Interstitial Lung Disease Outcome Prediction Using Quantitative Densitometry Indices on Baseline Chest Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
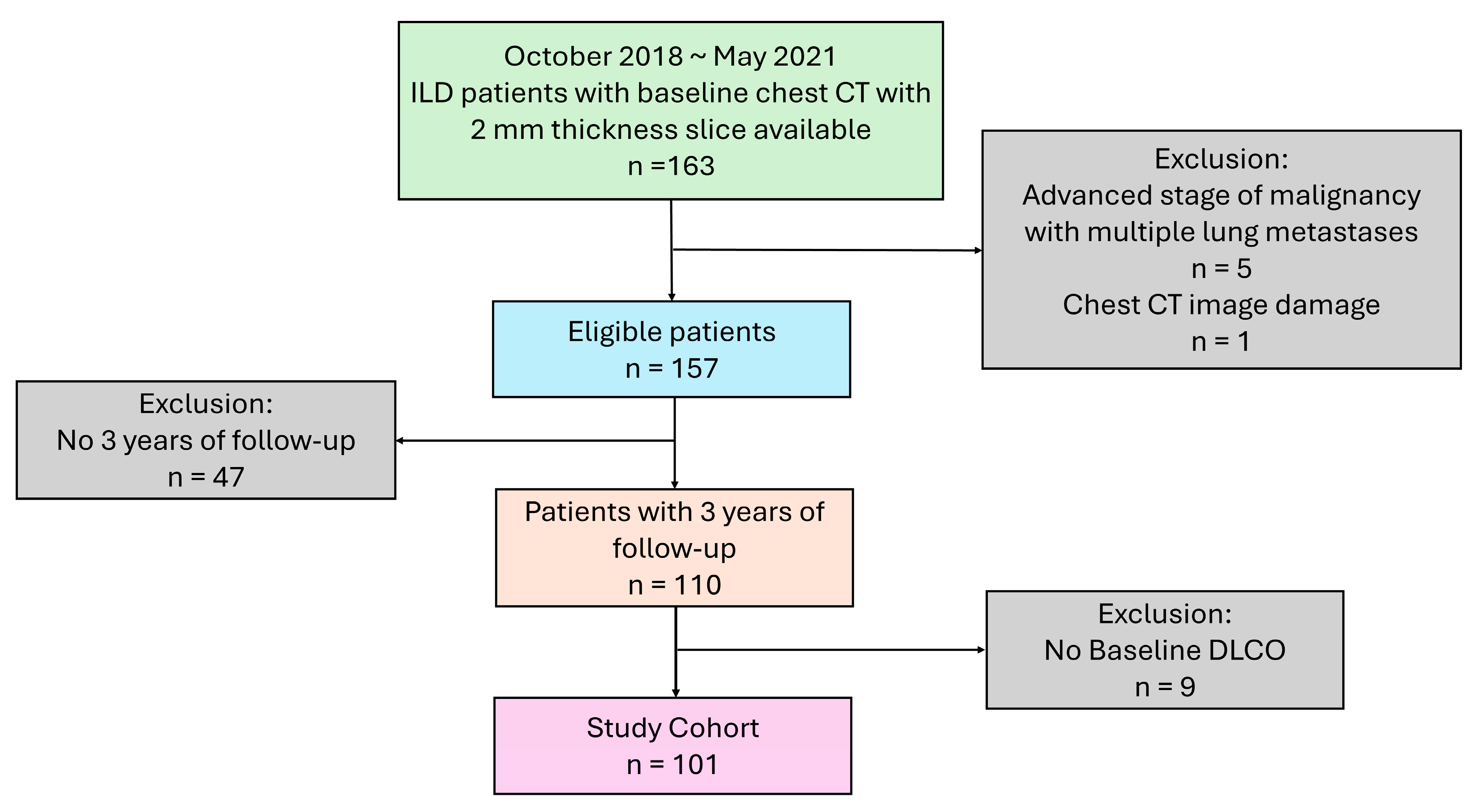
2.1. Study Population
2.2. CT Image Acquisition
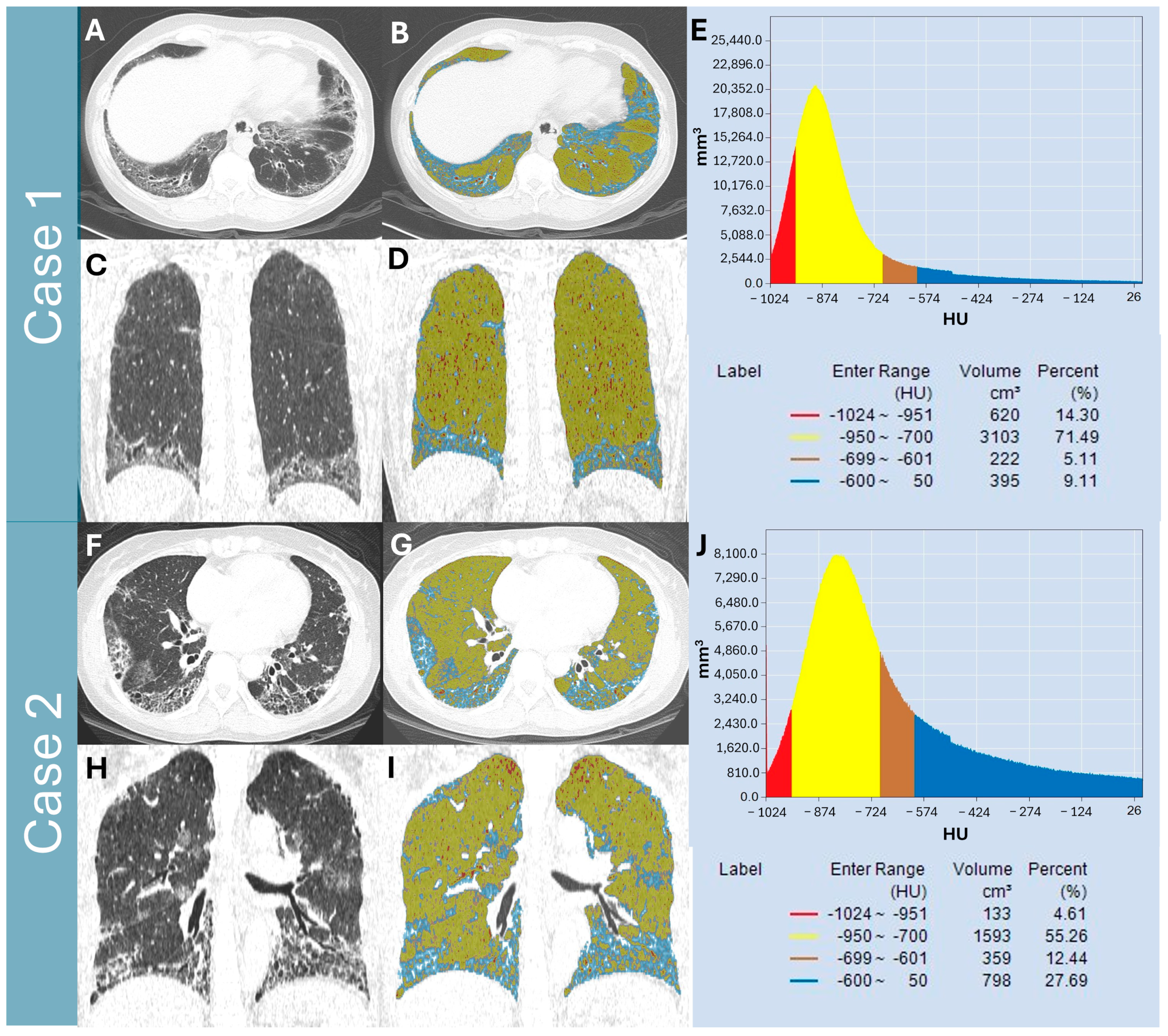
2.3. Quantitative Lung Parenchymal Radiodensity Measurement
- (1)
- Total lung volume (TLVcm3): defined as the volume from −1024 HU to −200 HU and automatically segmented by the software.
- (2)
- Normal lung volume % (NLV%): defined as the percentage of lung volume with attenuation between −950 and −700 [14].
