Peripheral Odontogenic Keratocyst of the Gingiva: A Systematic Review of the Literature and Case Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Analysis
2.1.1. Protocol and Registration
2.1.2. Search Strategy
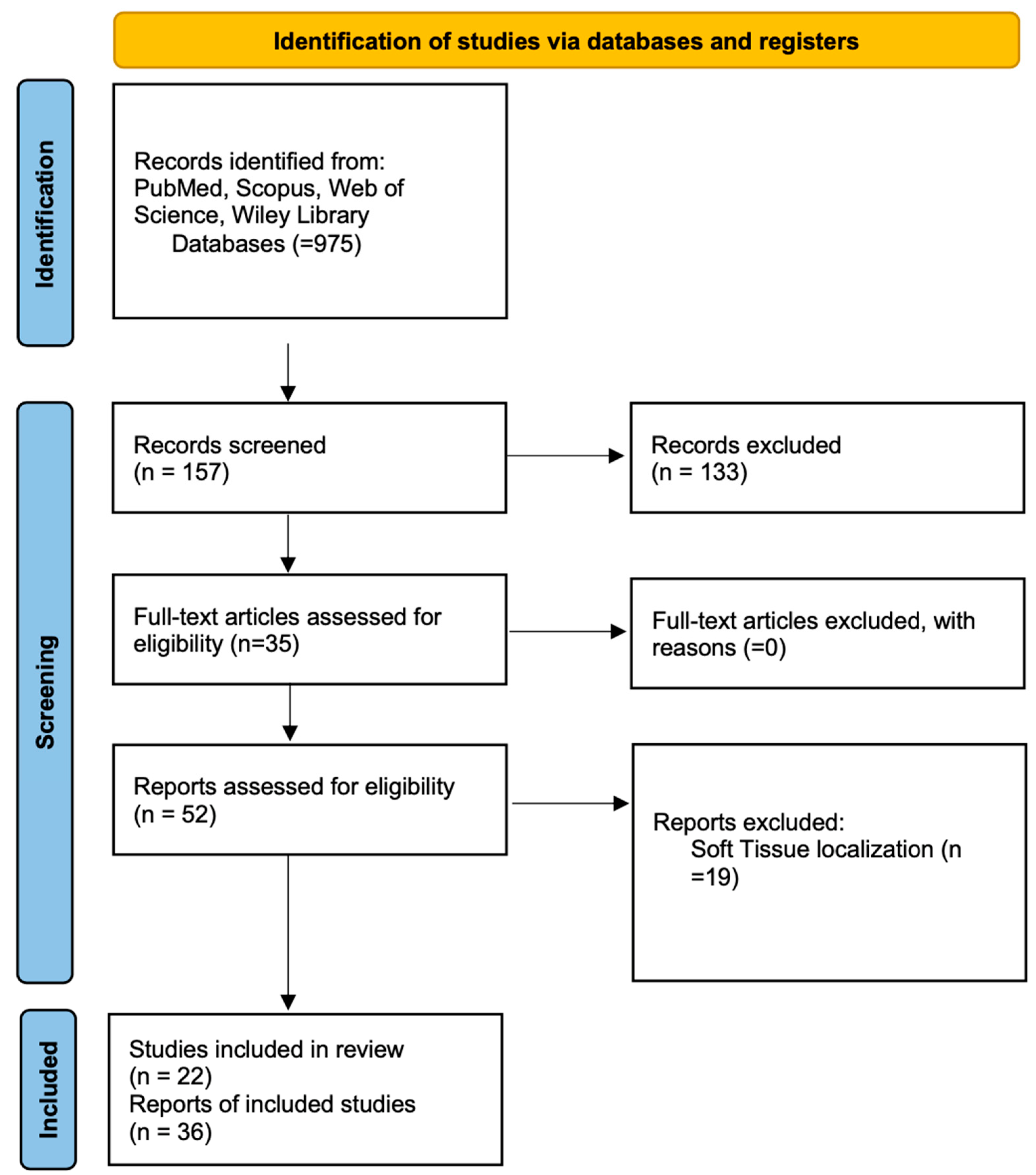
3. Results
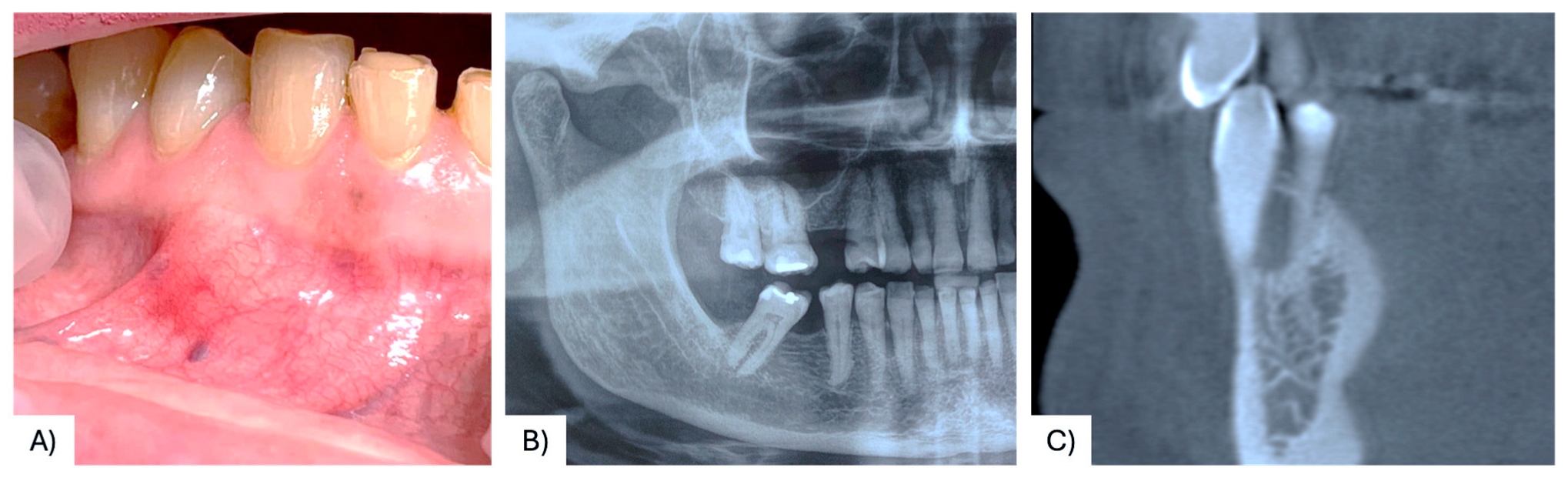
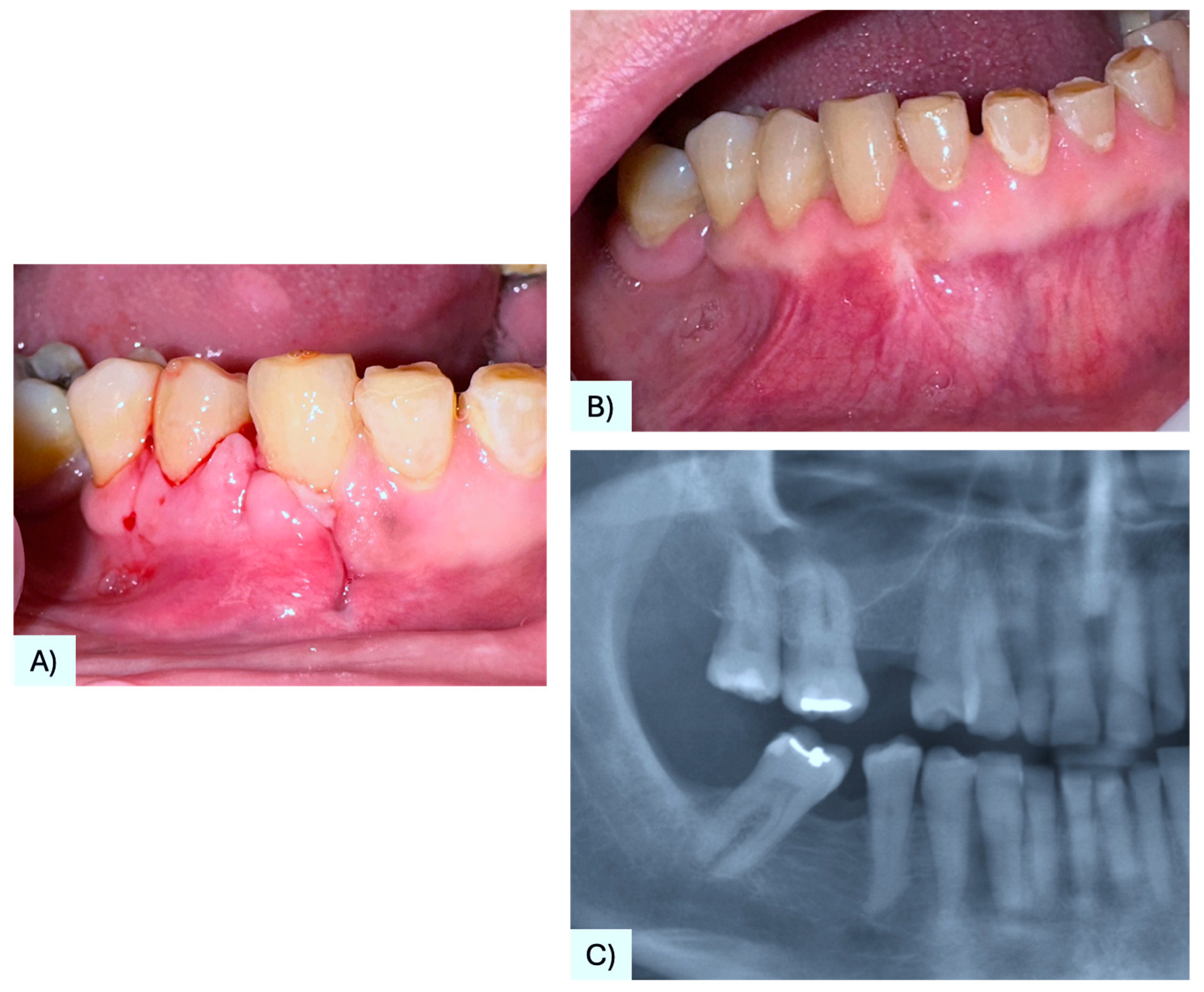
3.1. Case Presentation
3.2. Literature Analysis
3.3. Quality Assessment and Risk of Bias
4. Discussion
4.1. Pathogenesis
4.2. Clinical and Radiographical Features
4.3. Histopathological Features
4.4. Differential Diagnosis of Peripheral Odontogenic Lesion
4.5. Recurrences and Prognosis
4.6. Treatment Modalities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Mendoza, I.L.; Aguirre-Urizar, J.M.; Villatoro-Ugalde, V.; Magaña-Quiñones, J.; Lana-Ojeda, J.; Mosqueda-Taylor, A. Peripheral odontogenic keratocyst: Clinicopathological and immunohistochemical characterization. Oral Dis. 2022, 28, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Romero, M.; Serrera-Figallo, M.; Alberdi-Navarro, J.; Cabezas-Talavero, J.; Romero-Ruiz, M.; Torres-Lagares, D.; Aguirre-Urizar, J.; Gutierrez-Perez, J. Maxillary peripheral keratocystic odontogenic tumor. A clinical case report. J. Clin. Exp. Dent. 2017, 9, e167–e171. [Google Scholar] [CrossRef]
- Brooks, J.K.; Sultan, A.S.; Rabkin, M.P.; Sands, J.M.; Everett, D.E.; Price, J.B.; Basile, J.R. Recurrent peripheral odontogenic keratocyst: Review of the literature and presentation of a novel case initially masquerading as an atypical infected lateral periodontal cyst. J Stomatol. Oral Maxillofac. Surg. 2024, 125, 101540. [Google Scholar] [CrossRef] [PubMed]
- Villota, M.H.-D.; Pampin-Martínez, M.M.; Moran-Soto, J.; Cebrián-Carretero, J.-L. Peripheral odontogenic keratocyst. A Case report. J. Clin. Exp. Dent. 2023, 15, e169–e172. [Google Scholar] [CrossRef]
- Shathur, A.; Patel, B.; Pitiyage, G.; Cameron, S.; Hyde, N. Odontogenic keratocyst located in the retromolar trigone. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, e82–e85. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yih, W.; Zieper, M.; Kratochvil, F.; Stewart, J. Immunohistochemical analysis of the expression of p53, bcl-2, and Ki-67 in peripheral odontogenic keratocyst and gingival cyst of the adult. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2000, 90, 493. [Google Scholar]
- Rodrigues, B.; Israel, M.; de Moura, K.; Pinheiro, G.; Carlos, R.; Pires, F. Peripheral odontogenic keratocyst: Report of two new cases and review of the literature. J. Clin. Exp. Dent. 2020, 12, e1005–e1010. [Google Scholar] [CrossRef]
- Precheur, H.V.; Krolls, S.O. An unusual presentation of an odontogenic keratocyst in the buccal space: Case report. J. Oral Maxillofac. Surg. 2009, 67, 2513–2515. [Google Scholar] [CrossRef] [PubMed]
- Stoelinga, P.J.; Cohen, M.M., Jr.; Morgan, A.F. The origin of keratocysts in the basal cell nevus syndrome. J. Oral. Surg. 1975, 33, 659–663. [Google Scholar] [PubMed]
- Buchner, A.; Hansen, L.S. The histomorphologic spectrum of the gingival cyst in the adult. Oral Surg. Oral Med. Oral Pathol. 1979, 48, 532–539. [Google Scholar] [CrossRef]
- Dayan, D.; Buchner, A.; Gorsky, M.; Harel-Raviv, M. The peripheral odontogenic keratocyst. Int. J. Oral Maxillofac. Surg. 1988, 17, 81–83. [Google Scholar] [CrossRef]
