Application of Nanogenerators in Lumbar Motion Monitoring: Fundamentals, Current Status, and Perspectives
Abstract
1. Introduction
2. Significance of Lumbar Motion Monitoring and Basic Components of Monitoring Devices
2.1. Basics of Lumbar Motion Monitoring Devices
2.2. Lumbar Motion Monitoring and Lumbar Spine Disorders
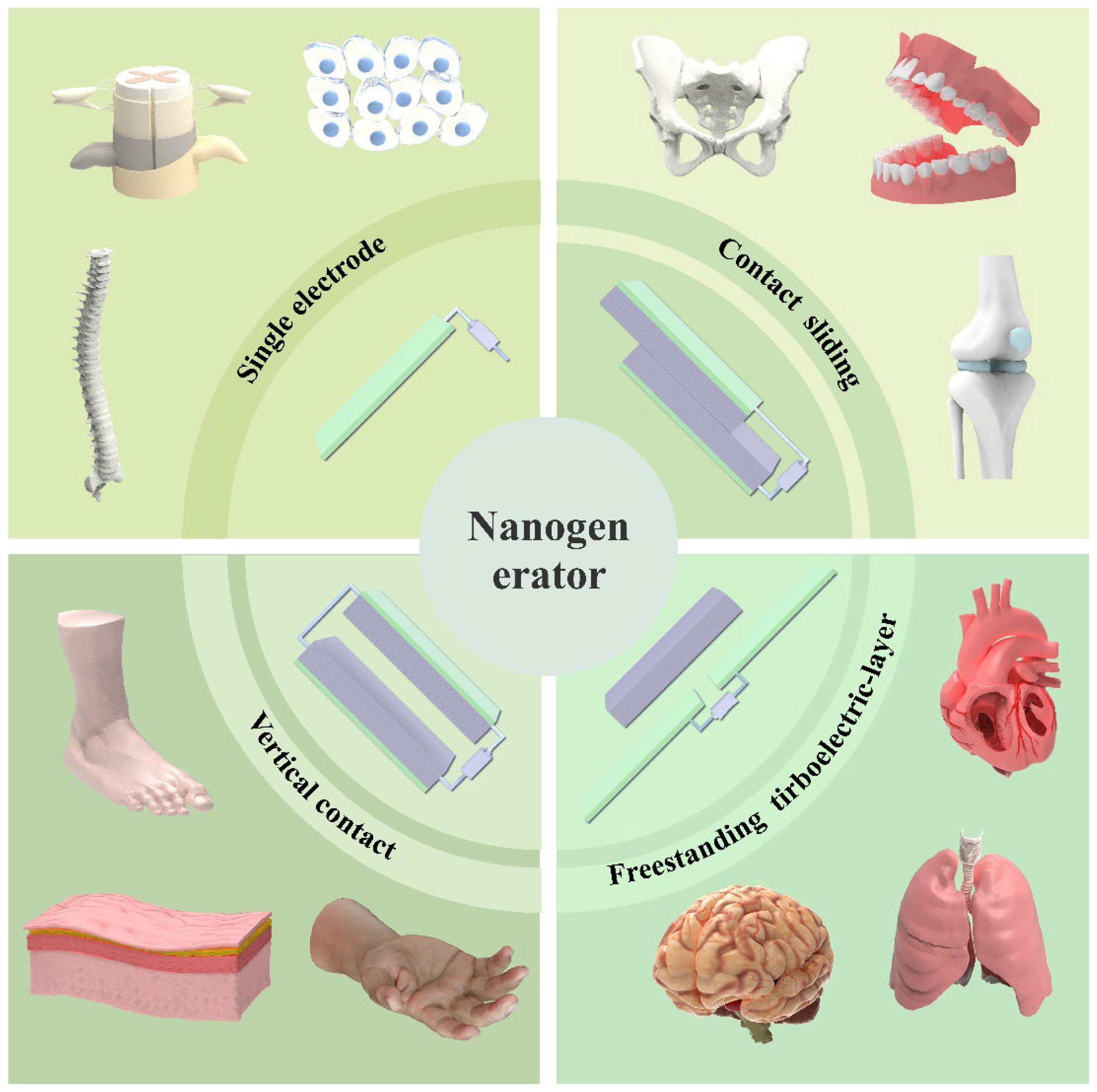
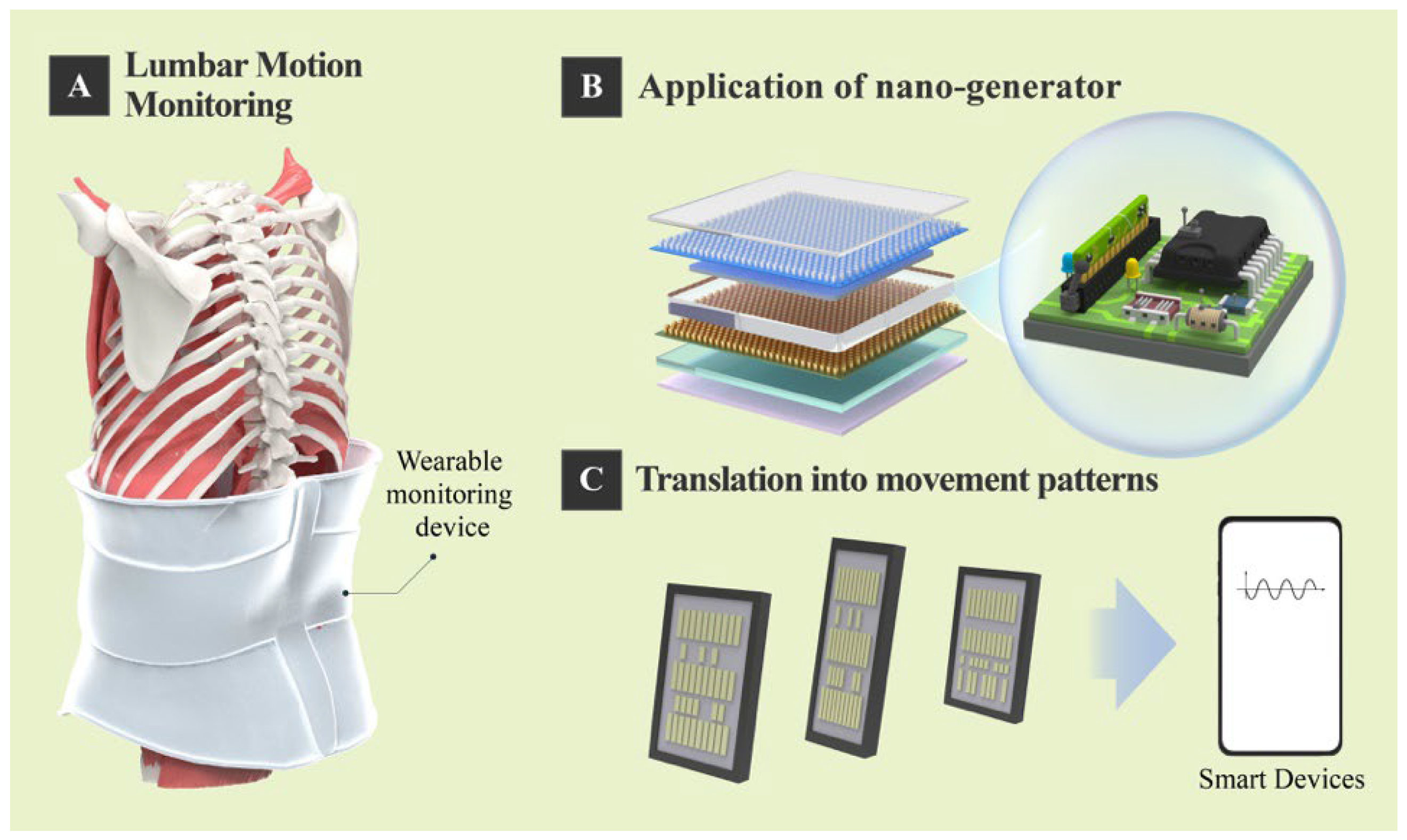
3. Integration of Nanogenerator Technology and Lumbar Motion Monitoring
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.L. Triboelectric Nanogenerators as New Energy Technology for Self-Powered Systems and as Active Mechanical and Chemical Sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J.H. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Moradi-Dastjerdi, R.; Behdinan, K.; Safaei, B.; Qin, Z. Static performance of agglomerated CNT-reinforced porous plates bonded with piezoceramic faces. Int. J. Mech. Sci. 2020, 188, 105966. [Google Scholar] [CrossRef]
- Moradi-Dastjerdi, R.; Behdinan, K. Dynamic performance of piezoelectric energy harvesters with a multifunctional nanocomposite substrate. Appl. Energy 2021, 293, 116947. [Google Scholar] [CrossRef]
- Xia, Z.; Feng, P.Y.; Jing, X.; Li, H.; Mi, H.Y.; Liu, Y. Design and Optimization Principles of Cylindrical Sliding Triboelectric Nanogenerators. Micromachines 2021, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Bae, J.; Lee, J.; Lee, C.-S.; Hong, S.; Wang, Z.L. Self-powered environmental sensor system driven by nanogenerators. Energy Environ. Sci. 2011, 4, 3359–3363. [Google Scholar] [CrossRef]
- Xu, S.; Qin, Y.; Xu, C.; Wei, Y.; Yang, R.; Wang, Z.L. Self-powered nanowire devices. Nat. Nanotechnol. 2010, 5, 366–373. [Google Scholar] [CrossRef]
- Yang, Y.; Jung, J.H.; Yun, B.K.; Zhang, F.; Pradel, K.C.; Guo, W.; Wang, Z.L. Flexible pyroelectric nanogenerators using a composite structure of lead-free KNbO(3) nanowires. Adv. Mater. 2012, 24, 5357–5362. [Google Scholar]
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric Biomaterials for Sensors and Actuators. Adv. Mater. 2019, 31, e1802084. [Google Scholar]
- Chen, J.; Zhu, G.; Yang, W.; Jing, Q.; Bai, P.; Yang, Y.; Hou, T.C.; Wang, Z.L. Harmonic-resonator-based triboelectric nanogenerator as a sustainable power source and a self-powered active vibration sensor. Adv. Mater. 2013, 25, 6094–6099. [Google Scholar]