- (3)
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
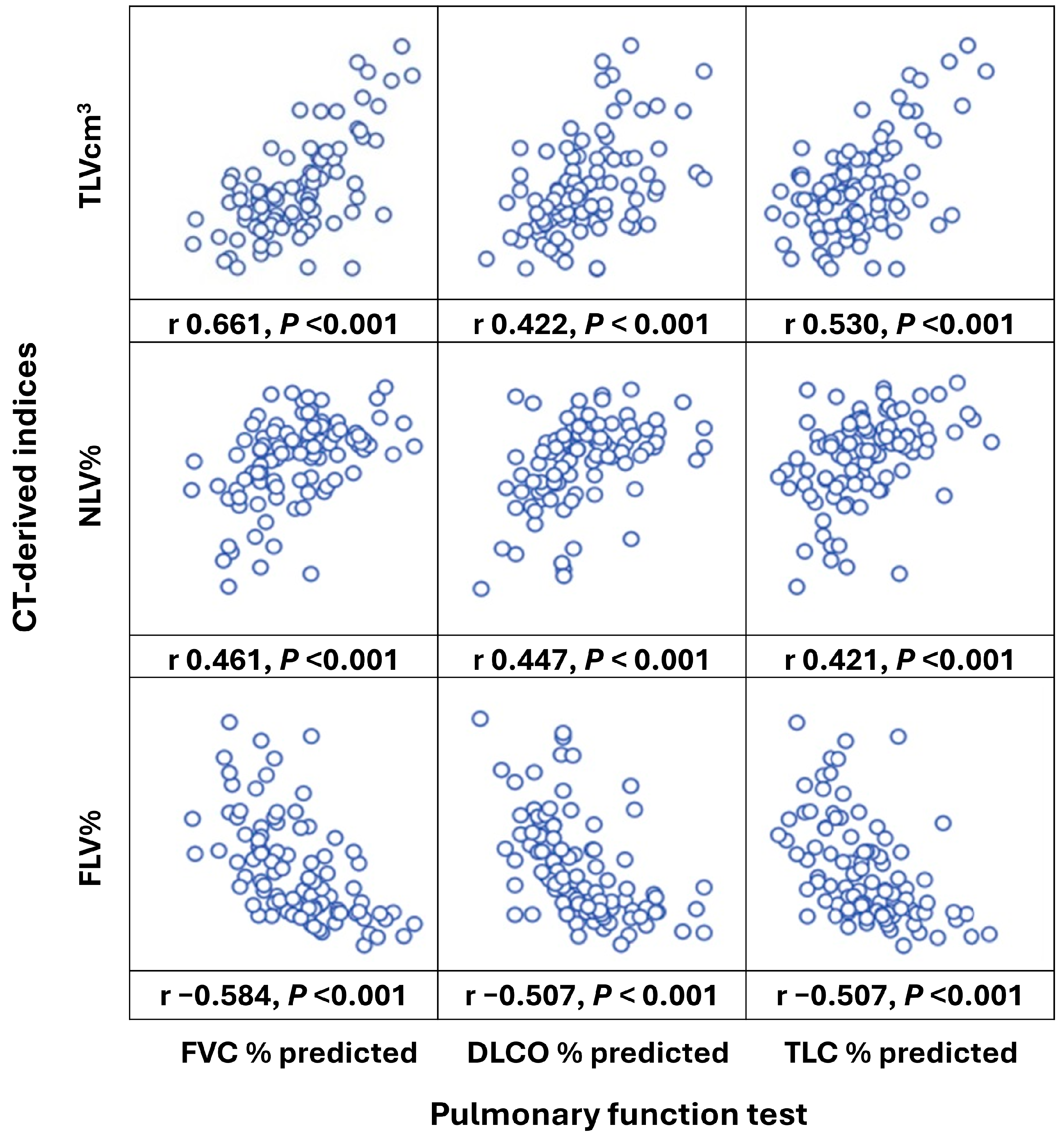
3.2. Correlations of Baseline PFT and CT-Derived Indices
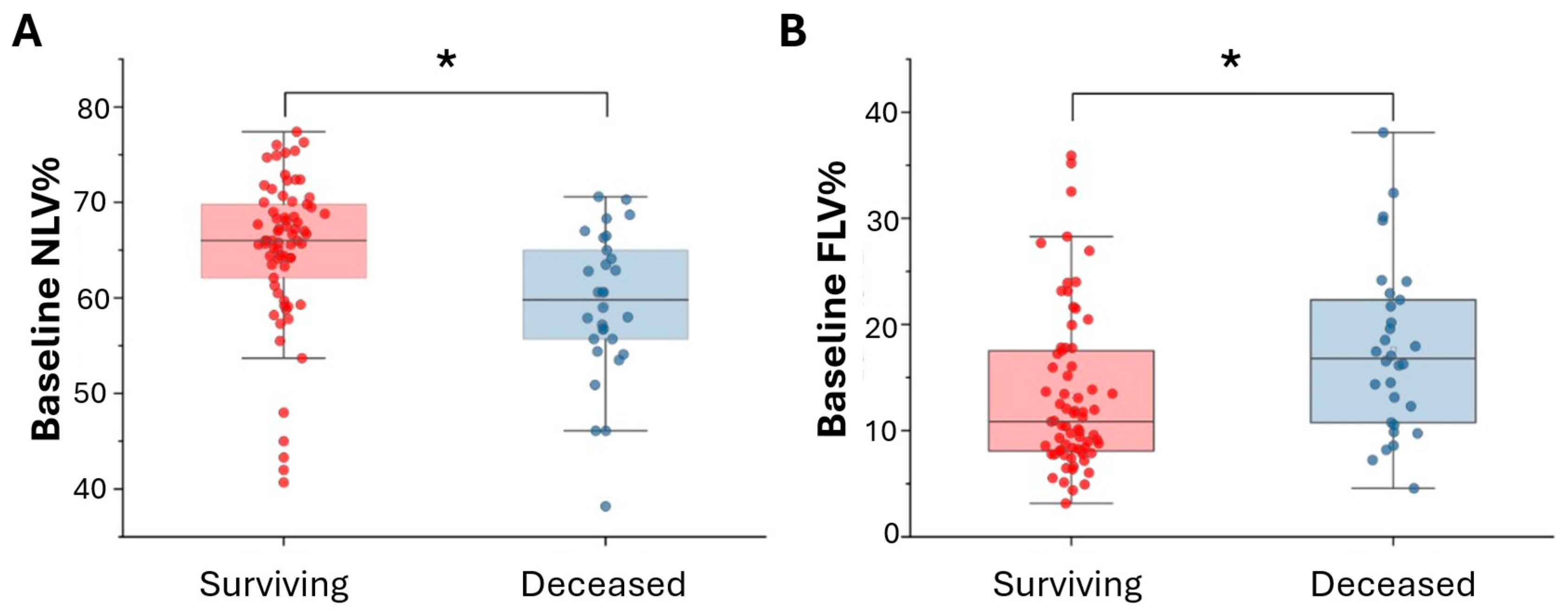
3.3. Comparison Analysis Between Surviving and Deceased Patients
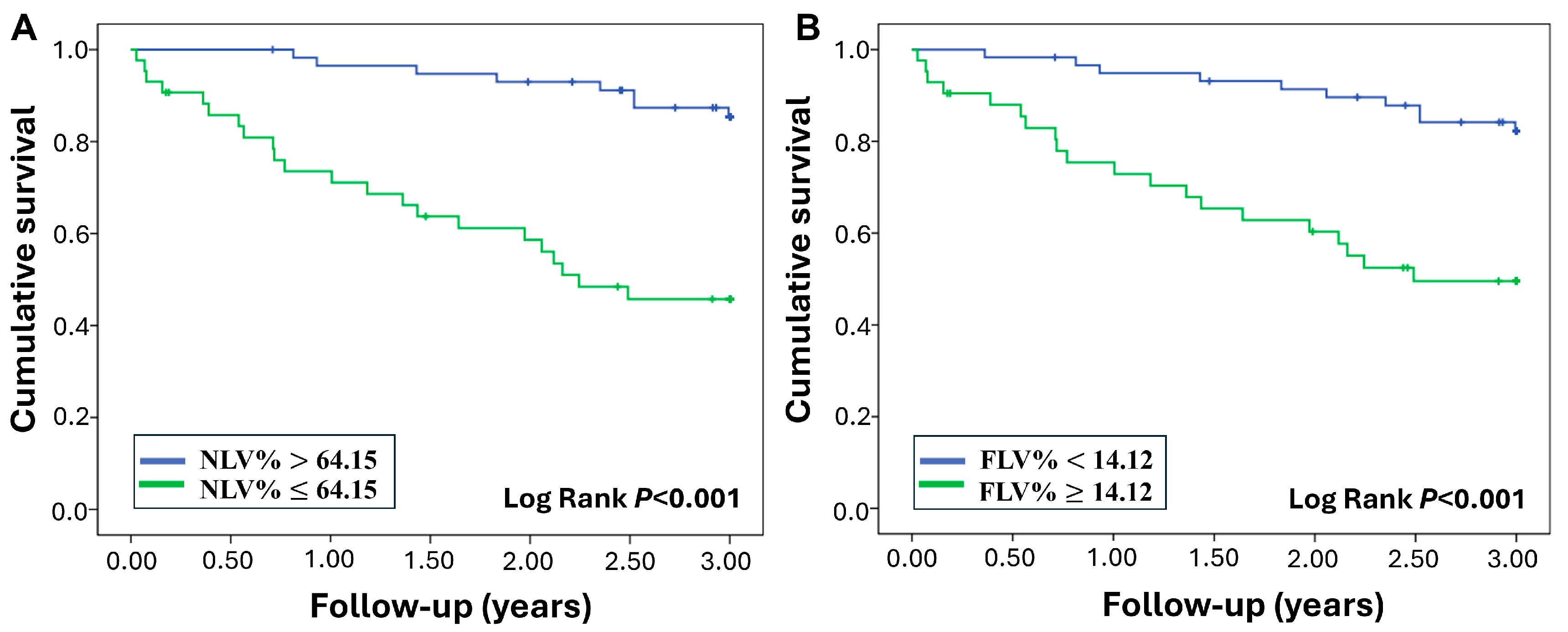
3.4. Mortality Prediction of Baseline CT-Derived Indices
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ILD | interstitial lung disease |
| GGO | ground glass opacity |
| TLVcm3 | total lung volume |
| NLV% | normal lung volume % |
| FLV% | fibrotic lung volume % |
| PFT | pulmonary function test |
| FVC | forced vital capacity |
| DLCO | diffusing capacity of lungs for carbon monoxide |
| IQR | interquartile range |
References
- Cottin, V.; Wollin, L.; Fischer, A.; Quaresma, M.; Stowasser, S.; Harari, S. Fibrosing interstitial lung diseases: Knowns and unknowns. Eur. Respir. Rev. 2019, 28, 180100. [Google Scholar] [CrossRef]
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef]
- Oldham, J.M.; Lee, C.T.; Wu, Z.; Bowman, W.S.; Pugashetti, J.V.; Dao, N.; Tonkin, J.; Seede, H.; Echt, G.; Adegunsoye, A.; et al. Lung function trajectory in progressive fibrosing interstitial lung disease. Eur. Respir. J. 2022, 59, 2101396. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Y. Global health inequalities in the burden of interstitial lung disease and pulmonary sarcoidosis from 1990 to 2021. BMC Public Health 2024, 24, 2892. [Google Scholar] [CrossRef]
- Nasser, M.; Larrieu, S.; Boussel, L.; Si-Mohamed, S.; Diaz, F.; Marque, S.; Massol, J.; Revel, D.; Thivolet-Bejui, F.; Chalabreysse, L.; et al. Epidemiology and mortality of non-idiopathic pulmonary fibrosis (IPF) progressive fibrosing interstitial lung disease (PF-ILD) using the French national health insurance system (SNDS) database in France: The PROGRESS study. Eur. Respir. J. 2020, 56 (Suppl. S64), 444. [Google Scholar]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Bernstein, E.J.; Bolster, M.B.; Chung, J.H.; Danoff, S.K.; George, M.D.; Khanna, D.; Guyatt, G.; Mirza, R.D.; Aggarwal, R.; et al. 2023 American College of Rheumatology (ACR)/American College of Chest Physicians (CHEST) Guideline for the Screening and Monitoring of Interstitial Lung Disease in People with Systemic Autoimmune Rheumatic Diseases. Arthritis Rheumatol 2024, 76, 1201–1213. [Google Scholar] [CrossRef]
- Ledda, R.E.; Campochiaro, C. High resolution computed tomography in systemic sclerosis: From diagnosis to follow-up. Rheumatol. Immunol. Res. 2024, 5, 166–174. [Google Scholar] [CrossRef]