- Chehade, A.; Daley, T.D.; Wysocki, G.P.; Miller, A.S. Peripheral odontogenic keratocyst. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 494–497. [Google Scholar] [CrossRef]
- Fardal, O.; Johannessen, A.C. Rare case of keratin-producing multiple gingival cysts. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Ide, F.; Shimoyama, T.; Horie, N. Peripheral odontogenic keratocyst: A report of 2 cases. J. Periodontol. 2002, 73, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Owings, J.R.; Muller, S. Peripheral odontogenic keratocyst: Report of two cases and review of the literature. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontology 2005, 99, 71–78. [Google Scholar] [CrossRef]
- Preston, R.D.; Narayana, N. Peripheral odontogenic keratocyst. J. Periodontol. 2005, 76, 2312–2315. [Google Scholar] [CrossRef]
- Mozaffari, E.; Marmor, D.S.; Alawi, F. Odontogenic keratocyst with a misleading clinical and radiologic appearance. Quintessence Int. 2008, 38, 837–841. [Google Scholar]
- Kinaia, B.M.; Kinaia, M.; Graham, J.; Villaneuva, N.P.; Van Winkle, D.; Dawood, A.; Neely, A.L. Odontogenic keratocyst management using guided tissue regeneration: Literature review—Two case reports. Clin. Adv. Periodontics. 2024, 1–10. [Google Scholar] [CrossRef]
- Faustino, S.E.S.; Pereira, M.C.; Rossetto, A.C.; Oliveira, D.T. Recurrent peripheral odontogenic keratocyst: A case report. Dentomaxillofacial Radiol. 2008, 37, 412–414. [Google Scholar] [CrossRef]
- Ide, F.; Mishima, K.; Saito, I.; Kusama, K. Rare peripheral odontogenic tumors: Report of 5 cases and comprehensive review of the literature. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 106, e22–e28. [Google Scholar] [CrossRef]
- Jinbu, Y.; Ueno, Y.; Obi, Y.; Ikeda, K.; Kusama, M.; Tsukinoki, K. Peripheral keratocystic odontogenic tumour: A case report. Oral Surg. 2009, 2, 95–98. [Google Scholar] [CrossRef]
- Vij, R.; Vij, H.; Gupta, V.; Sengupta, S. Odontogenic keratocyst: A peripheral variant. Niger. J. Clin. Pr. 2011, 14, 504–507. [Google Scholar] [CrossRef]
- Sakamoto, K.; Morita, K.-I.; Shimada, Y.; Omura, K.; Izumo, T.; Yamaguchi, A. Peripheral odontogenic keratocyst associated with nevoid basal cell carcinoma syndrome: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, e19–e23. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, J.; Zheng, J.W. Radiological and clinical features of peripheral keratocystic odontogenic tumor. Int. J. Clin. Exp. Med. 2014, 7, 300–306. [Google Scholar] [PubMed] [PubMed Central]
- Gröbe, A.; Hanken, H.; Blessmann, M.; Zustin, J.; Heiland, M.; Al-Dam, A. An odontogenic keratocystic tumor in the buccal space: An unusual site of origin and a review of the literature. In Vivo 2012, 26, 847–851. [Google Scholar]
- Yamamoto, K.; Matsusue, Y.; Kurihara, M.; Takahashi, Y.; Kirita, T. A keratocyst in the buccal mucosa with the features of keratocystic odontogenic tumor. Open Dent. J. 2013, 7, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Kaminagakura, E.; Almeida, J.; Carvalho, Y.; Franco, R.; Soares, F.; Rocha, R.; Kowalski, L. Keratocyst of the buccal mucosa: Case report and immunohistochemical comparative study with sporadic Intraosseous keratocystic odontogenic tumor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e387–e392. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, M.E.; Flores, I.L.; Karssemakers, L.H.; Bloemena, E. Intramuscular keratocyst as a soft tissue counterpart of keratocystic odontogenic tumor: Differential diagnosis by immunohistochemistry. Hum. Pathol. 2014, 45, 110–118. [Google Scholar] [CrossRef]