- Pan, S.; Zhang, Z. Fundamental theories and basic principles of triboelectric effect: A review. Friction 2018, 7, 2–17. [Google Scholar] [CrossRef]
- Hurdoganoglu, D.; Safaei, B.; Cheng, J.; Qin, Z.; Sahmani, S. A Comprehensive Review on the Novel Principles, Development and Applications of Triboelectric Nanogenerators. Appl. Mech. Rev. 2024, 76, 010802. [Google Scholar] [CrossRef]
- Shaislamov, U.; Kim, Y.; Kim, W.S.; Jeong, H.; Lee, H.-J.; Chun, W. Hybrid operation of triboelectric nanogenerator for electricity generation by a low-temperature differential heat engine. Int. J. Energy Res. 2017, 41, 1412–1421. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, Y.; Sun, N.; Li, G.; Liu, Y.; Chen, C.; Shi, J.; Xie, L.; Jiang, H.; Bao, D.; et al. A Wrinkled PEDOT:PSS Film Based Stretchable and Transparent Triboelectric Nanogenerator for Wearable Energy Harvesters and Active Motion Sensors. Adv. Funct. Mater. 2018, 28, 1803684. [Google Scholar] [CrossRef]
- Niu, S.; Wang, S.; Lin, L.; Liu, Y.; Zhou, Y.S.; Hu, Y.; Wang, Z.L. Theoretical study of contact-mode triboelectric nanogenerators as an effective power source. Energy Environ. Sci. 2013, 6, 3576–3583. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, Q.; Zhang, X.; Zhao, D.; Xia, X.; Wang, J.; Li, H.; Wang, Z.L.; Cheng, T. Contact-sliding-separation mode triboelectric nanogenerator. Energy Environ. Sci. 2023, 16, 3932–3941. [Google Scholar] [CrossRef]
- Niu, S.M.; Liu, Y.; Chen, X.Y.; Wang, S.H.; Zhou, Y.S.; Lin, L.; Xie, Y.N.; Wang, Z.L. Theory of freestanding triboelectric-layer-based nanogenerators. Nano Energy 2015, 12, 760–774. [Google Scholar] [CrossRef]
- Yu, J.R.; Gao, G.Y.; Huang, J.R.; Yang, X.X.; Han, J.; Zhang, H.; Chen, Y.H.; Zhao, C.L.; Sun, Q.J.; Wang, Z.L. Contact-electrification-activated artificial afferents at femtojoule energy. Nat. Commun. 2021, 12, 1581. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.L.; Chen, J.; Jing, Q.S.; Zhou, Y.S.; Wen, X.N.; Wang, Z.L. Single-Electrode-Based Sliding Triboelectric Nanogenerator for Self-Powered Displacement Vector Sensor System. ACS Nano 2013, 7, 7342–7351. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Ma, X.F.; Zhang, C.M.; Han, J.Q.; He, S.J.; Liu, K.M.; Jiang, S.H. Nanocellulose-based nanogenerators for sensor applications: A review. Int. J. Biol. Macromol. 2024, 259, 129268. [Google Scholar] [CrossRef]