- Ruaro, B.; Baratella, E.; Confalonieri, P.; Wade, B.; Marrocchio, C.; Geri, P.; Busca, A.; Pozzan, R.; Andrisano, A.G.; Cova, M.A.; et al. High-Resolution Computed Tomography: Lights and Shadows in Improving Care for SSc-ILD Patients. Diagnostics 2021, 11, 1960. [Google Scholar] [CrossRef]
- Taha, N.; D’Amato, D.; Hosein, K.; Ranalli, T.; Sergiacomi, G.; Zompatori, M.; Mura, M. Longitudinal functional changes with clinically significant radiographic progression in idiopathic pulmonary fibrosis: Are we following the right parameters? Respir. Res. 2020, 21, 119. [Google Scholar] [CrossRef]
- Saldana, D.C.; Hague, C.J.; Murphy, D.; Coxson, H.O.; Tschirren, J.; Peterson, S.; Sieren, J.P.; Kirby, M.; Ryerson, C.J. Association of Computed Tomography Densitometry with Disease Severity, Functional Decline, and Survival in Systemic Sclerosis-associated Interstitial Lung Disease. Ann. Am. Thorac. Soc. 2020, 17, 813–820. [Google Scholar] [CrossRef]
- Uppaluri, R.; Hoffman, E.A.; Sonka, M.; Hunninghake, G.W.; McLennan, G. Interstitial lung disease: A quantitative study using the adaptive multiple feature method. Am. J. Respir. Crit. Care Med. 1999, 159, 519–525. [Google Scholar] [CrossRef]
- Amorim, F.G.; Santos, E.R.D.; Verrastro, C.G.Y.; Kayser, C. Quantitative chest computed tomography predicts mortality in systemic sclerosis: A longitudinal study. PLoS ONE 2024, 19, e0310892. [Google Scholar] [CrossRef]
- Ohkubo, H.; Kanemitsu, Y.; Uemura, T.; Takakuwa, O.; Takemura, M.; Maeno, K.; Ito, Y.; Oguri, T.; Kazawa, N.; Mikami, R.; et al. Normal Lung Quantification in Usual Interstitial Pneumonia Pattern: The Impact of Threshold-based Volumetric CT Analysis for the Staging of Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0152505. [Google Scholar]
- Lederer, D.J.; Enright, P.L.; Kawut, S.M.; Hoffman, E.A.; Hunninghake, G.; van Beek, E.J.; Austin, J.H.; Jiang, R.; Lovasi, G.S.; Barr, R.G. Cigarette smoking is associated with subclinical parenchymal lung disease: The Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am. J. Respir. Crit. Care Med. 2009, 180, 407–414. [Google Scholar] [CrossRef]
- Carvalho, A.R.S.; Guimarães, A.R.; Sztajnbok, F.R.; Rodrigues, R.S.; Silva, B.R.A.; Lopes, A.J.; Zin, W.A.; Almeida, I.; França, M.M. Automatic Quantification of Interstitial Lung Disease From Chest Computed Tomography in Systemic Sclerosis. Front. Med. 2020, 7, 577739. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M. Interstitial Lung Disease: A Review. JAMA 2024, 331, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, K.; Handa, T.; Nagai, S.; Hirai, T.; Kubo, T.; Oguma, T.; Ito, I.; Ito, Y.; Watanabe, K.; Aihara, K.; et al. Clinical impact of high-attenuation and cystic areas on computed tomography in fibrotic idiopathic interstitial pneumonias. BMC Pulm. Med. 2015, 15, 74. [Google Scholar] [CrossRef]
