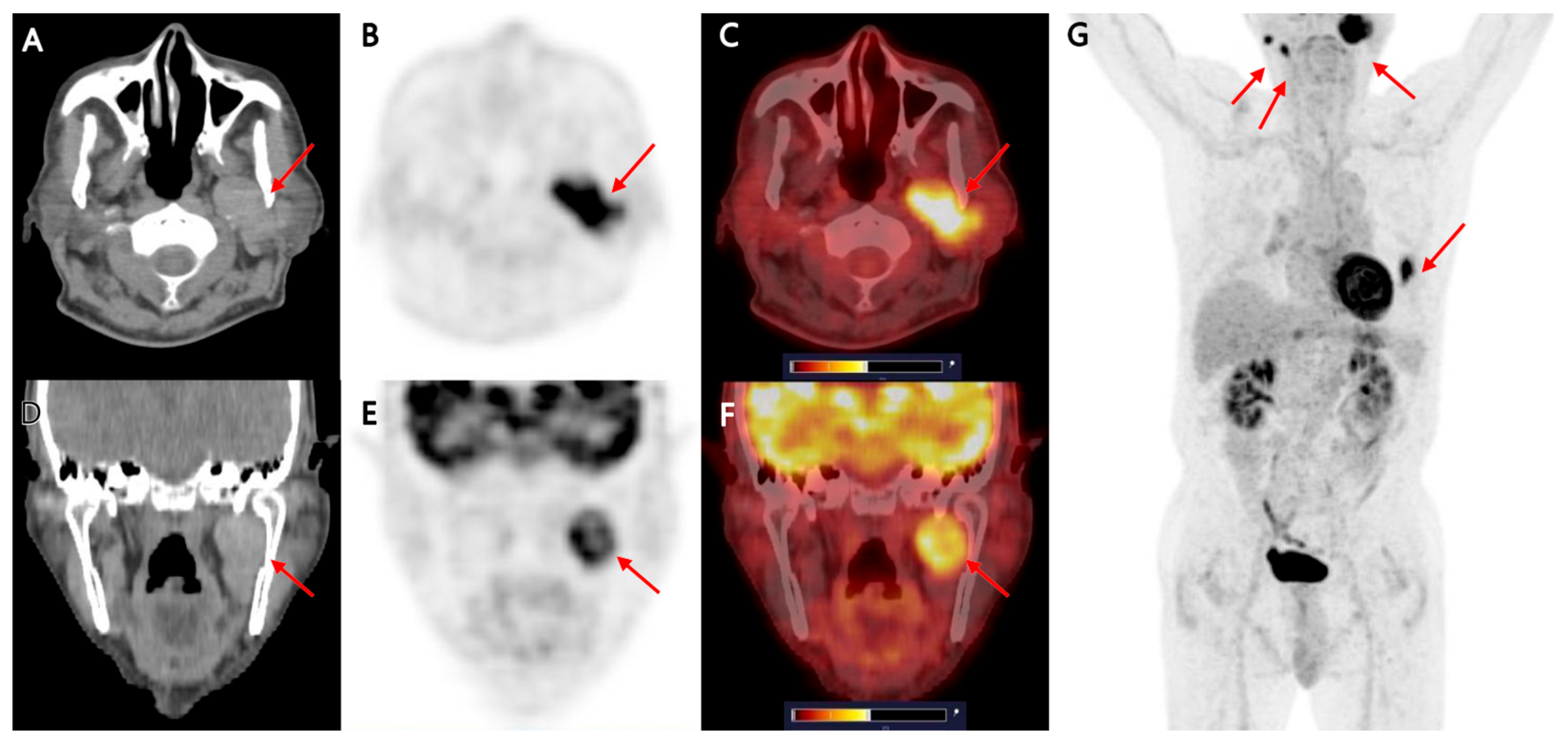
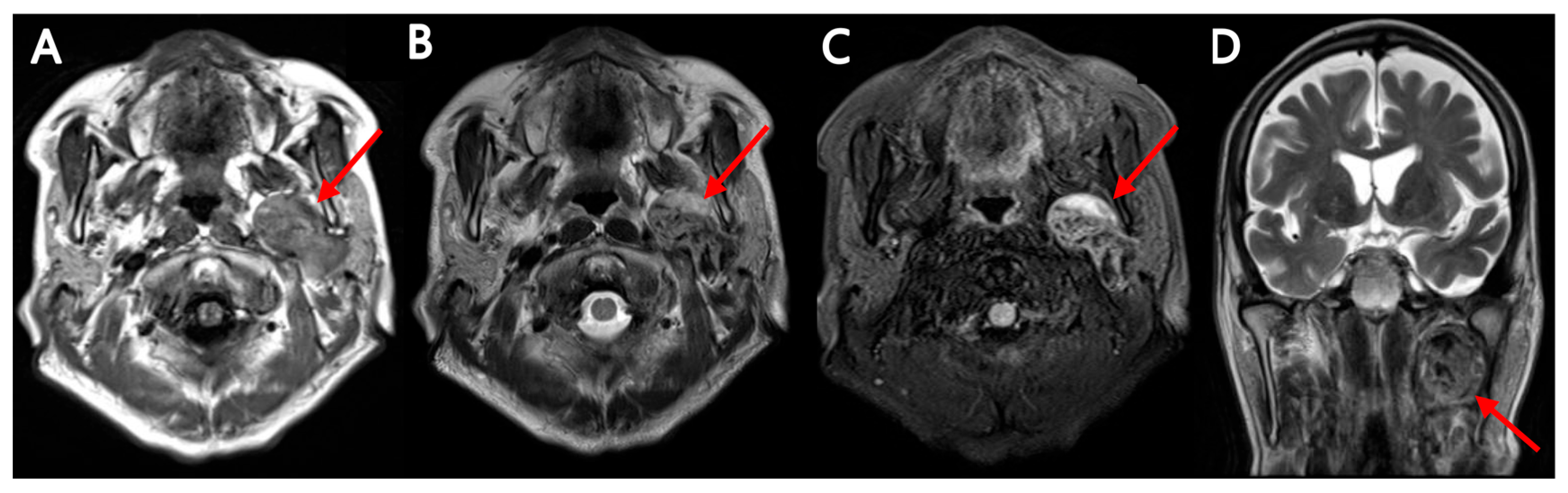
Multimodality Imaging of Warthin’s Tumor: PET/CT, Scintigraphy, MRI, and CT
Abstract


Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakahara, T.; Suzuki, T.; Hashimoto, J.; Shigematsu, N.; Tomita, T.; Ogawa, K.; Kubo, A. Role of salivary gland scintigraphy with Tc-99m pertechnetate in determining treatment of solitary parotid gland tumors: A retrospective study. Clin. Nucl. Med. 2007, 32, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Tanaka, T. Pathogenesis of Warthin′s Tumor: Neoplastic or Non-Neoplastic? Cancers 2024, 16, 912. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, A.; Tissot, H.; Jehanno, N.; Bakuła, Z.E.; Choussy, O.; Lesnik, M.; Badois, N.; Rougier, G.; Klijanienko, J. Fine needle aspiration as a diagnostic modality for Warthin tumors identified as fluorodeoxyglucose positron emission tomography/computed tomography-positive. Diagn. Cytopathol. 2024, 52, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Choi, D.S.; Kim, J.P.; Park, J.J.; Joo, Y.H.; Chung, P.S.; Kim, B.Y.; Ko, Y.H.; Jeong, H.S.; Kim, H.J. Two-phase computed tomography study of warthin tumor of parotid gland: Differentiation from other parotid gland tumors and its pathologic explanation. J. Comput. Assist. Tomogr. 2013, 37, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Motoori, K.; Hanazawa, T.; Nagai, Y.; Yamamoto, S.; Ueda, T.; Funatsu, H.; Ito, H. Warthin tumor of the parotid gland: Diagnostic value of MR imaging with histopathologic correlation. AJNR Am. J. Neuroradiol. 2004, 25, 1256–1262. [Google Scholar] [PubMed]
