Interactive Effect of C-Reactive Protein upon the Relationship Between Chlamydia trachomatis and Depression
Abstract
1. Introduction
Inflammatory Mechanisms Induced by Chlamydia
2. Methods
Statistical Analysis
3. Results
4. Discussion
4.1. Neuroimmune Interactions in Depression
4.2. Epidemiological Evidence Linking C. trachomatis, Inflammation, and Depression
4.3. Recommendations and Practical Implications
4.4. Potential Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Workowski, K.A. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections, 2022–2030; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240053779 (accessed on 1 May 2025).
- Chang, S.X.; Chen, K.K.; Liu, X.T.; Xia, N.; Xiong, P.S.; Cai, Y.M. Cross-sectional study of asymptomatic Neisseria gonorrhoeae and Chlamydia trachomatis infections in sexually transmitted disease related clinics in Shenzhen, China. PLoS ONE 2020, 15, e0234261. [Google Scholar] [CrossRef]
- Anderer, S. US Depression Rates Rise, with Variation Across Sex. JAMA 2025, 333, 2043–2044. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774. [Google Scholar] [CrossRef] [PubMed]
- Ferat-Osorio, E.; Maldonado-García, J.L.; Pavón, L. How inflammation influences psychiatric disease. World J. Psychiatry 2024, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. Burning down the house: Reinventing drug discovery in psychiatry for the development of targeted therapies. Mol. Psychiatry 2023, 28, 68–75. [Google Scholar] [CrossRef]
- Steffens, N.; Doyle, C.; Bressoud, P.F.; Ewald, H.A.S.; Ewald, P.W. Associations Between Chlamydia trachomatis Infection and Depression. Sex. Transm. Dis. 2025, 52, 554–558. [Google Scholar] [CrossRef]
- Peng, S.; Deng, J.; Zhou, Y.; Lu, Y.; Chen, Z.; Yan, W.; Huang, X. Causal associations between sexually transmitted infections, depression, and self-harm: A mendelian randomization and cross-sectional study. BMC Infect. Dis. 2024, 24, 1339. [Google Scholar] [CrossRef]