- Fan, F.R.; Tang, W.; Wang, Z.L. Flexible Nanogenerators for Energy Harvesting and Self-Powered Electronics. Adv. Mater. 2016, 28, 4283–4305. [Google Scholar] [CrossRef]
- Deng, W.L.; Zhou, Y.H.; Libanori, A.; Chen, G.R.; Yang, W.Q.; Chen, J. Piezoelectric nanogenerators for personalized healthcare. Chem. Soc. Rev. 2022, 51, 3380–3435. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Joe, P.; Tuzman, O.L.; Park, K.-I.; Lee, K.J.; Shi, Y.; Huang, Y.; Rogers, J.A. Recent progress in flexible and stretchable piezoelectric devices for mechanical energy harvesting, sensing and actuation. Extreme Mech. Lett. 2016, 9, 269–281. [Google Scholar] [CrossRef]
- Rao, D.; Scuderi, G.; Scuderi, C.; Grewal, R.; Sandhu, S.J. The Use of Imaging in Management of Patients with Low Back Pain. J. Clin. Imaging Sci. 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, M.; Dillon, C.; Erdil, M.; Ferguson, S.; Kaplan, J.; Krompinger, J.; Litt, M.; Murphy, M. Clinical and psychological correlates of lumbar motion abnormalities in low back disorders. Spine J. 2001, 1, 290–298. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, C.; Kim, K.; Cho, M.; Kim, H.C.; Chung, S.G. Lumbar Stability in Healthy Individuals and Low Back Pain Patients Quantified by Wall Plank-and-Roll Test. Phys. Med. Rehabil. 2019, 11, 483–494. [Google Scholar] [CrossRef]
- Dwornik, M.; Puszczałowska-Lizis, E.; Wójcik, M.; Szajkowski, S.; Graczykowski, M.; Szymański, D.; Marszałek, S. Efficacy of osteopathic manipulative treatment (93.6. ICD–9)—Systematic review. Med. Stud. 2024, 40, 289–307. [Google Scholar] [CrossRef]
- Goncharow, P.N.; Beaudette, S.M. Assessing Time-Varying Lumbar Flexion-Extension Kinematics Using Automated Pose Estimation. J. Appl. Biomech. 2022, 38, 355–360. [Google Scholar] [CrossRef]
- Morita, T.; Yoshimoto, M.; Terashima, Y.; Tanimoto, K.; Iesato, N.; Ogon, I.; Oshigiri, T.; Teramoto, A.; Emori, M.; Takashima, H.; et al. Do We Have Adequate Flexion-extension Radiographs for Evaluating Instability in Patients With Lumbar Spondylolisthesis? Spine (Phila Pa 1976) 2020, 45, 48–54. [Google Scholar] [CrossRef]
- Huijnen, I.P.J.; Schasfoort, F.C.; Smeets, R.; Sneekes, E.; Verbunt, J.A.; Bussmann, J.B.J. Subgrouping patients with chronic low back pain: What are the differences in actual daily life behavior between patients classified as avoider or persister? J. Back Musculoskelet. Rehabil. 2020, 33, 303–311. [Google Scholar] [CrossRef]