- Podolanczuk, A.J.; Oelsner, E.C.; Barr, R.G.; Bernstein, E.J.; Hoffman, E.A.; Easthausen, I.J.; Stukovsky, K.H.; RoyChoudhury, A.; Michos, E.D.; Raghu, G.; et al. High-Attenuation Areas on Chest Computed Tomography and Clinical Respiratory Outcomes in Community-Dwelling Adults. Am. J. Respir. Crit. Care Med. 2017, 196, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Nam, B.D.; Hwang, J.H.; Kim, H.-S. Clinical course and risk factors for development and progression of interstitial lung disease in primary Sjögren’s syndrome. Sci. Rep. 2023, 13, 9189. [Google Scholar] [CrossRef]
- Karacaoğlu, M.C.; Kızıldağ, B.; Okyar, B.; Çetin, G.Y.; Doğaner, A.; Atilla, N. Computed tomography-based quantitative scoring system for rheumatoid arthritis-associated interstitial lung disease: A retrospective diagnostic accuracy study for progressive fibrosis detection. Clin. Rheumatol. 2025, 44, 2669–2681. [Google Scholar] [CrossRef]
- Dixon, G.; Thould, H.; Wells, M.; Tsaneva-Atanasova, K.; Scotton, C.J.; Gibbons, M.A.; Barratt, S.L.; Rodrigues, J.C.L. A systematic review of the role of quantitative CT in the prognostication and disease monitoring of interstitial lung disease. Eur. Respir. Rev. 2025, 34, 240194. [Google Scholar] [CrossRef]
- Han, M.K. Clinical Correlations of Computed Tomography Imaging in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2013, 10 (Suppl. S1), S131–S137. [Google Scholar] [CrossRef]
- Okaya, T.; Shigeta, A.; Tanabe, N.; Tatsumi, K.; Yokota, H.; Nishiyama, A.; Naito, A.; Sekine, A.; Sugiura, T.; Sakao, S.; et al. Significance of Normal Lung Volume on Quantitative CT Imaging Analysis in Group 1 and Group 3 Pulmonary Hypertension. CHEST Pulm. 2024, 2, 100062. [Google Scholar] [CrossRef]
- Ohkubo, H.; Taniguchi, H.; Kondoh, Y.; Yagi, M.; Furukawa, T.; Johkoh, T.; Arakawa, H.; Fukuoka, J.; Niimi, A. A Volumetric Computed Tomography Analysis of the Normal Lung in Idiopathic Pulmonary Fibrosis: The Relationship with the Survival. Intern. Med. 2018, 57, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.E.; Chung, M.J.; Jung, M.P.; Choe, B.K.; Lee, K.S. Quantitative Computed Tomographic Indexes in Diffuse Interstitial Lung Disease: Correlation With Physiologic Tests and Computed Tomography Visual Scores. J. Comput. Assist. Tomogr. 2011, 35, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.J.; Bartholmai, B.J.; Wylam, M.E. Comparison of Total Lung Capacity Determined by Plethysmography With Computed Tomographic Segmentation Using CALIPER. J. Thorac. Imaging 2017, 32, 101–106. [Google Scholar] [CrossRef]
