Venous Thromboembolism in Inherited Platelet Disorders: A Clinical Challenge
Abstract
1. Introduction
2. Background on Thrombotic and Bleeding Risks in VTE Management
3. Role of Platelets in VTE
4. VTE in IPD Patients
5. Pro-Hemostatic Agents Used in IPD and Their Thrombotic Risk
5.1. Platelet Concentrates
5.2. Recombinant Activated FVII
5.3. Desmopressin
5.4. Antifibrinolytic Agents
5.5. Thrombopoietin Receptor Agonists
5.6. Red Blood Cell Transfusions
6. VTE in IPD Patients: Prevention and Management
7. Use of Anticoagulants in IPD: Balancing Risks and Benefits
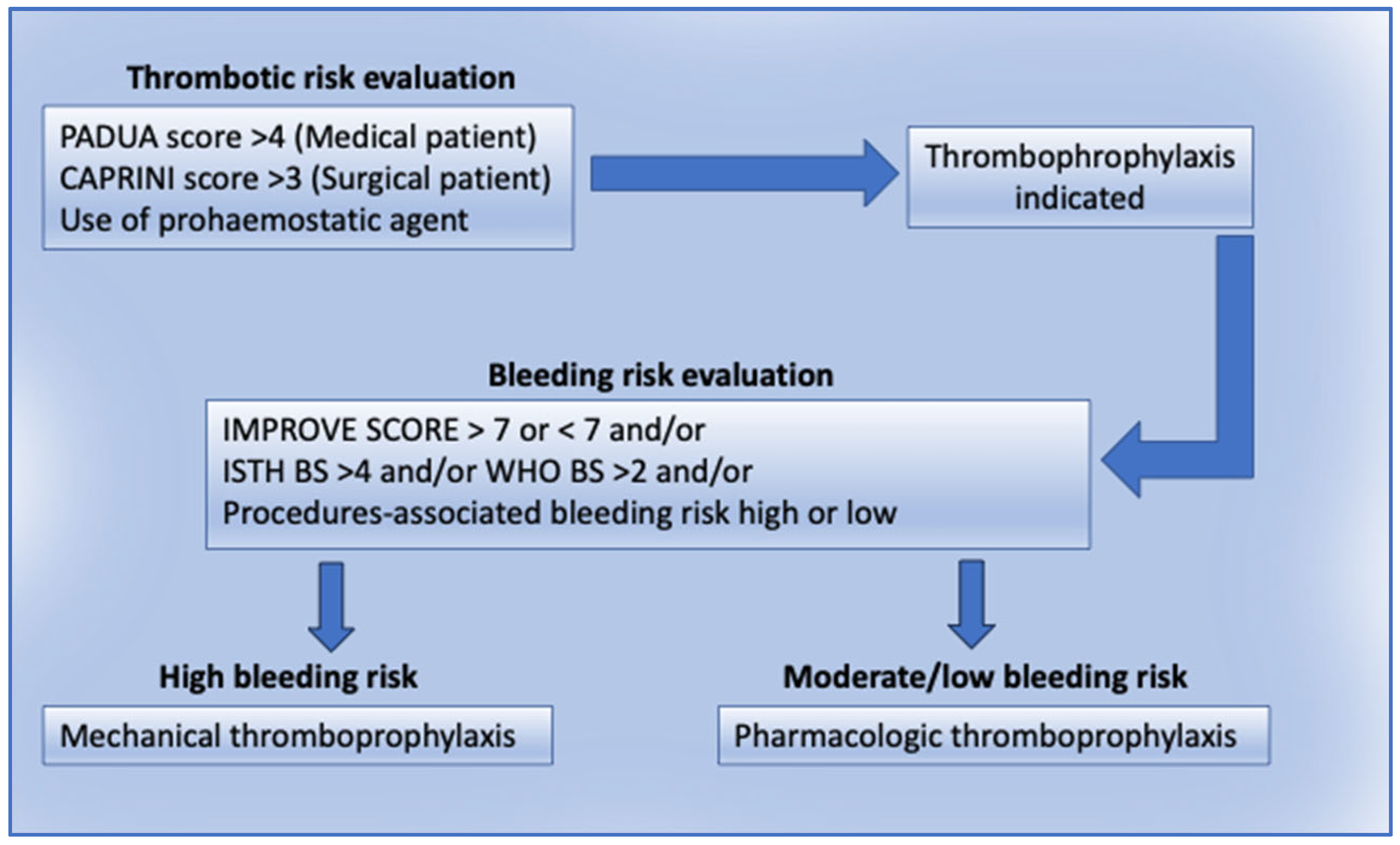
7.1. Thromboprophylaxis in IPD Patients
7.2. Anticoagulation in IPD Patients
8. Use of Antithrombotic Therapy in Patients with Other Inherited or Acquired Bleeding Disorders
9. How We Approach Thromboembolism in IPD Patients
Thromboprophylaxis to Prevent VTE
10. Future Perspectives and Unmet Needs
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACCP | American College of Chest Physician |
| LMWH | Low molecular weight heparin |
| WHO | World health organization |
| IPD | Inherited platelet disorders |
| IVC | Inferior vena cava |
| VTE | Venous thromboembolism |
| ISTH | International society of thrombosis and haemostasis |
| DOAC | Direct oral anticoagulant |
References
- Gresele, P.; Falcinelli, E.; Bury, L. Inherited platelet function disorders. Diagnostic approach and management. Hamostaseologie 2016, 36, 265–278. [Google Scholar]
- Gresele, P.; Orsini, S.; Noris, P.; Falcinelli, E.; Alessi, M.C.; Bury, L.; Borhany, M.; Santoro, C.; Glembotsky, A.C.; Cid, A.R.; et al. Validation of the ISTH/SSC bleeding assessment tool for inherited platelet disorders: A communication from the Platelet Physiology SSC. J. Thromb. Haemost. 2020, 18, 732–739. [Google Scholar] [CrossRef]
- Gresele, P.; Harrison, P.; Gachet, C.; Hayward, C.; Kenny, D.; Mezzano, D.; Mumford, A.D.; Nugent, D.; Nurden, A.T.; Cattaneo, M.; et al. Diagnosis of inherited platelet function disorders: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 314–322. [Google Scholar] [CrossRef]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, H.T.; Pedersen, L.; van Es, N.; Buller, H.R.; Horvath-Puho, E. Impact of venous thromboembolism on the mortality in patients with cancer: A population-based cohort study. Lancet Reg. Health Eur. 2023, 34, 100739. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Ainle, F.N.; Kevane, B. Which patients are at high risk of recurrent venous thromboembolism (deep vein thrombosis and pulmonary embolism)? Blood Adv. 2020, 4, 5595–5606. [Google Scholar] [CrossRef]
- Linnemann, B.; Hart, C. Laboratory Diagnostics in Thrombophilia. Hamostaseologie 2019, 39, 49–61. [Google Scholar] [CrossRef]