- Ghosh, N.; Chen, H.Y.; Stafford, I. The Association Between Depression and Sexually Transmitted Infections During Pregnancy Among Underserved Pregnant Patients. Sex. Transm. Dis. 2025, 52, 549–553. [Google Scholar] [CrossRef]
- Ling, H.; Luo, L.; Dai, X.; Chen, H. Fallopian tubal infertility: The result of Chlamydia trachomatis-induced fallopian tubal fibrosis. Mol. Cell. Biochem. 2022, 477, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Sousa, C.; Vale, N. Chlamydia trachomatis as a current health problem: Challenges and opportunities. Diagnostics 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The bidirectional relationship of depression and inflammation: Double trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Gottlieb, S.L.; Taylor, B.D.; Low, N.; Xu, F.; Ness, R.B. Risk of sequelae after Chlamydia trachomatis genital infection in women. J. Infect. Dis. 2010, 201, S134–S155. [Google Scholar] [CrossRef]
- Paavonen, J.; Turzanski Fortner, R.; Lehtinen, M.; Idahl, A. Chlamydia trachomatis, pelvic inflammatory disease, and epithelial ovarian cancer. J. Infect. Dis. 2021, 224 (Suppl. S2), S121–S127. [Google Scholar] [CrossRef]
- Brunham, R.C. Problems with understanding Chlamydia trachomatis immunology. J. Infect. Dis. 2022, 225, 2043–2049. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Yuan, H.; Chen, H. The role of tryptophan in Chlamydia trachomatis persistence. Front. Cell. Infect. Microbiol. 2022, 12, 931653. [Google Scholar] [CrossRef]
- Kroenke, K. PHQ-9: Global uptake of a depression scale. World Psychiatry 2021, 20, 135. [Google Scholar] [CrossRef]