- Nooijen, C.F.J.; de Groot, J.F.; Stam, H.J.; van den Berg-Emons, R.J.G.; Bussmann, H.B.J.; Fit Future, C. Validation of an activity monitor for children who are partly or completely wheelchair-dependent. J. Neuroeng. Rehabil. 2015, 12, 11. [Google Scholar] [CrossRef]
- Bloemen, M.A.T.; van den Berg-Emons, R.J.G.; Tuijt, M.; Nooijen, C.F.J.; Takken, T.; Backx, F.J.G.; Vos, M.; de Groot, J.F. Physical activity in wheelchair-using youth with spina bifida: An observational study. J. Neuroeng. Rehabil. 2019, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.; Vinothraj, R. Design and study of ultrasound-based automatic patient movement monitoring device for quantifying the intrafraction motion during teletherapy treatment. J. Appl. Clin. Med. Phys. 2012, 13, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.S.; Kim, H.; Ahn, J.H.; Park, Y.B.; Park, Y.J. Individual characteristics of reliable lumbar coupling motions. Eur. Spine J. 2015, 24, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, M.; O’Dwyer, T.; Connolly, J.; Condell, J.; Esquivel, K.M.; O’Shea, F.D.; Gardiner, P.; Wilson, F. Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Reliability Study in Axial Spondyloarthritis. Diagnostics 2021, 11, 490. [Google Scholar] [CrossRef]
- Chang, R.P.; Smith, A.; Kent, P.; Saraceni, N.; Hancock, M.; O’Sullivan, P.B.; Campbell, A. Concurrent validity of DorsaVi wireless motion sensor system Version 6 and the Vicon motion analysis system during lifting. BMC Musculoskelet. Disord. 2022, 23, 909. [Google Scholar] [CrossRef]
- Chu, M.Z.; Hu, C.; Zhu, L.; Lyu, J.J.; Wang, F.; Tao, X.J. Physical activity and sedentary behavior in peritoneal dialysis patients: A comparative analysis of ActiGraph GT3X data collected via wrist and waist with placement-specific cut-points. BMC Nephrol. 2025, 26, 178. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Xu, K.; Hossbach, M.; Demir, A.; Rajan, P.; Taylor, R.H.; Moghekar, A.; Foroughi, P.; Kazanzides, P.; et al. Wearable Mechatronic Ultrasound-integrated AR Navigation System for Lumbar Puncture Guidance. IEEE Trans. Med. Robot. Bionics 2023, 5, 966–977. [Google Scholar] [CrossRef]
- Laird, R.A.; Kent, P.; Keating, J.L. How consistent are lordosis, range of movement and lumbo-pelvic rhythm in people with and without back pain? BMC Musculoskelet. Disord. 2016, 17, 403. [Google Scholar] [CrossRef]