- Geerts, W.H.; Bergqvist, D.; Pineo, G.F.; Heit, J.A.; Samama, C.M.; Lassen, M.R.; Colwell, C.W. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133, 381S–453S. [Google Scholar] [CrossRef]
- Bozzato, S.; Galli, L.; Ageno, W. Thromboprophylaxis in surgical and medical patients. Semin. Respir. Crit. Care Med. 2012, 33, 163–175. [Google Scholar] [CrossRef]
- Cohen, A.T.; Tapson, V.F.; Bergmann, J.F.; Goldhaber, S.Z.; Kakkar, A.K.; Deslandes, B.; Huang, W.; Zayaruzny, M.; Emery, L.; Anderson, F.A., Jr.; et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet 2008, 371, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Garcia, D.A.; Wren, S.M.; Karanicolas, P.J.; Arcelus, J.I.; Heit, J.A.; Samama, C.M. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e227S–e277S. [Google Scholar] [CrossRef] [PubMed]
- Selby, R.; Borah, B.J.; McDonald, H.P.; Henk, H.J.; Crowther, M.; Wells, P.S. Impact of thromboprophylaxis guidelines on clinical outcomes following total hip and total knee replacement. Thromb. Res. 2012, 130, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Arcelus, J.I.; Villar, J.M.; Munoz, N. Should we follow the 9th ACCP guidelines for VTE prevention in surgical patients? Thromb. Res. 2012, 130 (Suppl S1), S4–S6. [Google Scholar] [CrossRef]
- Glise Sandblad, K.; Rosengren, A.; Schulman, S.; Roupe, M.; Sandstrom, T.Z.; Philipson, J.; Svennerholm, K.; Tavoly, M. Excess risk of bleeding in patients with venous thromboembolism on direct oral anticoagulants during initial and extended treatment versus population controls. J. Intern. Med. 2025, 297, 382–399. [Google Scholar] [CrossRef]
- Coyle, M.; Lynch, A.; Higgins, M.; Costello, M.; Judge, C.; O’Donnell, M.; Reddin, C. Risk of Intracranial Hemorrhage Associated With Direct Oral Anticoagulation vs Antiplatelet Therapy: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2449017. [Google Scholar] [CrossRef]
- Douketis, J.D.; Spyropoulos, A.C.; Murad, M.H.; Arcelus, J.I.; Dager, W.E.; Dunn, A.S.; Fargo, R.A.; Levy, J.H.; Samama, C.M.; Shah, S.H.; et al. Perioperative Management of Antithrombotic Therapy: An American College of Chest Physicians Clinical Practice Guideline. Chest 2022, 162, e207–e243. [Google Scholar] [CrossRef]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef]
- Muller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.A.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.H.; Renne, T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 2009, 139, 1143–1156. [Google Scholar] [CrossRef]
- Roberts, H.R.; Hoffman, M.; Monroe, D.M. A cell-based model of thrombin generation. Semin. Thromb. Hemost. 2006, 32 (Suppl. S1), 32–38. [Google Scholar] [CrossRef]
