The Hidden Signal: P Wave Morphology and In-Hospital Mortality in Acute Pulmonary Embolism
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Clinical Data Collection
2.3. Electrocardiographic Assessment
- Normal: Smooth, monophasic deflection of typical duration and amplitude.
- Biphasic: Initial positive deflection followed by a terminal negative component.
- Notched: Double-peaked P wave with two positive deflections.
- Peaked: Sharply pointed P wave exceeding normal amplitude thresholds.
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association Between P Wave Morphology and Mortality
3.3. Univariate Logistic Regression
3.4. Multivariable Logistic Regression
3.5. Effect Sizes and Clinical Interpretation
3.6. Subgroup Analysis
4. Discussion
4.1. Main Findings
4.2. Comparison with Existing Literature
4.3. Pathophysiological Considerations
- Right atrial overload and stretch: sudden increases in pulmonary arterial pressure due to thromboembolic obstruction elevate right ventricular afterload. This leads to right atrial pressure elevation, which is reflected on the surface ECG as peaked or biphasic P waves. The severity of such atrial strain may directly correlate with hemodynamic compromise and, consequently, increased risk of death [21].
- Electrical conduction abnormalities: abnormal P waves may also reflect delayed interatrial conduction or anisotropy of atrial depolarization. This electrical instability could predispose patients to atrial arrhythmias, which are known to complicate the course of PE and worsen prognosis [22].
- Indirect marker of right ventricular dysfunction: since right atrial pressure is closely tied to right ventricular function, abnormal P wave morphologies may act as a surrogate for right ventricular strain, a well-established determinant of PE-related mortality [21].
4.4. Clinical Implications
- Rapid bedside risk stratification: unlike imaging modalities or biomarker assays, ECG is universally available, non-invasive, and inexpensive. Recognition of biphasic or peaked P waves at presentation may provide clinicians with an immediate signal of elevated risk.