- O’Sullivan, K.; O’Sullivan, L.; O’Sullivan, P.; Dankaerts, W. Investigating the effect of real-time spinal postural biofeedback on seated discomfort in people with non-specific chronic low back pain. Ergonomics 2013, 56, 1315–1325. [Google Scholar] [CrossRef]
- Hodges, P.W.; van den Hoorn, W. A vision for the future of wearable sensors in spine care and its challenges: Narrative review. J. Spine Surg. 2022, 8, 103–116. [Google Scholar] [CrossRef]
- Mjøsund, H.L.; Boyle, E.; Kjaer, P.; Mieritz, R.M.; Skallgård, T.; Kent, P. Clinically acceptable agreement between the ViMove wireless motion sensor system and the Vicon motion capture system when measuring lumbar region inclination motion in the sagittal and coronal planes. BMC Musculoskelet. Disord. 2017, 18, 124. [Google Scholar] [CrossRef]
- Sahrmann, S.; Azevedo, D.C.; Dillen, L.V. Diagnosis and treatment of movement system impairment syndromes. Braz. J. Phys. Ther. 2017, 21, 391–399. [Google Scholar] [CrossRef]
- Rezaei, A.; Cheng, C.H.; Pignolo, R.J.; Lu, L.; Kaufman, K. Effects of Age and Muscle Activation on Three-Dimensional Spine Kinematics and Asymmetry in Elderly Adults. J. Clin. Med. 2025, 14, 1610. [Google Scholar] [CrossRef] [PubMed]
- du Rose, A. Have Studies that Measure Lumbar Kinematics and Muscle Activity Concurrently during Sagittal Bending Improved Understanding of Spinal Stability and Sub-System Interactions? A Systematic Review. Healthcare 2018, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Kemani, M.K.; Hägg, O.; Jakobsson, M.; Lundberg, M. Fear of Movement Is Related to Low Back Disability During a Two-Year Period in Patients Who Have Undergone Elective Lumbar Spine Surgery. World Neurosurg. 2020, 137, E416–E424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Zhang, G.Q.; Yao, S.; Peng, Y. Piezoelectric-triboelectric energy harvester with elastic double-side stoppers. Int. J. Mech. Sci. 2024, 280, 109561. [Google Scholar] [CrossRef]
- Pan, J.; Sun, W.L.; Li, X.; Hao, Y.T.; Bai, Y.; Nan, D. A noval transparent triboelectric nanogenerator as electronic skin for real-time breath monitoring. J. Colloid Interface Sci. 2024, 671, 336–343. [Google Scholar] [CrossRef]
- Zheng, Q.; Shi, B.; Fan, F.; Wang, X.; Yan, L.; Yuan, W.; Wang, S.; Liu, H.; Li, Z.; Wang, Z.L. In vivo powering of pacemaker by breathing-driven implanted triboelectric nanogenerator. Adv. Mater. 2014, 26, 5851–5856. [Google Scholar]