- Schlesinger, A.E.; White, D.K.; Mallory, G.B.; Hildeboldt, C.F.; Huddleston, C.B. Estimation of total lung capacity from chest radiography and chest CT in children: Comparison with body plethysmography. AJR. Am. J. Roentgenol. 1995, 165, 151–154. [Google Scholar] [CrossRef]
- Coates, A.L.; Peslin, R.; Rodenstein, D.; Stocks, J. Measurement of lung volumes by plethysmography. Eur. Respir. J. 1997, 10, 1415–1427. [Google Scholar] [CrossRef]
- Denison, D.M.; Morgan, M.D.; Millar, A.B. Estimation of regional gas and tissue volumes of the lung in supine man using computed tomography. Thorax 1986, 41, 620–628. [Google Scholar] [CrossRef]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Mura, M.; Porretta, M.A.; Bargagli, E.; Sergiacomi, G.; Zompatori, M.; Sverzellati, N.; Taglieri, A.; Mezzasalma, F.; Rottoli, P.; Saltini, C.; et al. Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: A 3-year prospective study. Eur. Respir. J. 2012, 40, 101–109. [Google Scholar] [CrossRef]
- Xu, W.; Wu, W.; Zhang, D.; Chen, Z.; Tao, X.; Zhao, J.; Wang, K.; Wang, X.; Zheng, Y.; Ye, S. A novel CT scoring method predicts the prognosis of interstitial lung disease associated with anti-MDA5 positive dermatomyositis. Sci. Rep. 2021, 11, 17070. [Google Scholar] [CrossRef] [PubMed]
- Ley-Zaporozhan, J.; Giannakis, A.; Norajitra, T.; Weinheimer, O.; Kehler, L.; Dinkel, J.; Ganter, C.; Ley, S.; Van Lunteren, C.; Eichinger, M.; et al. Fully Automated Segmentation of Pulmonary Fibrosis Using Different Software Tools. Respiration 2021, 100, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Henket, M.; Gester, F.; André, B.; Ernst, B.; Frix, A.N.; Smeets, D.; Van Eyndhoven, S.; Antoniou, K.; Conemans, L.; et al. Automated AI-based image analysis for quantification and prediction of interstitial lung disease in systemic sclerosis patients. Respir. Res. 2025, 26, 39. [Google Scholar] [CrossRef]
- Oh, A.S.; Lynch, D.A.; Swigris, J.J.; Baraghoshi, D.; Dyer, D.S.; Hale, V.A.; Koelsch, T.L.; Marrocchio, C.; Parker, K.N.; Teague, S.D.; et al. Deep Learning–based Fibrosis Extent on Computed Tomography Predicts Outcome of Fibrosing Interstitial Lung Disease Independent of Visually Assessed Computed Tomography Pattern. Ann. Am. Thorac. Soc. 2024, 21, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Chassagnon, G.; Vakalopoulou, M.; Régent, A.; Zacharaki, E.I.; Aviram, G.; Martin, C.; Marini, R.; Bus, N.; Jerjir, N.; Mekinian, A.; et al. Deep Learning-based Approach for Automated Assessment of Interstitial Lung Disease in Systemic Sclerosis on CT Images. Radiol. Artif. Intell. 2020, 2, e190006. [Google Scholar] [CrossRef]