- Makarla, S.; Bavle, R.M.; Muniswamappa, S.; Narasimhamurthy, S. A Large Extragnathic Keratocystic Odontogenic Tumour. Case Rep. Pathol. 2015, 2015, 723010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Witteveen, M.E.; Flores, I.L.; Karssemakers, L.H.; Bloemena, E. Odontogenic keratocysts located in the buccal mucosa: A description of two cases and review of the literature. SAGE Open Med. Case Rep. 2019, 7, 2050313X19849828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meleveetil, D.; Beena, V.; Cheriyan, L.; Angamuthu, K. Mucosal keratocyst of buccal mucosa: A rare entity. J. Oral Maxillofac. Pathol. 2020, 24, 589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, T. Recurrence of odontogenic keratocyst in the buccal space. BMJ Case Rep. 2022, 15, e246735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mustakim, K.R.D.; Sodnom-Ish, B.D.; Yoon, H.-J.D.; Kim, S.M.D. Odontogenic Keratocyst in the Masseter Muscle. J. Craniofac. Surg. 2022, 33, e275–e276. [Google Scholar] [CrossRef] [PubMed]
- Kochaji, N.; Alshami, G.; Haddad, B. Primary odontogenic keratocyst in the cheek muscles: Report of the 4th case in the world and review of peripheral OKC literature. Int. J. Surg. Case Rep. 2023, 106, 108161. [Google Scholar] [CrossRef]
- Gonçalves, T.O.d.F.; Daquer, A.K.; Teixeira, L.D.D.; Abrantes, T.C.; Belloti, O.; Maurity, A.; dos Santos, V.L.C.; Ferme, N.S.S.; Agostini, M.; Roza, A.L.O.C.; et al. Soft-tissue keratocyst: Report of 3 new cases from Brazil. Oral Maxillofac. Surg. 2024, 29, 1. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE Guidelines: Consensus—Based Clinical Case Reporting Guideline Development. Headache J. Head Face Pain 2013, 53, 1541–1547. [Google Scholar] [CrossRef]
- Loro, L.L.; Johannessen, A.C.; Skarstein, K.; Hellem, S. Peripheral odontogenic keratocyst causing bone resorption: Report of two cases. Oral Maxillofac. Surg. Cases 2023, 9, 100301. [Google Scholar] [CrossRef]
- Usui, H.; Sato, T.; Yamamoto, H.; Asada, K.; Nakayama, A.; Ishibashi, K. A case of peripheral keratocystic odontogenic tumor arising in the incisal gingiva of the mandible. Jpn. J. Oral Maxillofac. Surg. 2007, 53, 285–288. [Google Scholar] [CrossRef][Green Version]
- Forte, M.; d’Amati, A.; Manfuso, A.; Vittoli, M.; Girone, G.; Cascardi, E.; Capodiferro, S. Gingival Cyst of the Adult: A Case Description with a Relevant Literature Analysis. Reports 2024, 7, 51. [Google Scholar] [CrossRef]
- Handschel, J.G.; A Depprich, R.; Zimmermann, A.C.; Braunstein, S.; Kübler, N.R. Adenomatoid odontogenic tumor of mandible review of the literature and report of rare case. Head Face Med. 2005, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Decani, S.; Quatrale, M.; Caria, V.; Moneghini, L.; Varoni, E.M. Peripheral Ameloblastoma: A Case Report and Review of Literature. J. Clin. Med. 2024, 13, 6714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malali, V.V.; Satisha, T.; Jha, A.; Rath, S. Gingival cyst of adult: A rare case. J. Ind. Soc. Periodontol. 2012, 16, 465–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kerezoudis, N.P.; Donta-Bakoyianni, C.; Siskos, G. The lateral periodontal cyst: Aetiology, clinical significance and diagnosis. Endod. Dent. Traumatol. 2000, 16, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.W.; Damm, D.D.; Allen, C.M. Follicular Cysts of The Skin: Oral and Maxillofacial Pathology, 3rd ed.; Saunders: Philadelphia, PA, USA, 2008; pp. 32–34. [Google Scholar]