- Adjunct to established scores: while the PESI score and its simplified version are validated tools, they require integration of multiple variables and may not be readily available at the bedside. Incorporating P wave morphology could refine these scores and improve their predictive accuracy.
- Guidance for monitoring and therapy: patients identified as high-risk based on P wave abnormalities may warrant closer hemodynamic monitoring, earlier echocardiographic assessment of right ventricular function, and consideration of advanced therapies such as systemic thrombolysis or catheter-based interventions.
- Educational reinforcement: given that P wave morphology is often underemphasized in routine ECG interpretation, our findings highlight the need for clinicians to re-engage with basic ECG principles when evaluating patients with acute PE.
4.5. Strengths of the Study
4.6. Limitations
5. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabrhel, C.; Rosovsky, R.; Channick, R.; Jaff, M.R.; Weinberg, I.; Sundt, T.; Dudzinski, D.M.; Rodriguez-Lopez, J.; Parry, B.A.; Harshbarger, S.; et al. A Multidisciplinary Pulmonary Embolism Response Team. Chest 2016, 150, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D. Simplification of the Pulmonary Embolism Severity Index for Prognostication in Patients with Acute Symptomatic Pulmonary Embolism. Arch. Intern. Med. 2010, 170, 1383. [Google Scholar] [CrossRef]
- Silva, B.V.; Calé, R.; Menezes, M.N.; Jorge, C.; Pinto, F.J.; Caldeira, D. How to Predict Prognosis in Patients with Acute Pulmonary Embolism? Recent Advances. Kardiol. Pol. 2023, 81, 684–691. [Google Scholar] [CrossRef]
- Kukla, P.; Długopolski, R.; Krupa, E.; Furtak, R.; Szełemej, R.; Mirek-Bryniarska, E.; Jastrzębski, M.; Nowak, J.; Wańczura, P.; Bryniarski, L. Electrocardiography and Prognosis of Patients with Acute Pulmonary Embolism. Cardiol. J. 2011, 18, 648–653. [Google Scholar] [CrossRef]
- Nilsson, L.T.; Andersson, T.; Carlberg, B.; Johansson, L.Å.; Söderberg, S. Electrocardiographic Abnormalities and NT-proBNP Levels at Long-Term Follow-up of Patients with Dyspnea after Pulmonary Embolism. Scand. Cardiovasc. J. 2024, 58, 2373090. [Google Scholar] [CrossRef]