- Herault, J.P.; Perrin, B.; Jongbloet, C.; Pflieger, A.M.; Bernat, A.; Herbert, J.M. Effect of factor Xa inhibitors on the platelet-derived microparticles procoagulant activity in vitro and in vivo in rats. Thromb. Haemost. 2000, 84, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, E.M.; Kruglik, S.G.; Swamy, S.; Latysheva, N.; Osterud, B.; Guigner, J.M.; Sureau, F.; Bonneau, S.; Kuzmin, A.N.; Prasad, P.N.; et al. Extracellular vesicles from activated platelets possess a phospholipid-rich biomolecular profile and enhance prothrombinase activity. J. Thromb. Haemost. 2024, 22, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Apta, B.H.R.; Bonna, A.M.; Harper, M.T. Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci. Rep. 2019, 9, 13397. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef]
- Joglekar, M.V.; Ware, J.; Xu, J.; Fitzgerald, M.E.; Gartner, T.K. Platelets, glycoprotein Ib-IX, and von Willebrand factor are required for FeCl(3)-induced occlusive thrombus formation in the inferior vena cava of mice. Platelets 2013, 24, 205–212. [Google Scholar] [CrossRef][Green Version]
- Lisman, T.; Adelmeijer, J.; Heijnen, H.F.; de Groot, P.G. Recombinant factor VIIa restores aggregation of alphaIIbbeta3-deficient platelets via tissue factor-independent fibrin generation. Blood 2004, 103, 1720–1727. [Google Scholar] [CrossRef]
- Girolami, A.; Sambado, L.; Bonamigo, E.; Vettore, S.; Lombardi, A.M. Occurrence of thrombosis in congenital thrombocytopenic disorders: A critical annotation of the literature. Blood Coagul. Fibrinolysis 2013, 24, 18–22. [Google Scholar] [CrossRef]
- Seretny, M.; Senadheera, N.; Miller, E.; Keeling, D. Pulmonary embolus in Glanzmann’s thrombasthenia treated with warfarin. Haemophilia 2008, 14, 1138–1139. [Google Scholar] [CrossRef]
- Nurden, A.T. Should studies on Glanzmann thrombasthenia not be telling us more about cardiovascular disease and other major illnesses? Blood Rev. 2017, 31, 287–299. [Google Scholar] [CrossRef]
- Paciullo, F.; Bury, L.; Noris, P.; Falcinelli, E.; Melazzini, F.; Orsini, S.; Zaninetti, C.; Abdul-Kadir, R.; Obeng-Tuudah, D.; Heller, P.G.; et al. Antithrombotic prophylaxis for surgery-associated venous thromboembolism risk in patients with inherited platelet disorders. The SPATA-DVT Study. Haematologica 2020, 105, 1948–1956. [Google Scholar] [CrossRef]
- Dupuis, A.; Gachet, C. Inherited platelet disorders: Management of the bleeding risk. Transfus. Clin. Biol. 2018, 25, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Orsini, S.; Noris, P.; Bury, L.; Heller, P.G.; Santoro, C.; Kadir, R.A.; Butta, N.C.; Falcinelli, E.; Cid, A.R.; Fabris, F.; et al. Bleeding risk of surgery and its prevention in patients with inherited platelet disorders. Haematologica 2017, 102, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Blumberg, N.; Culakova, E.; Refaai, M.A.; Lyman, G.H. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch. Intern. Med. 2008, 168, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Bakal, M.; Ambriz, E.; Ortiz-Pivaral, L.; Kogut, K.; Rood, C.S.; Rauch, S.; Eskenazi, B.; Deardorff, J. Impacts of COVID-19 shelter in place across key life domains among immigrant farmworker Latina mothers and young adults. BMC Public Health 2024, 24, 2036. [Google Scholar] [CrossRef]
- Franchini, M. The use of recombinant activated factor VII in platelet disorders: A critical review of the literature. Blood Transfus. 2009, 7, 24–28. [Google Scholar]
- Rajpurkar, M.; Croteau, S.E.; Boggio, L.; Cooper, D.L. Thrombotic events with recombinant activated factor VII (rFVIIa) in approved indications are rare and associated with older age, cardiovascular disease, and concomitant use of activated prothrombin complex concentrates (aPCC). J. Blood Med. 2019, 10, 335–340. [Google Scholar] [CrossRef]
- Gresele, P.; Bury, L.; Falcinelli, E. Inherited Platelet Function Disorders: Algorithms for Phenotypic and Genetic Investigation. Semin. Thromb. Hemost. 2016, 42, 292–305. [Google Scholar] [CrossRef]
- Bargehr, C.; Knofler, R.; Streif, W. Treatment of Inherited Platelet Disorders: Current Status and Future Options. Hamostaseologie 2023, 43, 261–270. [Google Scholar] [CrossRef]
- Desborough, M.J.; Oakland, K.A.; Landoni, G.; Crivellari, M.; Doree, C.; Estcourt, L.J.; Stanworth, S.J. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: A systematic review and meta-analysis of randomized controlled trials. J. Thromb. Haemost. 2017, 15, 263–272. [Google Scholar] [CrossRef]
- Abad-Motos, A.; Garcia-Erce, J.A.; Gresele, P.; Paramo, J.A. Is tranexamic acid appropriate for all patients undergoing high-risk surgery? Curr. Opin. Crit. Care 2024, 30, 655–663. [Google Scholar]
- Ross, J.; Al-Shahi Salman, R. The frequency of thrombotic events among adults given antifibrinolytic drugs for spontaneous bleeding: Systematic review and meta-analysis of observational studies and randomized trials. Curr. Drug Saf. 2012, 7, 44–54. [Google Scholar] [CrossRef]
- Chornenki, N.L.J.; Um, K.J.; Mendoza, P.A.; Samienezhad, A.; Swarup, V.; Chai-Adisaksopha, C.; Siegal, D.M. Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: A systematic review and meta-analysis. Thromb. Res. 2019, 179, 81–86. [Google Scholar] [CrossRef]
- Pecci, A.; Gresele, P.; Klersy, C.; Savoia, A.; Noris, P.; Fierro, T.; Bozzi, V.; Mezzasoma, A.M.; Melazzini, F.; Balduini, C.L. Eltrombopag for the treatment of the inherited thrombocytopenia deriving from MYH9 mutations. Blood 2010, 116, 5832–5837. [Google Scholar] [CrossRef]