| Focused Question (PICO) | What Are the Clinical and Histopathological Features, in Relation to Other Similar Peripheral Lesions, of Peripheral Keratocysts that Will Allow a Better Understanding of Recurrence Rates and Long-Term Outcomes? |
|---|---|
| Search Strategy Population | Patients affected by peripheral odontogenic keratocyst, with extraosseous localization. |
| Intervention | Advanced diagnostic approaches and optimal treatment strategies. |
| Comparison | Differential diagnosis with other peripheral lesions. |
| Outcome | Clinical and histopathological features, recurrence rates, treatment options, and long-term outcomes |
| Database Electronic search | PubMed, Web of Science, Scopus and Wiley Library |
| Selection criteria Inclusion criteria | Studies at all levels of evidence, except expert opinion; Articles published in English reporting cases with histological diagnosis of POKC |
| Exclusion criteria | Animal studies; in Vitro study; not available articles |
| Nr. CASE | Author | Age/ Gender | Site | Sign/ Syntoms | Size | Radiographic Appearance | Surgical Appearance | Treatment | Outcomes | YEAR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Stoelinga PJ et al. [10] | NS | Posterior maxilla | NS | NS | NS | NS | NS | NS | 1975 |
| 2 | Amos Buchner et al. [11] | NS | NS | Swelling | NS | NS | NS | NS | NS | 1979 |
| 3 | Dan Dayan et al. [12] | 42 M | Maxilla | Swelling | 10 mm | None | Small fenestration | Enucleation, curettage | No evidence after 10 months | 1988 |
| 4 | Antoine Chehade et al. [13] | 37 M | Anterior Mandible | Swelling, Fluctuant | 3 × 3 mm | None | None | NS | NS | 1994 |
| 5 | Antoine Chehade et al. [13] | 66 F | Posterior Maxilla | Swelling | NS | None | None | NS | NS | 1994 |
| 6 | Antoine Chehade et al. [13] | 35 F | Posterior Mandible | Mobile nodule | 10 mm | None | None | NS | NS | 1994 |
| 7 | Antoine Chehade et al. [13] | 70 M | Posterior Mandible | Nodule | NS | None | None | NS | Recurred after 7 years | 1994 |
| 8 | Antoine Chehade et al. [13] | 57 F | Anterior Maxilla | Swelling | 7 × 5 mm | None | None | NS | NS | 1994 |
| 9 | Antoine Chehade et al. [13] | 42 M | Posterior Mandible | NS | NS | None | Focal fenestration | NS | NS | 1994 |
| 10 | Fardal and Johannessen [14] | 41 F | Mandibular and maxillary gingiva | NS | NS | NS | NS | NS | NS | 1994 |
| 11 | Yih, W. et al. [7] | NS | NS | NS | NS | NS | NS | NS | NS | 2000 |
| 12 | Yih, W. et al. [7] | NS | NS | NS | NS | NS | NS | NS | NS | 2000 |
| 13 | Yih, W. et al. [7] | NS | NS | NS | NS | NS | NS | NS | NS | 2000 |
| 14 | F. Ide et al. [15] | 38 F | Mandible gingiva of #3.2 | Asymptomatic fluctuant nodule | 3 mm | None | None | Excision | No evidence after 5 years | 2002 |
| 15 | F. Ide et al. [15] | 46 F | Mandible, gingiva of #4.4 | Asymptomatic fluctuant nodule | 5 mm | None | None | Excision | No evidence after 6 years | 2002 |