- Thillai, M.; Oldham, J.M.; Ruggiero, A.; Kanavati, F.; McLellan, T.; Saini, G.; Johnson, S.R.; Ble, F.X.; Azim, A.; Ostridge, K.; et al. Deep Learning-based Segmentation of Computed Tomography Scans Predicts Disease Progression and Mortality in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2024, 210, 465–472. [Google Scholar] [CrossRef]
- Mei, X.; Liu, Z.; Singh, A.; Lange, M.; Boddu, P.; Gong, J.Q.X.; Lee, J.; DeMarco, C.; Cao, C.; Platt, S.; et al. Interstitial lung disease diagnosis and prognosis using an AI system integrating longitudinal data. Nat. Commun. 2023, 14, 2272. [Google Scholar] [CrossRef]
- Watadani, T.; Sakai, F.; Johkoh, T.; Noma, S.; Akira, M.; Fujimoto, K.; Bankier, A.A.; Lee, K.S.; Müller, N.L.; Song, J.W.; et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology 2013, 266, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, M.; Sedie, A.D.; Romei, C.; Tavanti, L.; Da Rio, M.; Morganti, R.; Della Rossa, A.; Mosca, M. Lung ultrasound and high-resolution computed tomography quantitative variations during nintedanib treatment for systemic sclerosis-associated interstitial lung disease. Rheumatology 2024, 63, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, J.-P.; Van Rikxoort, E.M.; Setio, A.A.; Schaefer-Prokop, C.M.; van Ginneken, B.; Ciompi, F. Improving airway segmentation in computed tomography using leak detection with convolutional networks. Med. Image Anal. 2017, 36, 52–60. [Google Scholar] [CrossRef] [PubMed]
| All Patients | |
|---|---|
| Characteristics | (n = 101) |
| Demographics | |
| Females | 38 (37.62%) |
| Age (years) | 69.41 ± 12.26 |
| Smoking status | |
| Never | 59 (58.42%) |
| Ever | 42 (41.58%) |
| Baseline pulmonary function | |
| FVC (% predicted) | 76.10 ± 17.60 |
| DLCO (% predicted) | 64.20 ± 23.58 |
| TLC (% predicted) | 76.32 ± 16.58 |
| TLC (L) | 3.58 ± 0.97 |
| Baseline CT-derived index | |
| Total lung volume (cm3) | 3148.20 ± 921.16 |
| Normal lung volume (%) | 63.31 ± 8.15 |
| Fibrotic lung volume (%) | 14.62 ± 7.85 |
| Outcome | |
| Surviving | 71 (70.30%) |
| Deceased | 30 (29.70%) |
| Follow up (years) | 3.00 (1.99, 3.00) |
| Surviving | Deceased | ||
|---|---|---|---|
| (n = 71) | (n = 30) | p | |
| Age | 67.93 ± 12.80 | 72.87 ± 10.24 | 0.065 |
| Gender | |||
| Female | 30 (40.25%) | 8 (26.67%) | 0.140 |
| Male | 41 (57.75%) | 22 (73.33%) | |
| Smoking status | |||
| Never smoked | 41 (57.75%) | 18 (60.00%) | 0.834 |