- Gabelli, M.; Marzollo, A.; Notarangelo, L.D.; Basso, G.; Putti, M.C. Eltrombopag use in a patient with Wiskott-Aldrich syndrome. Pediatr. Blood Cancer 2017, 64, e26692. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Kusunoki, Y.; Kida, T.; Oda, S.; Yamano, A.; Nakayama, F.; Fukuda, S.; Ikeda, N.; Namba-Hamano, T.; Homma, M.; et al. A Case of Acute Kidney Injury During Eltrombopag Use Successfully Treated With Plasma Exchange in Addition to Antithrombotic Therapy. Kidney Med. 2025, 7, 101007. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Kitamura, M.; Sugiyama, S.; Sawase, A.; Yamashita, H.; Tsushima, H.; Irie, J.; Katafuchi, E.; Nakayama, T.; Mukae, H.; et al. A Case of Eltrombopag-Induced Thrombotic Microangiopathy Initiating Hemodialysis. Cureus 2024, 16, e75947. [Google Scholar] [CrossRef]
- Sood, N.; Kushnir, M.; Jayavelu, B. Acute Renal Thrombotic Microangiopathy Caused by Eltrombopag and Romiplostim in a Patient with Myelodysplastic Syndromes (MDS) and Underlying Antiphospholipid Syndrome. Eur. J. Case Rep. Intern. Med. 2024, 11, 004564. [Google Scholar] [CrossRef]
- Garra, W.; Carmi, O.; Kivity, S.; Levy, Y. Catastrophic antiphospholipid syndrome in lupus-associated immune thrombocytopenia treated with eltrombopag A case series and literature review. Medicine 2023, 102, e32949. [Google Scholar] [CrossRef]
- Khoreva, A.; Abramova, I.; Deripapa, E.; Rodina, Y.; Roppelt, A.; Pershin, D.; Larin, S.; Voronin, K.; Maschan, A.; Novichkova, G.; et al. Efficacy of romiplostim in treatment of thrombocytopenia in children with Wiskott-Aldrich syndrome. Br. J. Haematol. 2021, 192, 366–374. [Google Scholar] [CrossRef]
- Zaninetti, C.; Gresele, P.; Bertomoro, A.; Klersy, C.; De Candia, E.; Veneri, D.; Barozzi, S.; Fierro, T.; Alberelli, M.A.; Musella, V.; et al. Eltrombopag for the treatment of inherited thrombocytopenias: A phase II clinical trial. Haematologica 2020, 105, 820–828. [Google Scholar] [CrossRef]
- Gerrits, A.J.; Leven, E.A.; Frelinger, A.L., 3rd; Brigstocke, S.L.; Berny-Lang, M.A.; Mitchell, W.B.; Revel-Vilk, S.; Tamary, H.; Carmichael, S.L.; Barnard, M.R.; et al. Effects of eltrombopag on platelet count and platelet activation in Wiskott-Aldrich syndrome/X-linked thrombocytopenia. Blood 2015, 126, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Garfinkle, M.; Lawler, P.R.; Filion, K.B.; Eisenberg, M.J. Red blood cell transfusion and mortality among patients hospitalized for acute coronary syndromes: A systematic review. Int. J. Cardiol. 2013, 164, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Selak, V.; Kerr, A.; Poppe, K.; Wu, B.; Harwood, M.; Grey, C.; Jackson, R.; Wells, S. Annual Risk of Major Bleeding Among Persons Without Cardiovascular Disease Not Receiving Antiplatelet Therapy. JAMA 2018, 319, 2507–2520. [Google Scholar] [CrossRef] [PubMed]