- Li, L.; Zhao, Y.; Luo, D.; Yang, L.; Hu, L.; Zhao, X.; Wang, Y.; Liu, W. Diagnostic value of single-source dual-energy spectral computed tomography in differentiating parotid gland tumors: Initial results. Quant. Imaging Med. Surg. 2018, 8, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kang, H.J.; Lee, J.H.; Lee, M.K.; Kim, S.I.; Lee, Y.C.; Eun, Y.G. MRI and CT imaging characteristics in parotid tumors with false-negative fine-needle aspirations. Head Face Med. 2024, 20, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Takumi, K.; Nagano, H.; Kikuno, H.; Kumagae, Y.; Fukukura, Y.; Yoshiura, T. Differentiating malignant from benign salivary gland lesions: A multiparametric non-contrast MR imaging approach. Sci. Rep. 2021, 11, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Stoia, S.; Ciurea, A.; Băciuț, M.; Bran, S.; Armencea, G.; Boțan, E.; Lenghel, M.; Tamaș, T.; Mocan, R.; Leucuța, D.; et al. Comparative Diagnostic Accuracy of Ultrasound, MRI, and Fine-Needle Aspiration Biopsy in the Preoperative Evaluation of Parotid Gland Tumors. J. Clin. Med. 2025, 14, 1342. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, C.; Cassoni, A.; Pisciotti, M.L.; Pucci, R.; Veneroso, C.; Di Gioia, C.R.T.; De Felice, F.; Pantano, P.; Valentini, V. High Field MRI in Parotid Gland Tumors: A Diagnostic Algorithm. Cancers 2024, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Yamada, I.; Umehara, I.; Okada, N.; Shibuya, H. Diagnostic accuracy of technetium-99m-pertechnetate scintigraphy with lemon juice stimulation to evaluate Warthin′s tumor. J. Nucl. Med. 1998, 39, 43–46. [Google Scholar] [PubMed]
- Holgado, A.; León, X.; Llansana, A.; Valero, C.; Casasayas, M.; Fernandez-León, A.; Quer, M. Warthin′s Tumour as a Parotid Gland Incidentaloma Identified by PET-CT Scan in a Large Series of Cases. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 3046–3050. [Google Scholar] [CrossRef] [PubMed]