| 16 | A.C. Chi et al. [16] | 81 F | Maxilla, gingiva between #2.1, #2.2 | Asymptomatic fluctuant nodule | 10 mm | None | Superficial resorption | Enucleation, curettage | Recurrence after 6 months, re-excised, No evidence after 3 months | 2005 |
| 17 | A.C. Chi et al. [16] | 64 F | Mandible, gingiva between #3.4, #3.5 | Asymptomatic nodule | 15 mm | None | NS | Enucleation | No evidence after 21 months | 2005 |
| 18 | R.D. Preston et al. [17] | 83 F | Maxilla, gingiva between #2.3, #2.4 | Nodule | 7 mm | None | Superficial resorption | Enucleation, curettage | No evidence after 6 months | 2005 |
| 19 | E. Mozaffari et al. [18] | 82 F | Mandible, gingiva of #3.5 | Swelling with persistent mild pain | 7 × 5 mm | Radiolucency with adjacent radiopaque shadow | NS | Excisional biopsy | NS | 2007 |
| 20 | Usuii H. et al. [19] | 53 M | Maxilla, gingiva of #2.3 | Asymptomatic fluctuant swelling | 6 mm | NS | Alveolar bone resorption | Excision | No evidence after 6 years | 2007 |
| 21 | S E S Faustino et al. [20] | 57 F | Mandible, gingiva between #3.4, #3.5 | Asymptomatic non-mobile nodule | 5 mm | Radiolucency with a defined margin area | Cortical resorption | Enucleation, curettage | Recurrence after 1 year, re-enucleated No evidence to date | 2008 |
| 22 | F. Ide et al. [21] | 53 M | Mandible, gingiva between #3.2, #3.3 | Fluctuant nodule | 6 mm | Radiolucency | Superficial resorption | NS | No evidence after 7 years | 2008 |
| 23 | Jinbu, Y et al. [22] | 63 M | Mandible, gingiva of #4.5 | Painful, fluctuant nodule | 15 mm | Radiolucency with ill-defined margin area | Alveolar bone resorption | Resection | No evidence after 1 year | 2009 |
| 24 | H. Vij et al. [23] | 56 M | Maxilla, Hard palate gingiva from #2.1 to #4.4 | Discharging swelling | 25 × 20 mm | None | None | Excisional biopsy | NS | 2011 |
| 25 | K. Sakamoto et al. [24] | 24 F | Mandible, gingiva of #4.3 | Pigmented asymptomatic papule | 3 mm | None | None | Excisional biopsy | NS | 2014 |
| 26 | María del Carmen Vázquez-Romero et al. [2] | 32 M | Maxilla, gingiva between #2.2, #2.3 | Asymptomatic fluctuant nodule | 4 mm | Hypodense lesion (CBCT) | Cortical resorption | Excisional biopsy, Curettage, Bone drill | No evidence after 1 year | 2017 |
| 27 | B.T. Rodrigues et al. [8] | 43 F | Maxilla, gingiva between #1.5, #1.6 | Asymptomatic nodule | 15 mm | None | Superficial resorption | Excisional biopsy | No evidence after 4 years | 2020 |
| 28 | B.T. Rodrigues et al. [8] | 63 F | Mandible, gingiva between #3.1, #4.1 | Asymptomatic elevated lesion | 10 mm | None | None | Excisional biopsy | Not returned for follow-ups | 2020 |
| 29 | Lafuente-Ibáñez de Mendoza et al. [1] | 61 F | Maxilla, gingiva between #2.1, #2.2 | Asymptomatic fluctuant nodule | 10 mm | None | NS | Excision | No evidence after 20 months | 2022 |
| 30 | Lafuente-Ibáñez de Mendoza et al. [1] | 74 F | Mandible, gingiva between #3.3, #3.4 | Fluctuant nodule | 15 mm | None | NS | Excision | No evidence after 4 years | 2022 |
| 31 | Lafuente-Ibáñez de Mendoza et al. [1] | 14 M | Maxilla, gingiva between #2.4, #2.5 | Asymptomatic nodule | 20 mm | None | NS | Excision | Already recurred after 2 years, No evidence after 12 months | 2022 |