- Meijer, F.M.M.; Kies, P.; Jongbloed, M.R.M.; Van Wijngaarden, S.E.; Swenne, C.A.; Man, S.; Schalij, M.J.; De Vries-Bouwstra, J.K.; Vliegen, H.W. ECG Derived Ventricular Gradient Exceeds Echocardiography in the Early Detection of Pulmonary Hypertension in Scleroderma Patients. Int. J. Cardiol. 2018, 273, 203–206. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, M.J.; Cho, Y.; Kim, J.; Lee, E.; Son, D.; Kim, S.-Y.; Soh, M.S. Screening for RV Dysfunction Using Smartphone ECG Analysis App: Validation Study with Acute Pulmonary Embolism Patients. J. Clin. Med. 2024, 13, 4792. [Google Scholar] [CrossRef]
- Gokhale, T.A.; Riek, N.T.; Medoff, B.; Ji, R.Q.; Rivera-Lebron, B.; Sejdic, E.; Akcakaya, M.; Saba, S.F.; Al-Zaiti, S.; Toma, C. Artificial Intelligence-Driven Electrocardiogram Analysis for Risk Stratification in Pulmonary Embolism. Eur. Heart J.-Digit. Health 2025, 6, 989–996. [Google Scholar] [CrossRef]
- Magnani, J.W.; Gorodeski, E.Z.; Johnson, V.M.; Sullivan, L.M.; Hamburg, N.M.; Benjamin, E.J.; Ellinor, P.T. P Wave Duration Is Associated with Cardiovascular and All-Cause Mortality Outcomes: The National Health and Nutrition Examination Survey. Heart Rhythm 2011, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Asadi Anar, M.; Ansari, A.; Erabi, G.; Rahmanian, M.; Movahedi, M.; Chichagi, F.; Deravi, N.; Taghavi, F.; Kazemi, B.; Javanshir, E.; et al. Prognostic Value of Fragmented QRS in Acute Pulmonary Embolism: A Cross-Sectional-Analytic Study of the Iranian Population. Am. J. Cardiovasc. Dis. 2023, 13, 21–28. [Google Scholar] [PubMed]
- Digby, G.C.; Kukla, P.; Zhan, Z.; Pastore, C.A.; Piotrowicz, R.; Schapachnik, E.; Zareba, W.; Bayés De Luna, A.; Pruszczyk, P.; Baranchuk, A.M. The Value of Electrocardiographic Abnormalities in the Prognosis of Pulmonary Embolism: A Consensus Paper. Noninvasive Electrocardiol. 2015, 20, 207–223. [Google Scholar] [CrossRef]