- Selak, V.; Jackson, R.; Poppe, K.; Wu, B.; Harwood, M.; Grey, C.; Pylypchuk, R.; Mehta, S.; Kerr, A.; Wells, S. Predicting Bleeding Risk to Guide Aspirin Use for the Primary Prevention of Cardiovascular Disease: A Cohort Study. Ann. Intern. Med. 2019, 170, 357–364. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Tosetto, A.; Abshire, T.; Arnold, D.M.; Coller, B.; James, P.; Neunert, C.; Lillicrap, D. ISTH/SSC bleeding assessment tool: A standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J. Thromb. Haemost. 2010, 8, 2063–2065. [Google Scholar] [CrossRef]
- Gresele, P.; Falcinelli, E.; Bury, L.; Pecci, A.; Alessi, M.C.; Borhany, M.; Heller, P.G.; Santoro, C.; Cid, A.R.; Orsini, S.; et al. The ISTH bleeding assessment tool as predictor of bleeding events in inherited platelet disorders: Communication from the ISTH SSC Subcommittee on Platelet Physiology. J. Thromb. Haemost. 2021, 19, 1364–1371. [Google Scholar] [CrossRef]
- Sandrock-Lang, K.; Wentzell, R.; Santoso, S.; Zieger, B. Inherited platelet disorders. Hamostaseologie 2016, 36, 178–186. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Ghiotto, L.; Pontalto, L.; Casini, A.; Castaman, G.; Abdul-Kadir, R.; Berntorp, E.; Bodo, I.; Degenaar-Dujardin, M.; Fijnvandraat, K.; et al. Mild or moderate hemophilia is not always a mild or moderate bleeding disorder: Back to the clinical phenotype. Hemasphere 2025, 9, e70111. [Google Scholar] [CrossRef]
- Ambaglio, C.; Zane, F.; Russo, M.C.; Preti, P.S.; Scudeller, L.; Klersy, C.; Gamba, G.; Squizzato, A. Preoperative bleeding risk assessment with ISTH-BAT and laboratory tests in patients undergoing elective surgery: A prospective cohort study. Haemophilia 2021, 27, 717–723. [Google Scholar] [CrossRef]
- Klok, F.A.; Hosel, V.; Clemens, A.; Yollo, W.D.; Tilke, C.; Schulman, S.; Lankeit, M.; Konstantinides, S.V. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur. Respir. J. 2016, 48, 1369–1376. [Google Scholar] [CrossRef]
- Lambert, C.; Maitland, H.; Ghanima, W. Risk-based and individualised management of bleeding and thrombotic events in adults with primary immune thrombocytopenia (ITP). Eur. J. Haematol. 2024, 112, 504–515. [Google Scholar] [CrossRef]
- Dennis, M.; Sandercock, P.; Graham, C.; Forbes, J.; Collaboration, C.T.; Smith, J. The Clots in Legs Or sTockings after Stroke (CLOTS) 3 trial: A randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol. Assess. 2015, 19, 1–90. [Google Scholar]
- Agu, O.; Hamilton, G.; Baker, D. Graduated compression stockings in the prevention of venous thromboembolism. Br. J. Surg. 1999, 86, 992–1004. [Google Scholar] [CrossRef]