| 32 | Lado Lako Loro et al. [25] | 71 M | Maxilla, gingiva between #1.3, #1.4 | Asymptomatic nodule | 10 mm | Radiolucency with a defined margin area | Alveolar bone resorption | Excision, curettage | No evidence after 10 months | 2023 |
| 33 | Lado Lako Loro et al. [25] | 33 F | Maxilla, gingiva between #1.3, #1.5 | Asymptomatic nodule | 3 mm | None | Alveolar bone resorption | Excision | No evidence after 10 months | 2023 |
| 34 | Brooks JK et al. [3] | 70 F | Maxilla, gingiva between #1.2, #1.3 | Non-mobile papule | 4 mm | Radiolucency with a defined margin area | Bone defect | Enucleation | Recurred after 8 months, re-enucleated, recurred after 6 months, Re-enucleated, no further evidence after 4 months | 2024 |
| 35 | Kinaia et al. [19] | 60 F | Mandible; gingi val tissue between #3.1–3.2 | Asymptomatic swelling | 10 × 9 × 5 mm | Radiolucency with a defined margin area | Loss of the cortical plate | Excisional biopsy of the cystic lesion combined with guided tissue regeneration | No recurrences at 6 months follow-up | 2024 |
| 36 | Kinaia et al. [19] | 62 M | Maxilla; gingiva betwee#14–15 | Asymptomatic swelling | 5 × 8 mm | Diffuse radiolucency | No bone alteration | Excisional biopsy of the cystic lesion combined with guided tissue regeneration | No recurrences at 36 months follow-up | 2024 |
| 37 | Present case | 68 F | Maxilla, between #4.3, #4.4 | Asymptomatic nodule | 15 mm | Radiolucency with a defined margin area | Alveolar bone resorption | Excisional biopsy, curettage | No recurrence at 6 months follow-up | 2025 |
| CASE | AUTHOR | AGE/GENDER | SITE | SIGN/SYMPTOMS | SIZE | Radiographic Appearance | Surgical Appearance | Treatment | Outcome | YEAR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Precheur and Krolls [9] | 59 M | Left cheek | Firm, slightly tender, mobile, 3–4 cm mass | NS | NS | NS | Incisional biopsy | NS | 2009 |
| 2 | Precheur and Krolls [9] | 60 M | Left cheek | Painless Swelling | 3 × 2 × 2 cm | NS | NS | Excision | No recurrences | 2010 |
| 3 | Gröbe A et al. [26] | 52 M | Right buccal mucosa | Asymptomatic fluctuant nodule | 20 × 20 mm | None | None | Excision | No evidence after 4 months | 2012 |
| 4 | Yamamoto K et al. [27] | 74 M | Right buccal mucosa | Asymptomatic fluctuant nodule | 30 × 25 mm | Defined margin lesion (CBCT) | None | Excision | No evidence after 4 years | 2013 |
| 5 | Kaminagakura E et al. [28] | NS | Left buccal mucosa | NS | NS | NS | NS | NS | No recurrences after 12 years | 2013 |
| 6 | Abé T. et al. [29] | 46 M | Left temporalis muscle | Painful fluctuant nodule | 21 mm | Loculated lesion (CBCT) | Bone resorption | Excision | No evidence after 12 years | 2014 |
| 7 | Zhu L et al. [25] | 44 M | Soft palate | Asymptomatic nodule | 30 × 40 mm | NS | NS | Resection | NS | 2014 |
| 8 | Zhu L et al. [25] | 69 M | Right buccal mucosa | Asymptomatic non-mobile nodule | 20 mm | NS | NS | Resection | NS | 2014 |
| 9 | Makarla et al. [30] | Right buccal space | NS | NS | NS | NS | NS | No after 24 months | 2015 | |
| 10 | Witteveen et al. [31] | NS | Right buccal space | NS | NS | NS | NS | NS | No recurrence after 4 years | 2018 |
| 11 | Witteveen et al. [31] | NS | Left buccal space | NS | NS | NS | NS | NS | No recurrence after 1 year | 2018 |
| 12 | Beena et al. [32] | NS | Right buccal space | NS | NS | NS | NS | NS | No recurrences after 6 months | 2020 |