- Di Marca, S.; Cilia, C.; Campagna, A.; D’Arrigo, G.; ElHafeez, S.A.; Tripepi, G.; Puccia, G.; Pisano, M.; Mastrosimone, G.; Terranova, V.; et al. Comparison of Wells and Revised Geneva Rule to Assess Pretest Probability of Pulmonary Embolism in High-Risk Hospitalized Elderly Adults. J. Am. Geriatr. Soc. 2015, 63, 1091–1097. [Google Scholar] [CrossRef]
- García Gómez, L.C.; Castilla Guerra, L.; Gandullo Moro, M.; Cano Alba, R.; Paniagua García, M.; Colmenero Camacho, M.Á. Embolia pulmonar en los pacientes muy ancianos. Un reto diagnóstico. Rev. Clínica Española 2019, 219, 310–314. [Google Scholar] [CrossRef]
- Sławek-Szmyt, S.; Araszkiewicz, A.; Jankiewicz, S.; Smukowska-Gorynia, A.; Grygier, M.; Janus, M.; Lesiak, M.; Mularek-Kubzdela, T. Association of Electrocardiographic Signs of Right Ventricular Hypertrophy and Clot Localization in Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2022, 11, 625. [Google Scholar] [CrossRef]
- Escobar, C.; Jiménez, D.; Martí, D.; Lobo, J.L.; Díaz, G.; Gallego, P.; Vidal, R.; Barrios, V.; Sueiro, A. Prognostic value of electrocardiographic findings in hemodynamically stable patients with acute symptomatic pulmonary embolism. Rev. Esp. Cardiol. 2008, 61, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.D.; Macedo, F.Y.; Hamzeh, I.R.; Birnbaum, Y. Correlation of Right Atrial Enlargement on ECG to Right Atrial Volume by Echocardiography in Patients with Pulmonary Hypertension. J. Electrocardiol. 2017, 50, 555–560. [Google Scholar] [CrossRef]
- Geibel, A.; Zehender, M.; Kasper, W.; Olschewski, M.; Klima, C.; Konstantinides, S.V. Prognostic Value of the ECG on Admission in Patients with Acute Major Pulmonary Embolism. Eur. Respir. J. 2005, 25, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Ribeiro, A.L.P.; Platonov, P.G.; Cygankiewicz, I.; Soliman, E.Z.; Gorenek, B.; Ikeda, T.; Vassilikos, V.P.; Steinberg, J.S.; Varma, N.; et al. P Wave Parameters and Indices: A Critical Appraisal of Clinical Utility, Challenges, and Future Research—A Consensus Document Endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardiology. Circ. Arrhythmia Electrophysiol. 2022, 15, e010435. [Google Scholar] [CrossRef] [PubMed]