- Min, S.; He, P.; Zhou, Q.; Chen, H. The dual role of cytokine responses to Chlamydia trachomatis infection in host pathogen crosstalk. Microb. Pathog. 2022, 173, 105812. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Darville, T. Pelvic inflammatory disease due to Neisseria gonorrhoeae and Chlamydia trachomatis: Immune evasion mechanisms and pathogenic disease pathways. J. Infect. Dis. 2021, 224 (Suppl. S2), S39–S46. [Google Scholar] [CrossRef]
- Fletcher, J.B.; Swendeman, D.; Reback, C.J. Mental health disorders among HIV-positive men who have sex with men engaged in care in Los Angeles, California. AIDS Care 2019, 31, 744–752. [Google Scholar] [CrossRef]
- Fond, G.; Hamdani, N.; Kapczinski, F.; Boukouaci, W.; Drancourt, N.; Dargel, A.; Oliveira, J.; Le Guen, E.; Marlinge, E.; Tamouza, R.; et al. Effectiveness and tolerance of anti-inflammatory drugs’ add-on therapy in major mental disorders: A systematic qualitative review. Acta Psychiatr. Scand. 2014, 129, 163–179. [Google Scholar] [CrossRef]
- Welk, G.; Lamoureux, N.R.; Zeng, C.; Zhu, Z.; Berg, E.; Wolff-Hughes, D.L.; Troiano, R.P. Equating NHANES monitor based physical activity to self-reported methods to enhance ongoing surveillance efforts. Med. Sci. Sports Exerc. 2023, 55, 1034. [Google Scholar] [CrossRef]
- Willett, C.L. Why Correlation Doesn’t Imply Causation: Improving Undergraduates Understanding of Research Design. Ph.D. Dissertation, University of Pittsburgh, Pittsburgh, PA, USA, 2023. [Google Scholar]
- Lu, Y.; Liu, B.P.; Tan, C.T.; Pan, F.; Larbi, A.; Ng, T.P. Lifetime pathogen burden, inflammatory markers, and depression in community-dwelling older adults. Brain Behav. Immun. 2022, 102, 124–134. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Population (n = 25,938) | C. trachomatis (+) (n = 271) | C. trachomatis (−) (n = 25,667) |
|---|---|---|---|
| C-reactive Protein | 9.2 (8.6–9.7) | 12.4 (6.6–18.3) | 9.1 (8.6–9.7) |
| Smoking Status | |||
| Never Smoked | 53.6 (52.3–54.7) | 50.3 (42.6–57.9) | 53.6 (52.4–54.8) |