- Urbankova, J.; Quiroz, R.; Kucher, N.; Goldhaber, S.Z. Intermittent pneumatic compression and deep vein thrombosis prevention. A meta-analysis in postoperative patients. Thromb. Haemost. 2005, 94, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Douketis, J.D.; Gianni, M.; Lim, W.; Crowther, M.A. Meta-analysis: Anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann. Intern. Med. 2007, 146, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.; Etxeandia-Ikobaltzeta, I.; Yang, S.; Germini, F.; Gupta, S.; Agarwal, A.; Ventresca, M.; Tang, S.; Morgano, G.P.; Wang, M.; et al. Benefits and harms of direct oral anticoagulation and low molecular weight heparin for thromboprophylaxis in patients undergoing non-cardiac surgery: Systematic review and network meta-analysis of randomised trials. BMJ 2022, 376, e066785. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Al-Hameed, F.; Burns, K.E.A.; Mehta, S.; Alsolamy, S.J.; Alshahrani, M.S.; Mandourah, Y.; Almekhlafi, G.A.; Almaani, M.; Al Bshabshe, A.; et al. Adjunctive Intermittent Pneumatic Compression for Venous Thromboprophylaxis. N. Engl. J. Med. 2019, 380, 1305–1315. [Google Scholar] [CrossRef]
- Swan, D.; Newland, A.; Rodeghiero, F.; Thachil, J. Thrombosis in immune thrombocytopenia-current status and future perspectives. Br. J. Haematol. 2021, 194, 822–834. [Google Scholar] [CrossRef]
- Windyga, J.; Lassila, R. Antithrombotic therapy in patients with inherited bleeding disorders: Practical considerations. Pol. Arch. Intern. Med. 2025, 135, 16993. [Google Scholar] [CrossRef]
- Falanga, A.; Leader, A.; Ambaglio, C.; Bagoly, Z.; Castaman, G.; Elalamy, I.; Lecumberri, R.; Niessner, A.; Pabinger, I.; Szmit, S.; et al. EHA Guidelines on Management of Antithrombotic Treatments in Thrombocytopenic Patients With Cancer. Hemasphere 2022, 6, e750. [Google Scholar] [CrossRef]
- Smith, S.B.; Geske, J.B.; Maguire, J.M.; Zane, N.A.; Carter, R.E.; Morgenthaler, T.I. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest 2010, 137, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lv, M.; Wu, S.; Jiang, S.; Xu, W.; Qian, J.; Chen, M.; Fang, Z.; Zeng, Z.; Zhang, J. Editor’s Choice—Severe Bleeding Risks of Direct Oral Anticoagulants in the Prevention and Treatment of Venous Thromboembolism: A Network Meta-Analysis of Randomised Controlled Trials. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Li, H.; Huang, W.; Zhao, L.; Guo, W. Comparative differences in the risk of major gastrointestinal bleeding among different direct oral anticoagulants: An updated traditional and Bayesian network meta-analysis. Front. Pharmacol. 2022, 13, 1049283. [Google Scholar] [CrossRef] [PubMed]