- Han, S.; Hu, Y.; Wei, J.; Li, S.; Yang, P.; Mi, H.; Liu, C.; Shen, C. A Semi-Interpenetrating Poly(Ionic Liquid) Network-Driven Low Hysteresis and Transparent Hydrogel as a Self-Powered Multifunctional Sensor. Adv. Funct. Mater. 2024, 34, 2401607. [Google Scholar]
- Wang, W.; Guo, P.; Liu, X.; Chen, M.; Li, J.; Hu, Z.; Li, G.; Chang, Q.; Shi, K.; Wang, X.; et al. Fully Polymeric Conductive Hydrogels with Low Hysteresis and High Toughness as Multi-Responsive and Self-Powered Wearable Sensors. Adv. Funct. Mater. 2024, 34, 2316346. [Google Scholar] [CrossRef]
- Shao, B.; Lu, M.H.; Wu, T.C.; Peng, W.C.; Ko, T.Y.; Hsiao, Y.C.; Chen, J.Y.; Sun, B.; Liu, R.; Lai, Y.C. Large-area, untethered, metamorphic, and omnidirectionally stretchable multiplexing self-powered triboelectric skins. Nat. Commun. 2024, 15, 1238. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Cho, S.; Choe, A.; Yeom, J.; Ro, Y.G.; Kim, J.; Kang, D.H.; Lee, S.; Ko, H. Soft Sensors and Actuators for Wearable Human-Machine Interfaces. Chem. Rev. 2024, 124, 1464–1534. [Google Scholar] [PubMed]
- Dong, L.; Wang, M.; Wu, J.; Zhu, C.; Shi, J.; Morikawa, H. Stretchable, Adhesive, Self-Healable, and Conductive Hydrogel-Based Deformable Triboelectric Nanogenerator for Energy Harvesting and Human Motion Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9126–9137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, T.; Cai, J.; Xu, L.; He, T.; Wang, T.; Tian, Y.; Li, L.; Peng, Y.; Lee, C. Wearable Triboelectric Sensors Enabled Gait Analysis and Waist Motion Capture for IoT-Based Smart Healthcare Applications. Adv. Sci. 2022, 9, e2103694. [Google Scholar]
- Moon, J.; Minaya, N.H.; Le, N.A.; Park, H.C.; Choi, S.I. Can Ensemble Deep Learning Identify People by Their Gait Using Data Collected from Multi-Modal Sensors in Their Insole? Sensors 2020, 20, 4001. [Google Scholar] [CrossRef]
- Liu, L.; Guo, X.G.; Lee, C. Promoting smart cities into the 5G era with multi-field Internet of Things (IoT) applications powered with advanced mechanical energy harvesters. Nano Energy 2021, 88, 106304. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Z.; Li, H. Application of internet of things data processing based on machine learning in community sports detection. Prev. Med. 2023, 173, 107603. [Google Scholar] [CrossRef]