| Formerly Smoked | 20.6 (19.8–21.4) | 15.6 (8.4–22.7) | 20.6 (19.8–21.5) |
| Current Smoker | 25.8 (24.8–26.9) | 34.1 (25.9–42.2) | 25.8 (24.7–26.6) |
| Chronic Kidney Disease (CKD) | 7.4 (7.0–7.8) | 7.8 (4.4–13.6) | 7.4 (7.0–7.8) |
| Cardiovascular Disease (CVD) * | 3.7 (3.4–4.1) | 1.6 (0.7–3.6) | 3.8 (3.4–4.1) |
| Diabetes ** | 5.4 (5.0–5.7) | 1.7 (0.8–3.6) | 5.4 (5.0–5.8) |
| BMI | |||
| BMI ≥ 18.5 and BMI < 25 | 31.7 (30.7–32.6) | 36.9 (29.2–44.6) | 31.6 (30.6–32.6) |
| BMI ≥ 25 and BMI < 29.9 | 33.1 (32.3–34.0) | 28.9 (22.3–35.6) | 33.2 (32.3–34.0) |
| BMI ≥ 30 and BMI < 34.9 | 19.6 (18.9–20.2) | 17.8 (10.0–25.7) | 19.6 (18.9–20.20) |
| BMI ≥ 35.0 and BMI < 39.9 | 9.0 (8.5–9.4) | 8.5 (5.2–13.6) | 9.0 (8.5–9.4) |
| BMI ≥ 40.0 | 6.7 (6.2–7.1) | 7.8 (5.0–11.9) | 6.7 (6.2–7.1) |
| Age ** | 39.6 (0.13) | 30.2 (30.4) | 39.6 (0.13) |
| Gender Female | 50.5 (49.9–51.1) | 62.1 (54.6–70.0) | 50.4 (50.1–51.2) |
| Family Poverty–Income Ratio (PIR < 2) ** | 32.5 (31.0–34.0) | 51.0 (42.1–59.8) | 32.4 (30.9–33.9) |
| Ethnicity ** | |||
| Non-Hispanic White | 67.9 (65.7–70.1) | 37.7 (29.5–45.9) | 68.1 (65.9–70.3) |
| Non-Hispanic Black | 11.5 (10.3–12.7) | 35.5 (28.7–42.3) | 11.3 (10.1–12.5) |
| Hispanic | 14.6 (12.8–16.2) | 21.5 (15.3–27.7) | 14.5 (12.8–16.2) |
| Other | 6.0 (5.5–6.6) | 5.3 (2.7–10.0) | 6.0 (5.5–6.6) |
| Education Level ** | |||
| Some High School | 15.9 (14.8–17.0) | 23.5 (17.9–29.2) | 15.8 (14.7–17.0) |
| High School Graduate | 22.5 (21.4–23.6) | 26.0 (19.1–32.3) | 22.5 (21.4–23.6) |
| Some College or Above | 61.6 (59.8–63.3) | 50.8 (43.4–58.2) | 61.7 (59.9–63.4) |
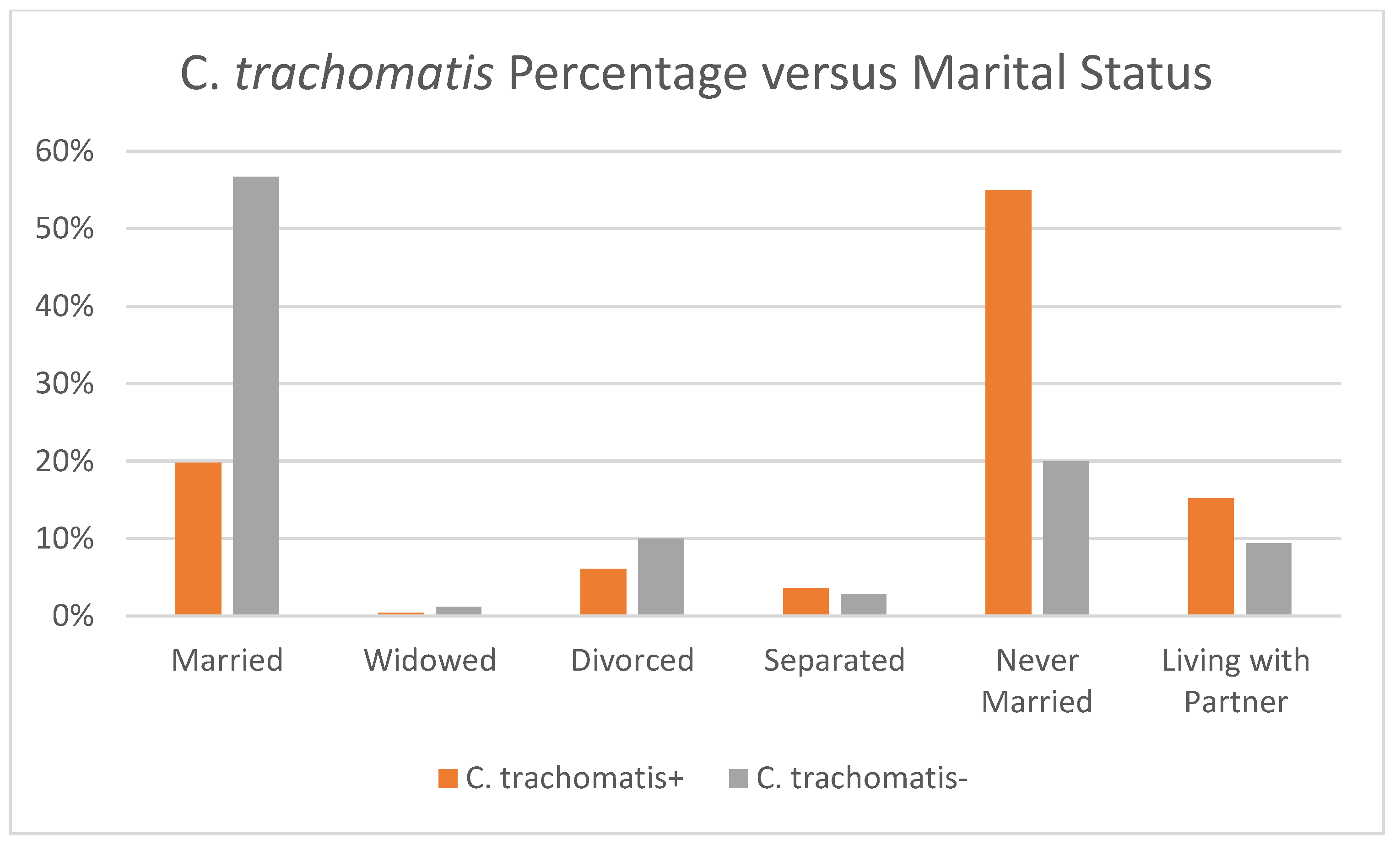
| Marital Status ** | |||
| Married | 56.4 (55.1–57.7) | 19.8 (12.0–27.5) | 56.7 (55.3–58.0) |
| Widowed | 1.2 (1.0–1.3) | 0.4 (0.10–1.6) | 1.2 (1.0–1.3) |
| Divorced | 10.0 (9.5–10.5) | 6.1 (3.1–11.6) | 10.0 (9.5–10.6) |