- Raghubeer, N.; Lahri, S.; Hendrikse, C. The Value of Electrocardiography in Predicting Inpatient Mortality in Patients with Acute Pulmonary Embolism: A Cross Sectional Analysis. Afr. J. Emerg. Med. 2024, 14, 65–69. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, Y.; He, H.; Li, Q.; Wang, Z.; Chen, Y.; Zhu, L.; Zheng, S.; Yang, F.; Zhai, Z.; et al. ECG Abnormalities and Biomarkers Enable Rapid Risk Stratification in Normotensive Patients with Acute Pulmonary Embolism. Clin. Respir. J. 2025, 19, e70060. [Google Scholar] [CrossRef]
- Douedi, S.; Douedi, H. P Wave. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Levis, J.T. ECG Diagnosis: Pulmonary Embolism. Perm. J. 2011, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Horie, M. Biphasic P Wave in Inferior Leads and the Development of Atrial Fibrillation. J. Arrhythm. 2015, 31, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, M.D.; Covino, M.; Passaro, A.; Benedetto, M.; D’Angelo, L.; Galizia, G.; Fabbri, I.S.; Pagano, T.; Portoraro, A.; Guarino, M.; et al. Predicting In-Hospital Mortality in Pulmonary Embolism Patients: Development and External Validation of the PATHOS Score. Clin. Exp. Emerg. Med. 2022, 10, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, I.; Kenttä, T.V.; Passi, J.; Haukilahti, M.A.E.; Eranti, A.; Holkeri, A.; Aro, A.L.; Kerola, T.; Noponen, K.; Seppänen, T.; et al. Prognostic Value of P-Wave Morphology in General Population. Europace 2023, 25, 164–174. [Google Scholar] [CrossRef]

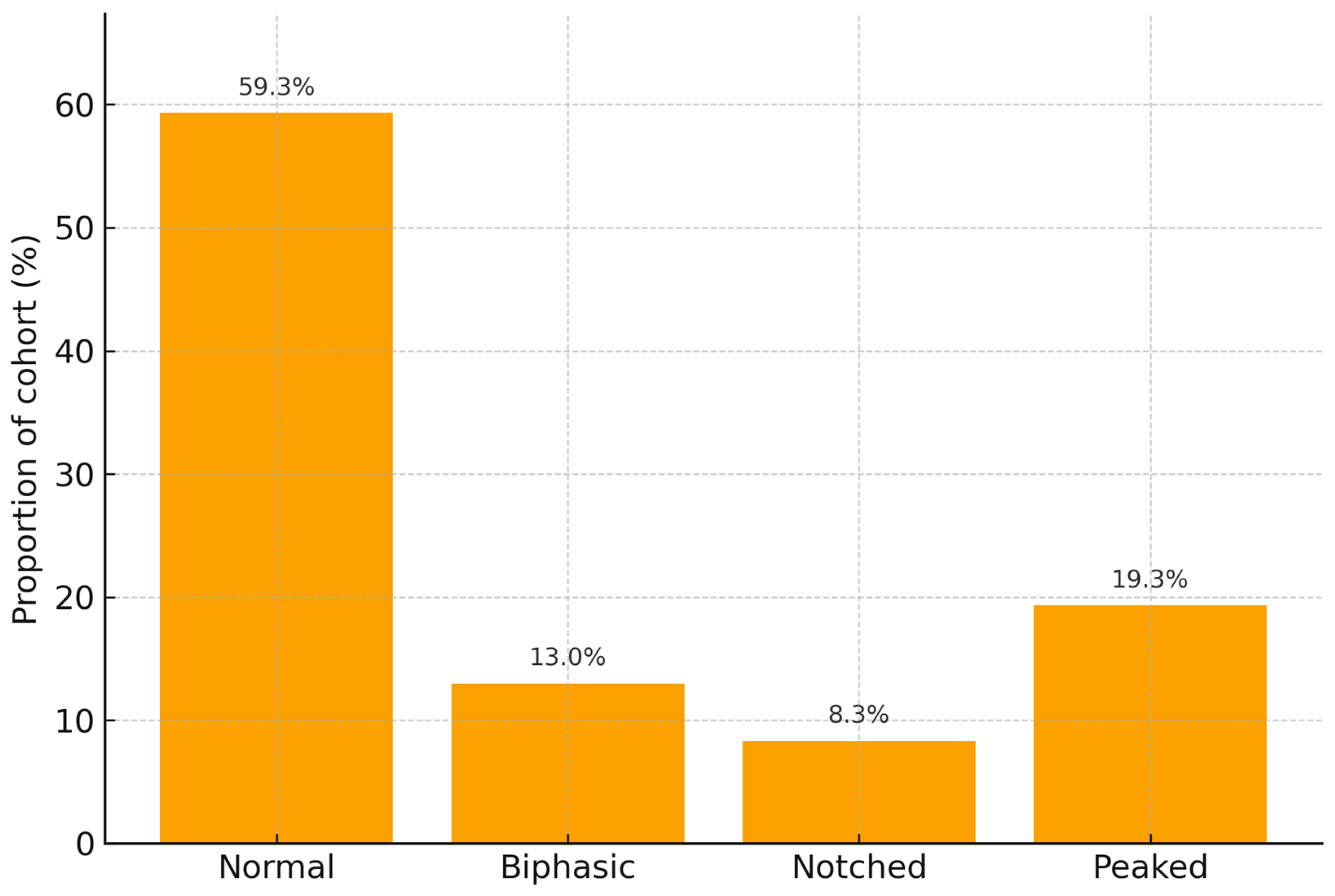

| P Wave Shape | n | Age (Years, Mean ± SD) | Male Sex n (%) | PESI (Median, IQR) | Oxygen Saturation (%) | Mortality n (%) |
|---|---|---|---|---|---|---|
| Normal | 178 | 65.2 ± 11.0 | 98 (55.1%) | 84 (72–98) | 92.3 ± 4.0 | 5 (2.8%) |
| Biphasic | 39 | 65.1 ± 13.3 | 24 (61.5%) | 85 (74–96) | 93.1 ± 3.9 | 12 (30.8%) |
| Notched | 25 | 63.2 ± 14.1 | 13 (52.0%) | 89 (79–97) | 91.2 ± 4.1 | 1 (4.0%) |
| Peaked | 58 | 62.3 ± 11.9 | 32 (55.2%) | 88 (72–100) | 92.8 ± 4.3 | 10 (17.2%) |
| p-value | - | 0.324 | 0.870 | 0.691 | 0.246 | <0.001 |
| P Wave Shape | Odds Ratio (OR) | 95% CI | p-Value |
|---|---|---|---|
| Biphasic | 15.38 | 5.02–47.10 | <0.001 |
| Notched | 1.44 | 0.16–12.87 | 0.743 |
| Peaked | 7.21 | 2.35–22.10 | 0.001 |