- Uminski, K.; Xu, Y.; Zahrai, A.; Hodgson, A.; Wang, T.F.; Duffett, L.; Tinmouth, A.; Khalife, R. Management strategies in persons with inherited haemophilia requiring antithrombotic therapy: A scoping review. Haemophilia 2024, 30, 16–50. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Bor, M.V. Management of Recurrent Venous Thromboembolism in Severe Immune Thrombocytopenia: A Case Report and a Review of the Literature. Hamostaseologie 2024, 44, 393–398. [Google Scholar] [CrossRef]
- Martin, K.; Key, N.S. How I treat patients with inherited bleeding disorders who need anticoagulant therapy. Blood 2016, 128, 178–184. [Google Scholar] [CrossRef]
- Uminski, K.; Wang, T.F.; Duffett, L.; Tinmouth, A.; Khalife, R. Management of Persons With Haemophilia Requiring Antithrombotic Therapy: A Canadian Modified Delphi Consensus Study. Haemophilia 2025, 31, 441–449. [Google Scholar] [CrossRef]
- Fabris, F.; Scandellari, R.; Vettore, S.; Scapin, M.; Bizzaro, N.; Randi, M.L. Intriguing outcome of JAK2V617F mutation seen in a patient with MYH9-related hereditary macrothrombocytopenia. Thromb. Haemost. 2010, 104, 858–859. [Google Scholar]
- Heller, P.G.; Pecci, A.; Glembotsky, A.C.; Savoia, A.; Negro, F.D.; Balduini, C.L.; Molinas, F.C. Unexplained recurrent venous thrombosis in a patient with MYH9-related disease. Platelets 2006, 17, 274–275. [Google Scholar] [CrossRef]
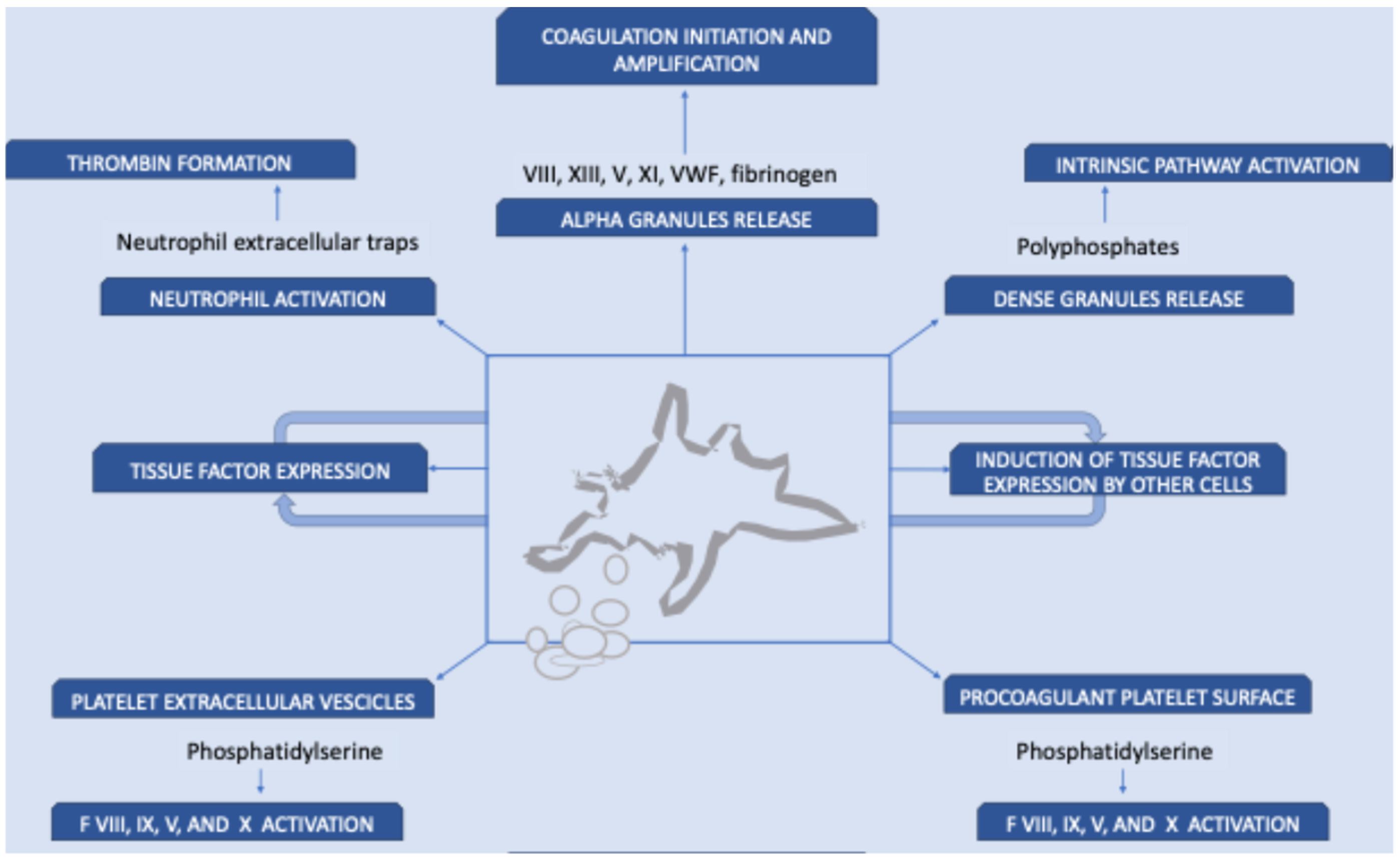
| Disorder | Genetic Defect/Main Feature | Bleeding Risk | VTE Cases Reported |
|---|---|---|---|
| Glanzmann Thrombasthenia (GT) | αIIbβ3 integrin deficiency | High | 10 cases [27,30] |
| Bernard-Soulier Syndrome | GPIb-IX-V complex deficiency | High | 1 case [30] |
| MYH9-Related Disorders | MYH9 gene variation | Moderate | 2 cases [27] |
| Mild (ISTH BAT BS 0–4) | Moderate (ISTH BAT BS 5–10) | Severe (ISTH BAT BS >11) |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paciullo, F.; Rovere-Querini, P.; Bury, L.; Falcinelli, E.; Gresele, P. Venous Thromboembolism in Inherited Platelet Disorders: A Clinical Challenge. Diagnostics 2025, 15, 2667. https://doi.org/10.3390/diagnostics15212667
Paciullo F, Rovere-Querini P, Bury L, Falcinelli E, Gresele P. Venous Thromboembolism in Inherited Platelet Disorders: A Clinical Challenge. Diagnostics. 2025; 15(21):2667. https://doi.org/10.3390/diagnostics15212667
Chicago/Turabian StylePaciullo, Francesco, Patrizia Rovere-Querini, Loredana Bury, Emanuela Falcinelli, and Paolo Gresele. 2025. "Venous Thromboembolism in Inherited Platelet Disorders: A Clinical Challenge" Diagnostics 15, no. 21: 2667. https://doi.org/10.3390/diagnostics15212667
APA StylePaciullo, F., Rovere-Querini, P., Bury, L., Falcinelli, E., & Gresele, P. (2025). Venous Thromboembolism in Inherited Platelet Disorders: A Clinical Challenge. Diagnostics, 15(21), 2667. https://doi.org/10.3390/diagnostics15212667