| Separated | 2.8 (2.6–3.1) | 3.6 (2.0–6.4) | 2.8 (2.5–3.1) |
| Never Married | 20.2 (19.1–21.4) | 55.0 (46.3–63.7) | 20.0 (18.8–21.1) |
| Living with Partner | 9.4 (8.6–10.0) | 15.2 (9.6–20.7) | 9.4 (8.8–10.0) |
| Depression ** | 8.0 (7.4–8.6) | 15.3 (9.7–20.9) | 7.9 (7.3–8.5) |
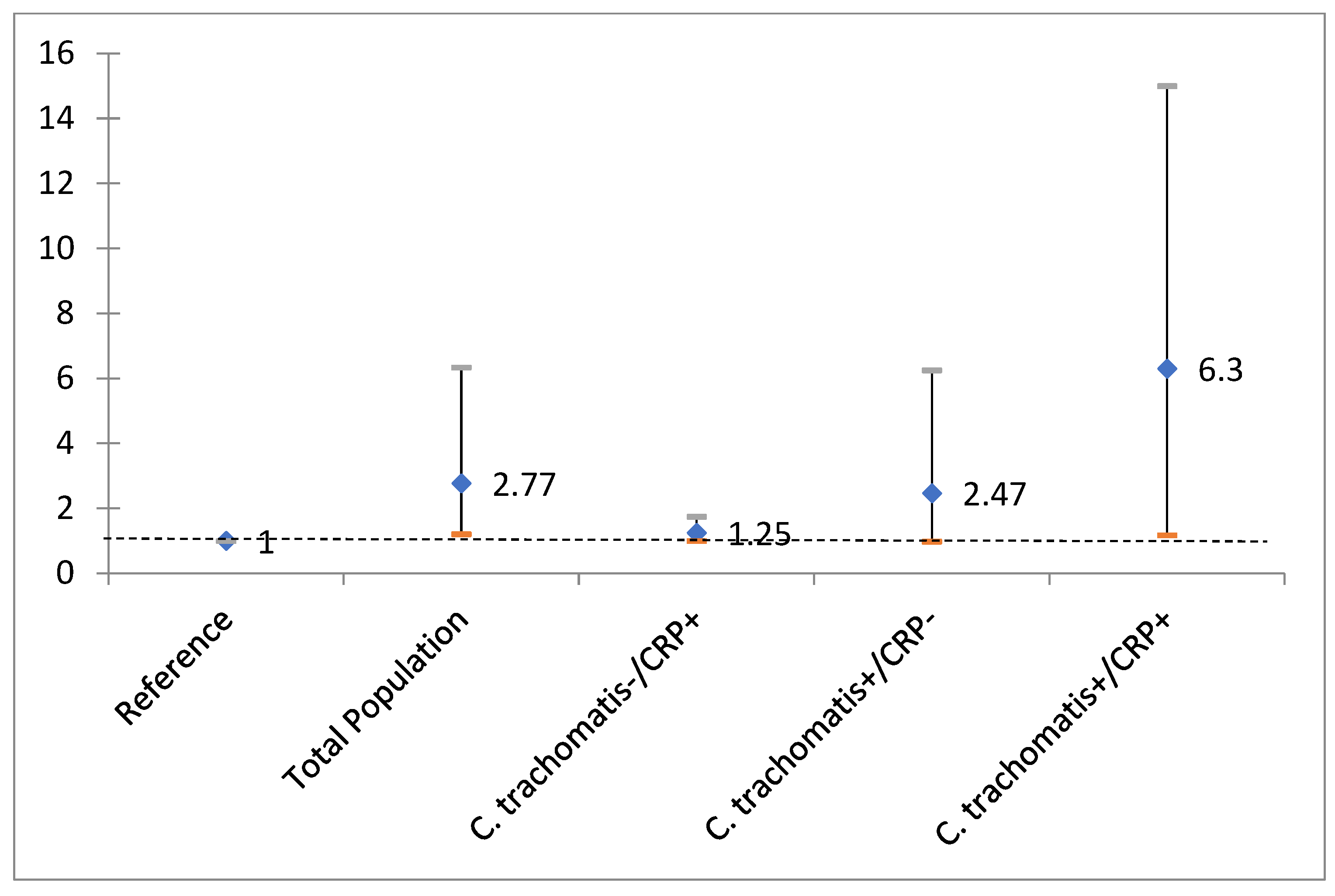
| Total Population OR (95% CI) | C. trachomatisydia− High CRP+ OR (95% CI) | C. trachomatis+ High CRP− OR (95% CI) | C. trachomatis+ High CRP+ OR (95% CI) | |
|---|---|---|---|---|
| Chlamydia/CRP | 2.77 (1.21–6.34) * | 1.25 (1.00–1.75) | 2.47 (0.98–6.25) | 6.30 (1.18–33.49) * |
| Smoking Status | ||||
| Never Smoked (Ref.) | Ref | Ref | Ref | Ref |
| Former Smoker | 1.18 (0.83–1.67) | 1.16 (0.82–1.63) | 1.13 (0.78–1.62) | 1.39 (0.70–2.75) |
| Current Smoker | 2.54 (2.06–3.13) ** | 2.52 (2.05–3.10) ** | 2.63 (2.10–3.30) ** | 1.76 (0.98–3.16) |
| Chronic Kidney Disease (CKD) | 1.05 (0.79–1.40) | 1.06 (0.79–1.41) | 0.93 (0.69–1.26) | 1.54 (0.73–3.23) |
| Cardiovascular Disease (CVD) | 2.23 (1.55–3.51) ** | 2.31 (1.53–3.48) ** | 2.47 (1.64–3.72) ** | 1.90 (0.87–4.17) |
| Diabetes | 1.33 (0.87–2.03) | 1.30 (0.85–1.99) | 1.34 (0.88–2.04) | 1.15 (0.53–2.49) |
| BMI | ||||
| Ref BMI ≥ 18.5 and BMI < 25 | Ref | Ref | Ref | Ref |
| BMI ≥ 25 and BMI < 29.9 | 1.00 (0.79–1.26) | 1.00 (0.80–1.26) | 0.95 (0.76–1.20) * | 1.21 (0.37–3.92) |
| BMI ≥ 30 and BMI < 34.9 | 1.32 (0.98–1.77) | 1.32 (0.97–1.80) | 1.39 (1.01–1.91) | 0.61 (0.26–1.46) |
| BMI ≥ 35.0 and BMI < 39.9 | 1.27 (0.91–1.78) | 1.21 (0.86–1.69) | 1.18 (0.79–1.76) | 0.89 (0.30–2.66) |
| BMI ≥ 40.0 | 1.81 (1.20–2.72) ** | 1.70 (1.08–2.68) | 1.85 (1.12–3.06) | 1.11 (0.41–3.00) |
| Age | 1.02 (1.01–1.03) ** | 1.02 (1.01–1.03) ** | 1.02 (1.01–1.03) ** | 1.02 (0.99–1.05) |
| Gender (Ref. Female) | 1.90 (1.55–2.33) ** | 1.86 (1.51–2.29) ** | 1.90 (1.56–2.32) ** | 1.73 (0.94–3.21) |