- Klug, T.E.; Hillerup, S.; Dias, A.H.; Gormsen, L.C.; Kristensen, P.N. Incidental [18F]FDG-avid focuses in parotid glands on PET/CT. Acta Otolaryngol. 2024, 144, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Nolli, T.; Bannister, M. Parotid incidentalomas: A systematic review. J. Laryngol. Otol. 2021, 135, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Minoshima, S.; Kawata, T.; Motoori, K.; Nakano, K.; Kazama, T.; Uno, T.; Okamoto, Y.; Ito, H. Diagnostic value of FDG PET and salivary gland scintigraphy for parotid tumors. Clin. Nucl. Med. 2005, 30, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Yasuda, S.; Shohtsu, A.; Ide, M. Four cases of Warthin′s tumor of the parotid gland detected with FDG PET. Ann. Nucl. Med. 1998, 12, 47–50. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, M.; Yi, H.; Kim, I. Multimodality Imaging of Warthin’s Tumor: PET/CT, Scintigraphy, MRI, and CT. Diagnostics 2025, 15, 2666. https://doi.org/10.3390/diagnostics15212666
Cheon M, Yi H, Kim I. Multimodality Imaging of Warthin’s Tumor: PET/CT, Scintigraphy, MRI, and CT. Diagnostics. 2025; 15(21):2666. https://doi.org/10.3390/diagnostics15212666
Chicago/Turabian StyleCheon, Miju, Hyunkyung Yi, and Injoong Kim. 2025. "Multimodality Imaging of Warthin’s Tumor: PET/CT, Scintigraphy, MRI, and CT" Diagnostics 15, no. 21: 2666. https://doi.org/10.3390/diagnostics15212666
APA StyleCheon, M., Yi, H., & Kim, I. (2025). Multimodality Imaging of Warthin’s Tumor: PET/CT, Scintigraphy, MRI, and CT. Diagnostics, 15(21), 2666. https://doi.org/10.3390/diagnostics15212666