| Baseline FVC % predicted | 79.34 ± 16.05 | 68.43 ± 18.95 | 0.004 |
| Baseline DLCO % predicted | 68.87 ± 22.37 | 53.13 ± 22.99 | 0.002 |
| Baseline TLV% predicted | 78.33 ± 15.87 | 71.52 ± 17.51 | 0.063 |
| Baseline TLV (L) | 3.66 ± 0.94 | 3.39 ± 1.03 | 0.217 |
| Baseline CT-derive index | |||
| TLVcm3 | 3223.55 ± 941.68 | 2969.87 ± 859.48 | 0.208 |
| NLV% | 65.02 ± 7.82 | 59.27 ± 7.61 | 0.001 |
| FLV% | 13.34 ± 7.48 | 17.64 ± 7.98 | 0.011 |
| Variables | HR | 95% CI | Raw p | Adjusted p |
|---|---|---|---|---|
| Female | − | |||
| Male | 1.74 | 0.78–3.91 | 0.179 | 1.432 |
| Age | 1.03 | 0.10–1.06 | 0.079 | 0.632 |
| Never smoker | − | |||
| Ever smoker | 0.97 | 0.47–2.01 | 0.932 | 7.456 |
| Baseline DLCO% predicted | 0.97 | 0.95–0.99 | 0.000 | 0.000 |
| Baseline FVC% predicted | 0.96 | 0.94–0.99 | 0.001 | 0.008 |
| Baseline TLVcm3 | 1.00 | 1.00–1.00 | 0.205 | 1.640 |
| Baseline NLA% | 0.94 | 0.91–0.97 | 0.000 | 0.000 |
| Baseline FLA% | 1.06 | 1.02–1.10 | 0.005 | 0.040 |
| Variables | HR | 95% CI | p |
|---|---|---|---|
| Female | − | ||
| Male | 2.70 | 0.92–7.91 | 0.070 |
| Age | 1.02 | 0.97–1.06 | 0.480 |
| Never smoker | − | ||
| Ever smoker | 0.64 | 0.26–1.56 | 0.326 |
| Baseline FVC | 0.98 | 0.95–1.01 | 0.229 |
| Baseline DLCO | 0.97 | 0.95–0.99 | 0.007 |
| Baseline NLA% | 0.88 | 0.78–0.99 | 0.034 |
| Baseline FLA% | 0.90 | 0.79–1.02 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-T.; Huang, T.-H.; Lin, C.-Y.; Ho, H.; Tsai, Y.-S.; Lin, C.-Y.; Wang, C.-K. Interstitial Lung Disease Outcome Prediction Using Quantitative Densitometry Indices on Baseline Chest Computed Tomography. Diagnostics 2025, 15, 2665. https://doi.org/10.3390/diagnostics15212665
Huang L-T, Huang T-H, Lin C-Y, Ho H, Tsai Y-S, Lin C-Y, Wang C-K. Interstitial Lung Disease Outcome Prediction Using Quantitative Densitometry Indices on Baseline Chest Computed Tomography. Diagnostics. 2025; 15(21):2665. https://doi.org/10.3390/diagnostics15212665
Chicago/Turabian StyleHuang, Li-Ting, Tang-Hsiu Huang, Chung-Ying Lin, Hao Ho, Yi-Shan Tsai, Chia-Ying Lin, and Chien-Kuo Wang. 2025. "Interstitial Lung Disease Outcome Prediction Using Quantitative Densitometry Indices on Baseline Chest Computed Tomography" Diagnostics 15, no. 21: 2665. https://doi.org/10.3390/diagnostics15212665
APA StyleHuang, L.-T., Huang, T.-H., Lin, C.-Y., Ho, H., Tsai, Y.-S., Lin, C.-Y., & Wang, C.-K. (2025). Interstitial Lung Disease Outcome Prediction Using Quantitative Densitometry Indices on Baseline Chest Computed Tomography. Diagnostics, 15(21), 2665. https://doi.org/10.3390/diagnostics15212665