| Family Poverty–Income Ratio (Ref: PIR < 2) | 2.55 (2.12–3.06) ** | 2.60 (2.16–3.11) ** | 2.53 (2.01–3.18) ** | 3.00 (1.53–5.89) |
| Ethnicity | ||||
| Non-Hispanic White | Ref | Ref | Ref | Ref |
| Non-Hispanic Black | 1.04 (0.84–1.28) | 1.01 (0.80–1.27) | 1.20 (0.93–1.55) | 0.43 (0.22–0.86) |
| Hispanic | 0.96 (0.75–1.24) | 0.97 (0.75–1.27) | 0.98 (0.74–1.30) | 0.74 (0.43–1.29) |
| Other | 0.85 (0.47–1.52) | 0.87 (0.49–1.56) | 0.90 (0.49–1.65) | 0.39 (0.06–2.53) |
| Education Level | ||||
| Some College or Above | Ref | Ref | Ref | Ref |
| Some High School | 1.22 (0.98–1.53) | 1.21 (0.97–1.53) | 1.21 (0.92–1.60) | 1.29 (0.69–2.41) |
| High School Graduate | 1.27 (1.02–1.58) ** | 1.26 (1.01–1.57) ** | 1.23 (1.00–1.01) | 1.46 (0.70–3.04) |
| Marital Status | 1.54 (1.28–1.87) ** | 1.58 (1.29–1.92) ** | 1.55 (1.26–1.91) ** | 1.59 (0.91–2.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, K.; Davis, W.S.; Banerjee, S. Interactive Effect of C-Reactive Protein upon the Relationship Between Chlamydia trachomatis and Depression. Diagnostics 2025, 15, 2638. https://doi.org/10.3390/diagnostics15202638
Banerjee K, Davis WS, Banerjee S. Interactive Effect of C-Reactive Protein upon the Relationship Between Chlamydia trachomatis and Depression. Diagnostics. 2025; 15(20):2638. https://doi.org/10.3390/diagnostics15202638
Chicago/Turabian StyleBanerjee, Kay, W. Sumner Davis, and Sri Banerjee. 2025. "Interactive Effect of C-Reactive Protein upon the Relationship Between Chlamydia trachomatis and Depression" Diagnostics 15, no. 20: 2638. https://doi.org/10.3390/diagnostics15202638
APA StyleBanerjee, K., Davis, W. S., & Banerjee, S. (2025). Interactive Effect of C-Reactive Protein upon the Relationship Between Chlamydia trachomatis and Depression. Diagnostics, 15(20), 2638. https://doi.org/10.3390/diagnostics15202638