- Wang, W.; Yao, D.; Wang, H.; Ding, Q.; Luo, Y.; Ding, H.; Yu, J.; Zhang, H.; Tao, K.; Zhang, S.; et al. A Breathable, Stretchable, and Self-Calibrated Multimodal Electronic Skin Based on Hydrogel Microstructures for Wireless Wearables. Adv. Funct. Mater. 2024, 34, 2316339. [Google Scholar] [CrossRef]
- Hatta, F.F.; Haniff, M.; Mohamed, M.A. Enhanced-Performance Triboelectric Nanogenerator Based on Polydimethylsiloxane/Barium Titanate/Graphene Quantum Dot Nanocomposites for Energy Harvesting. ACS Omega 2024, 9, 5608–5615. [Google Scholar] [CrossRef]
- Sagar, P.; Kumar, B. PANI-reinforced-ZnS/PDMS-based flexible hybrid piezo-triboelectric nanogenerator for self-powered wearable electronics and sensing. Mater. Res. Bull. 2025, 190, 113482. [Google Scholar] [CrossRef]
- Wang, Z.X.; Duan, Y.B.; Liu, C.Y.; Wang, L.H.; Zhang, Z.Y.; Zhao, W.; Zhang, X.M.; Zhang, Y.C.; Fu, P.; Cai, H.L.; et al. High-Performance Mechano-Sensitive Piezoelectric Nanogenerator from Post-Treated Nylon-11,11 Textiles for Energy Harvesting and Human Motion Monitoring. ACS Appl. Mater. Interfaces 2025, 17, 8312–8326. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Zhang, X.T.; Liu, X.Y.; Lei, J.B. Evaluating the Consistency of Current Mainstream Wearable Devices in Health Monitoring: A Comparison Under Free-Living Conditions. J. Med. Internet Res. 2017, 19, e68. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; He, H.; Tong, J.; Wang, T.; Wang, S.; Sun, Z.; Li, W.; Zhou, S. Application of Nanogenerators in Lumbar Motion Monitoring: Fundamentals, Current Status, and Perspectives. Diagnostics 2025, 15, 2657. https://doi.org/10.3390/diagnostics15202657
Zhao Y, He H, Tong J, Wang T, Wang S, Sun Z, Li W, Zhou S. Application of Nanogenerators in Lumbar Motion Monitoring: Fundamentals, Current Status, and Perspectives. Diagnostics. 2025; 15(20):2657. https://doi.org/10.3390/diagnostics15202657
Chicago/Turabian StyleZhao, Yudong, Hongbin He, Junhao Tong, Tianchang Wang, Shini Wang, Zhuoran Sun, Weishi Li, and Siyu Zhou. 2025. "Application of Nanogenerators in Lumbar Motion Monitoring: Fundamentals, Current Status, and Perspectives" Diagnostics 15, no. 20: 2657. https://doi.org/10.3390/diagnostics15202657
APA StyleZhao, Y., He, H., Tong, J., Wang, T., Wang, S., Sun, Z., Li, W., & Zhou, S. (2025). Application of Nanogenerators in Lumbar Motion Monitoring: Fundamentals, Current Status, and Perspectives. Diagnostics, 15(20), 2657. https://doi.org/10.3390/diagnostics15202657