| 13 | Shatur A et al. [5] | 76 M | Retromolar trigone | Painful fluctuant swelling | 50 × 40 mm | None | None | Excisional biopsy | NS | 2021 |
| 14 | Watanabe et al. [33] | NS | Right buccal space | NS | NS | NS | NS | NS | Two recurrences after 4 years | 2022 |
| 15 | Mustakim et al. [34] | NS | Massetere muscle | NS | NS | NS | NS | NS | No recurrences after 5 years | 2022 |
| 16 | María Hornillos-de Villota et al. [4] | 58 F | Soft tissue of the posterior margin of the mandibular ramus | NS | 26 × 19 mm | Loculated lesion (CBCT) | NS | Cystectomy by cervical approach | Already occurred twice, no evidence after 3 months | 2023 |
| 17 | Kochaji et al. [35] | 17 M | Right cheek | Painless swelling | 27 × 15 × 10 mm | None | NS | Biopsy | 6 months | 2023 |
| 18 | Thayanne Oliveira de Freitas Gonçalves et al. [36] | 58 M | Right buccal mucosa | Painful swelling | 5 cm | None | NS | Biopsy | No recurrences at 18 months | 2025 |
| 19 | Thayanne Oliveira de Freitas Gonçalves et al. [36] | 44 M | Left buccal mucosa | Painful swelling | NS | None | NS | Biopsy | No recurrences at 12 months | 2025 |
| 20 | Thayanne Oliveira de Freitas Gonçalves et al. [36] | 74 F | Left buccal mucosa | Painful swelling | 13 mm | None | NS | Biopsy | Lost follow-up | 2025 |
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | |
|---|---|---|---|---|---|---|---|---|
| Stoelinga PJ et al. [10] |  |  |  |  |  |  |  |  |
| Amos Buchner et al. [11] |  |  |  |  |  |  |  |  |
| Dan Dayan et al. [12] |  |  |  |  |  |  |  |  |
| Antoine Chehade et al. [13] |  |  |  |  |  |  |  |  |
| Antoine Chehade et al. [13] |  |  |  |  |  |  |  |  |
| Antoine Chehade et al. [13] |  |  |  |  |  |  |  |  |
| Antoine Chehade et al. [13] |  |  |  |  |  |  |  |  |
| Antoine Chehade et al. [13] |  |  |  |  |  |  |  |  |
| Antoine Chehade et al. [13] |  |  |  |  |  |  |  |  |
| Fardal and Johannessen [14] |  |  |  |  |  |  |  |  |
| Yih, W. et al. [7] |  |  |  |  |  |  |  |  |
| Yih, W. et al. [7] |  |  |  |  |  |  |  |  |
| Yih, W. et al. [7] |  |  |  |  |  |  |  |  |
| F. Ide et al. [15] |  |  |  |  |  |  |  |  |
| F. Ide et al. [15] |  |  |  |  |  |  |  |  |
| A.C. Chi et al. [16] |  |  |  |  |  |  |  |  |
| A.C. Chi et al. [16] |  |  |  |  |  |  |  |  |
| R.D. Preston et al. [17] |  |  |  |  |  |  |  |  |
| E. Mozaffari et al. [18] |  |  |  |  |  |  |  |  |
| Usuii H. et al. [19] |  |  |  |  |  |  |  |  |
| S E S Faustino et al. [20] |  |  |  |  |  |  |  |  |
| F. Ide et al. [21] |  |  |  |  |  |  |  |  |
| Jinbu, Y et al. [22] |  |  |  |  |  |  |  |  |
| H. Vij et al. [23] |  |  |  |  |  |  |  |  |
| K. Sakamoto et al. [24] |  |  |  |  |  |  |  |  |
| María del Carmen Vázquez-Romero et al. [2] |  |  |  |  |  |  |  |  |
| B.T. Rodrigues et al. [8] |  |  |  |  |  |  |  |  |
| B.T. Rodrigues et al. [8] |  |  |  |  |  |  |  |  |
| Lafuente-Ibáñez de Mendoza et al. [1] |  |  |  |  |  |  |  |  |
| Lafuente-Ibáñez de Mendoza et al. [1] |  |  |  |  |  |  |  |  |
| Lafuente-Ibáñez de Mendoza et al. [1] |  |  |  |  |  |  |  |  |
| Lado Lako Loro et al. [25] |  |  |  |  |  |  |  |  |
| Lado Lako Loro et al. [25] |  |  |  |  |  |  |  |  |
| Brooks JK et al. [3] |  |  |  |  |  |  |  |  |
| Kinaia et al. [19] |  |  |  |  |  |  |  |  |
| Kinaia et al. [19] |  |  |  |  |  |  |  |  |
 Yes,
Yes,  No;
No;  Unsure.