| Predictor | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|
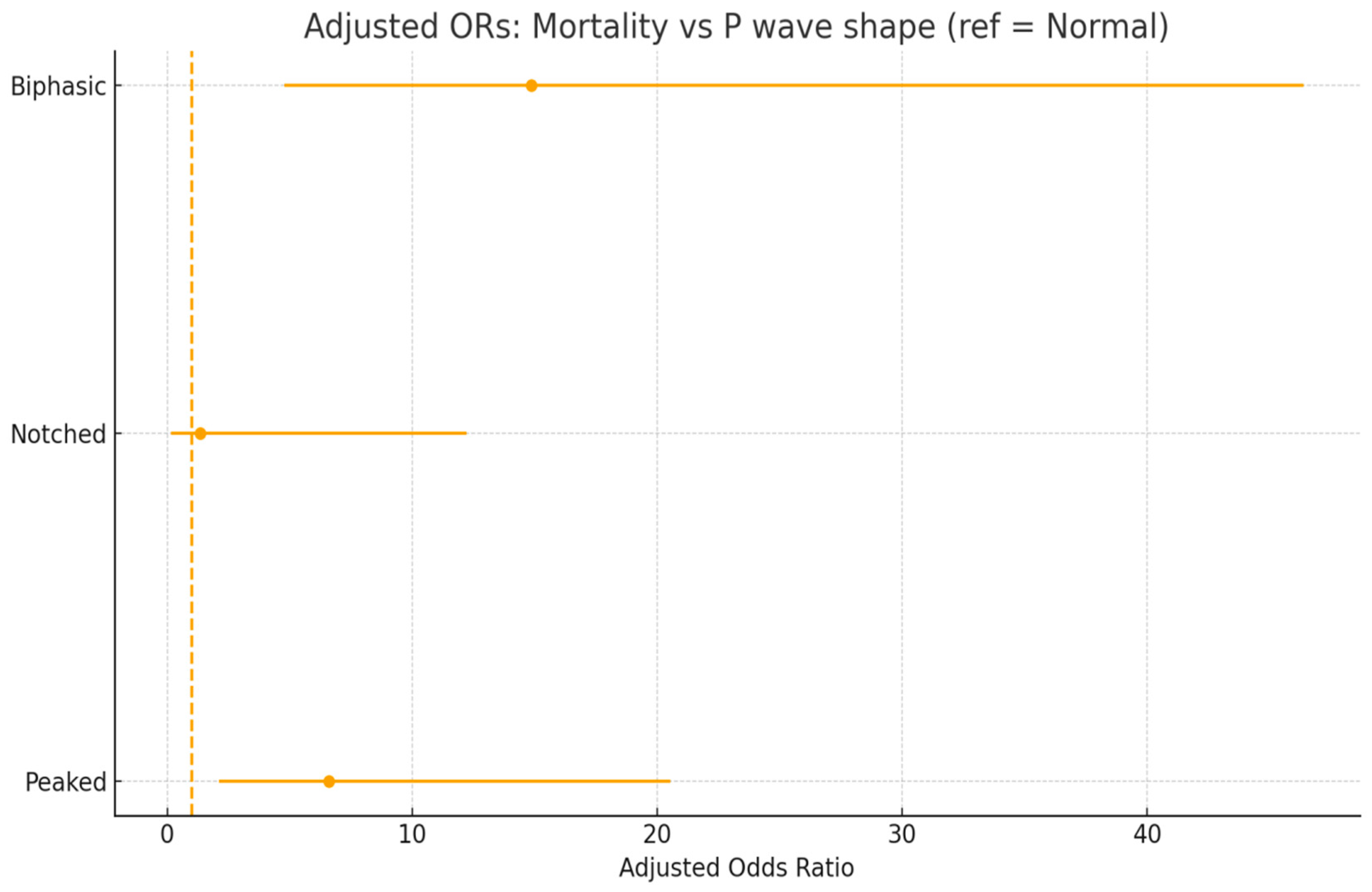
| Biphasic P wave | 14.87 | 4.77–46.37 | <0.001 |
| Notched P wave | 1.34 | 0.15–12.23 | 0.793 |
| Peaked P wave | 6.58 | 2.11–20.53 | 0.001 |
| Age | 0.97 | 0.94–1.01 | 0.154 |
| Male sex | 1.94 | 0.78–4.82 | 0.155 |
| PESI score | 1.02 | 0.99–1.04 | 0.197 |
| Oxygen saturation | 1.03 | 0.93–1.15 | 0.573 |
| P Wave Shape | Mortality n/N (%) | Absolute Risk Difference vs. Normal | Univariate OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Normal | 5/178 (2.8%) | Reference | Reference | Reference |
| Biphasic | 12/39 (30.8%) | +28.0% | 15.38 (5.02–47.10) | 14.87 (4.77–46.37) |
| Peaked | 10/58 (17.2%) | +14.4% | 7.21 (2.35–22.10) | 6.58 (2.11–20.53) |
| Notched | 1/25 (4.0%) | +1.2% | 1.44 (0.16–12.87) | 1.34 (0.15–12.23) |
| P Wave Morphology | Mortality % (PESI I–III) | p Value | Mortality % (PESI IV–V) | p Value |
|---|---|---|---|---|
| Normal | 3.6% | — | 9.8% | — |
| Biphasic | 18.2% | 0.014 | 38.5% | <0.001 |
| Peaked | 9.1% | 0.037 | 26.9% | 0.002 |
| Notched | 4.5% | 0.712 | 8.0% | 0.655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinezan, C.; Buzle, A.M.; Hiceag, M.L.; Rus, C.B. The Hidden Signal: P Wave Morphology and In-Hospital Mortality in Acute Pulmonary Embolism. Diagnostics 2025, 15, 2636. https://doi.org/10.3390/diagnostics15202636
Cinezan C, Buzle AM, Hiceag ML, Rus CB. The Hidden Signal: P Wave Morphology and In-Hospital Mortality in Acute Pulmonary Embolism. Diagnostics. 2025; 15(20):2636. https://doi.org/10.3390/diagnostics15202636
Chicago/Turabian StyleCinezan, Corina, Alexandra Manuela Buzle, Maria Luiza Hiceag, and Camelia Bianca Rus. 2025. "The Hidden Signal: P Wave Morphology and In-Hospital Mortality in Acute Pulmonary Embolism" Diagnostics 15, no. 20: 2636. https://doi.org/10.3390/diagnostics15202636
APA StyleCinezan, C., Buzle, A. M., Hiceag, M. L., & Rus, C. B. (2025). The Hidden Signal: P Wave Morphology and In-Hospital Mortality in Acute Pulmonary Embolism. Diagnostics, 15(20), 2636. https://doi.org/10.3390/diagnostics15202636






