vidolova N, Metodiev D, Stamenov G, Hadjidekova S. Spatial Distribution of Senescent Cells and Their Proximity to Immune Subsets in the Human Endometrium During the Implantation Window. Diagnostics. 2025; 15(21):2679. https://doi.org/10.3390/diagnostics15212679
Chicago/Turabian StyleParvanov, Dimitar, Rumiana Ganeva, Margarita Ruseva, Maria Handzhiyska, Jinahn Safir, Lachezar Jelezarsky, Nina Vidolova, Dimitar Metodiev, Georgi Stamenov, and Savina Hadjidekova. 2025. "Spatial Distribution of Senescent Cells and Their Proximity to Immune Subsets in the Human Endometrium During the Implantation Window" Diagnostics 15, no. 21: 2679. https://doi.org/10.3390/diagnostics15212679
APA StyleParvanov, D., Ganeva, R., Ruseva, M., Handzhiyska, M., Safir, J., Jelezarsky, L., Vidolova, N., Metodiev, D., Stamenov, G., & Hadjidekova, S. (2025). Spatial Distribution of Senescent Cells and Their Proximity to Immune Subsets in the Human Endometrium During the Implantation Window. Diagnostics, 15(21), 2679. https://doi.org/10.3390/diagnostics15212679