Unsure.| POKC | LPA | Peripheral Giant Cells Granuloma | GCA | Inflammatory Cyst | Odontogenic Adenomatoid Tumor | Peripheral Ameloblastoma | |
|---|---|---|---|---|---|---|---|
| Clinical features | Asymptomatic, slow-growing swelling of soft tissue | Small, asymptomatic, lateral to the roots of teeth | Firm, soft, nodular, or sessile or pediculate mass; surface occasionally ulcerated, bluish-red | Slow and painless swelling, usually solitary and small (measuring about 3–4 mm) nodules or vesicles, bluish | Painless or painful swelling in correspondence with a necrotic tooth (negative at vitality test) | Slow-growing, asymptomatic gingival swelling | Exophytic, sessile, solid gingival lesion (>1 cm); larger, more aggressive, with pain and swelling |
| Radiographic appearance | No evidence; sometimes uni or multi-loculated radiolucency | Unilocular radiolucency between the roots of vital erupted teeth, mostly premolars, canines and incisives; sometimes multilocular | Nonspecific, with foci of bone metaplasia in some cases; often widening of the periodontal ligament space | No bone involvement; possible bone resorption due to cyst pressure | Radiotransparent lesion encompassing one or more roots of a necrotic tooth | Resorption of the cortical cortex; tooth displacement without root resorption; in 78% of cases, radio-opacities | Bone expansion with corticated radiolucency |
| Incidence | 12.5–13.6% | 0.4–0.7 (of all cysts), 0.6–2.4% of odontogenic cysts | NR | 3.2% | 63.7% of all odontogenic cysts | 3–7% of all odontogenic tumors | 1–10% of all ameloblastoma |
| Histology | Uniform thin stratified squamous epithelium with a palisaded basal cell layer, superficial keratosis, and flat epithelial-connective tissue interface | Thin, nonkeratinized epithelium resembling reduced enamel epithelium. Epithelial lining exhibits focal thickenings or plaques, with clear glycogen-containing epithelial cells. | Non-encapsulated mass of tissue composed of a reticular and fibrillar connective tissue stroma containing abundant young connective tissue cells of ovoid or fusiform shape, and multinucleated giant cells | Subepithelial connective tissue wall covered by a thin, squamous or cuboidal epithelium with, in some points, glycogen-rich clear cells | Thin cell layers of nonkeratinized stratified squamous epithelium are associated with inflamed fibrous connective tissue and inflammatory infiltrates | Clusters of epithelial, fusiform or cuboidal cells, also with characteristics of duct-like spaces and epithelial bands with cancellous or cribriform configuration | Odontogenic epithelium organized in islands and chords, showing a follicular pattern and similar to the odontogenic islands of the central variant |
| Localization | Posterior mandibular region | Between the tooth roots, usually premolars | Interdental papilla, edentulous alveolar margin, or at the marginal gum level | Vestibular attached gingiva in the mandibular canine and first premolar areas | Necrotic teeth | Maxillary region, associated with osteo-included teeth (mostly canines) | Gingiva or alveolar crest |
| Intraosseous OKC | Peripheral OKC | |
|---|---|---|
| Gender | Males | Females |
| Age | Wide range | Wide range |
| Localization | Posterior mandible | Anterior, upper maxilla |
| Multiple presentation | 10% | 0% |
| Gorlin-Goltz Syndrome | 5–10% | 9% |
| Recurrence | Up to 62% | 12.5% |
| Clinical aspect | Small asymptomatic lesion | Asymptomatic, slow-growing swelling of soft tissue |
| Radiographic appearance | Well-defined unilocular radiolucency; in 20% of cases, multilocular radiolucency; 30% associated with an unerupted tooth | No evidence; either uni or multi-loculated radiolucency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forte, M.; Manfuso, A.; D’Albis, G.; Cianciotta, G.; Cascardi, E.; Pinto, G.; Ingravallo, G.; Favia, G.; d’Amati, A.; Limongelli, L.; et al. Peripheral Odontogenic Keratocyst of the Gingiva: A Systematic Review of the Literature and Case Report. Diagnostics 2025, 15, 2616. https://doi.org/10.3390/diagnostics15202616
Forte M, Manfuso A, D’Albis G, Cianciotta G, Cascardi E, Pinto G, Ingravallo G, Favia G, d’Amati A, Limongelli L, et al. Peripheral Odontogenic Keratocyst of the Gingiva: A Systematic Review of the Literature and Case Report. Diagnostics. 2025; 15(20):2616. https://doi.org/10.3390/diagnostics15202616
Chicago/Turabian StyleForte, Marta, Alfonso Manfuso, Giuseppe D’Albis, Giulia Cianciotta, Eliano Cascardi, Grazia Pinto, Giuseppe Ingravallo, Gianfranco Favia, Antonio d’Amati, Luisa Limongelli, and et al. 2025. "Peripheral Odontogenic Keratocyst of the Gingiva: A Systematic Review of the Literature and Case Report" Diagnostics 15, no. 20: 2616. https://doi.org/10.3390/diagnostics15202616
APA StyleForte, M., Manfuso, A., D’Albis, G., Cianciotta, G., Cascardi, E., Pinto, G., Ingravallo, G., Favia, G., d’Amati, A., Limongelli, L., & Capodiferro, S. (2025). Peripheral Odontogenic Keratocyst of the Gingiva: A Systematic Review of the Literature and Case Report. Diagnostics, 15(20), 2616. https://doi.org/10.3390/diagnostics15202616











