Assessment of Intra-Individual Variability and Reproducibility in Pancreatic EUS-Guided Elastography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Setting and Study Population
2.3. Endoscopic Ultrasound Elastography Protocol
2.4. Variable Definitions and Data Collection
2.5. Outcomes
2.6. Statistical Analysis
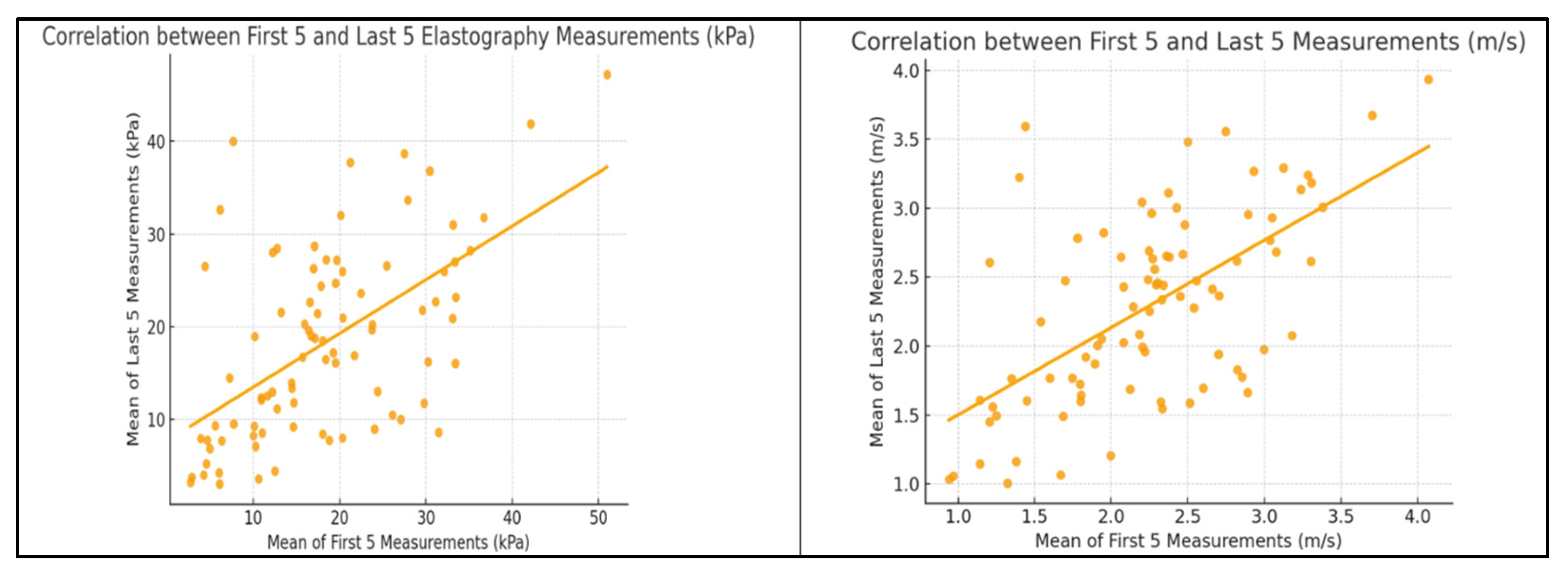
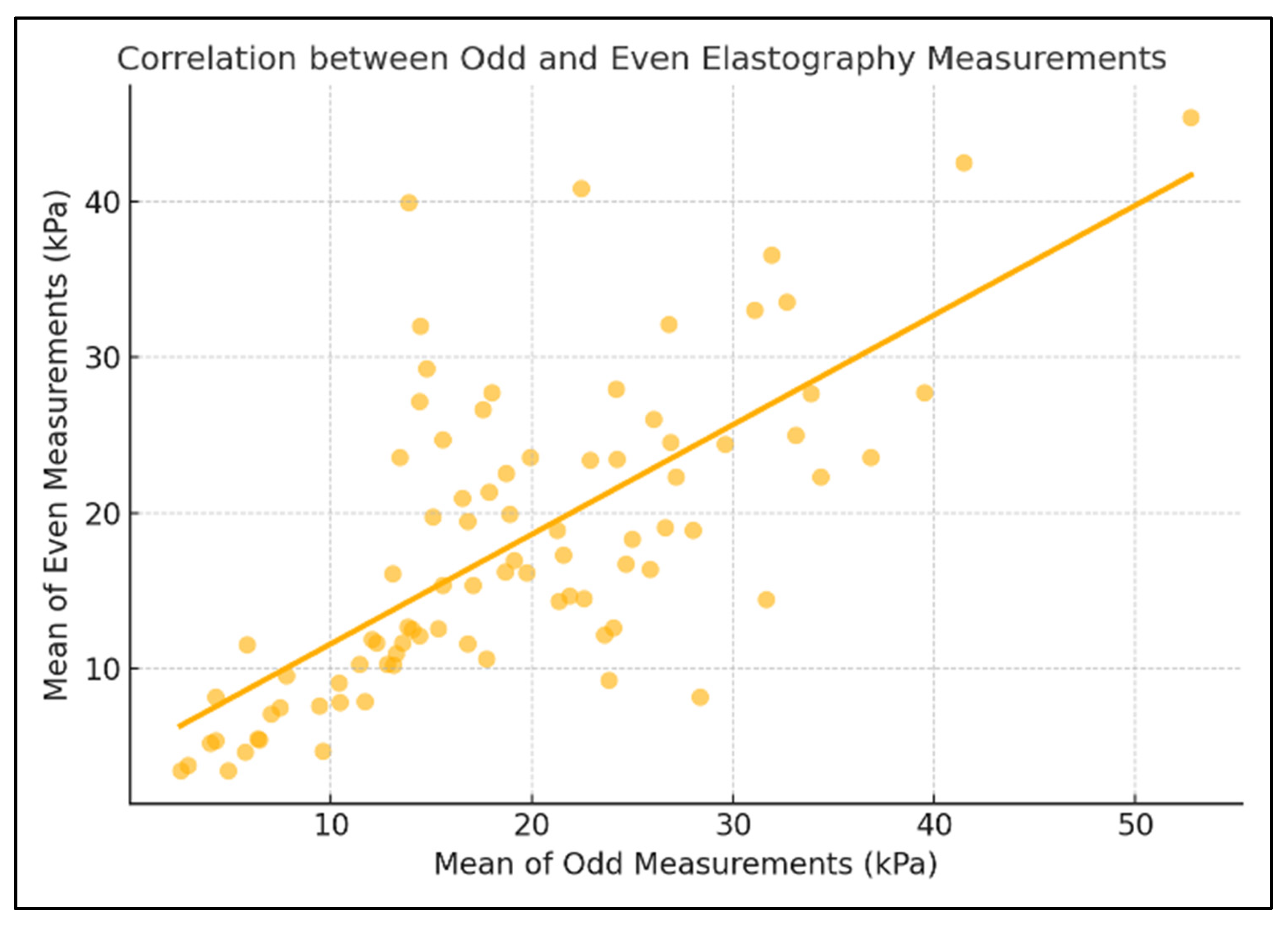
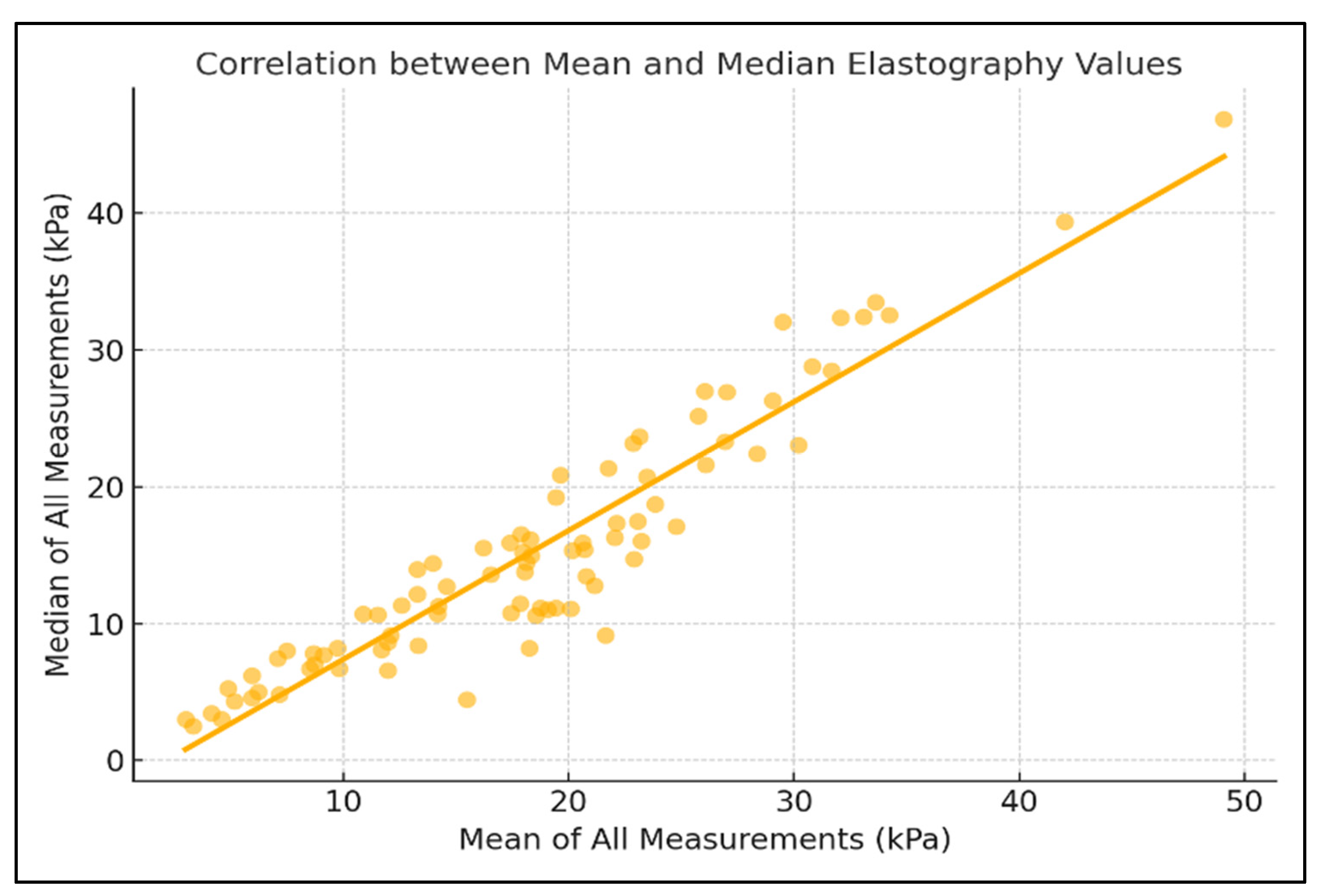
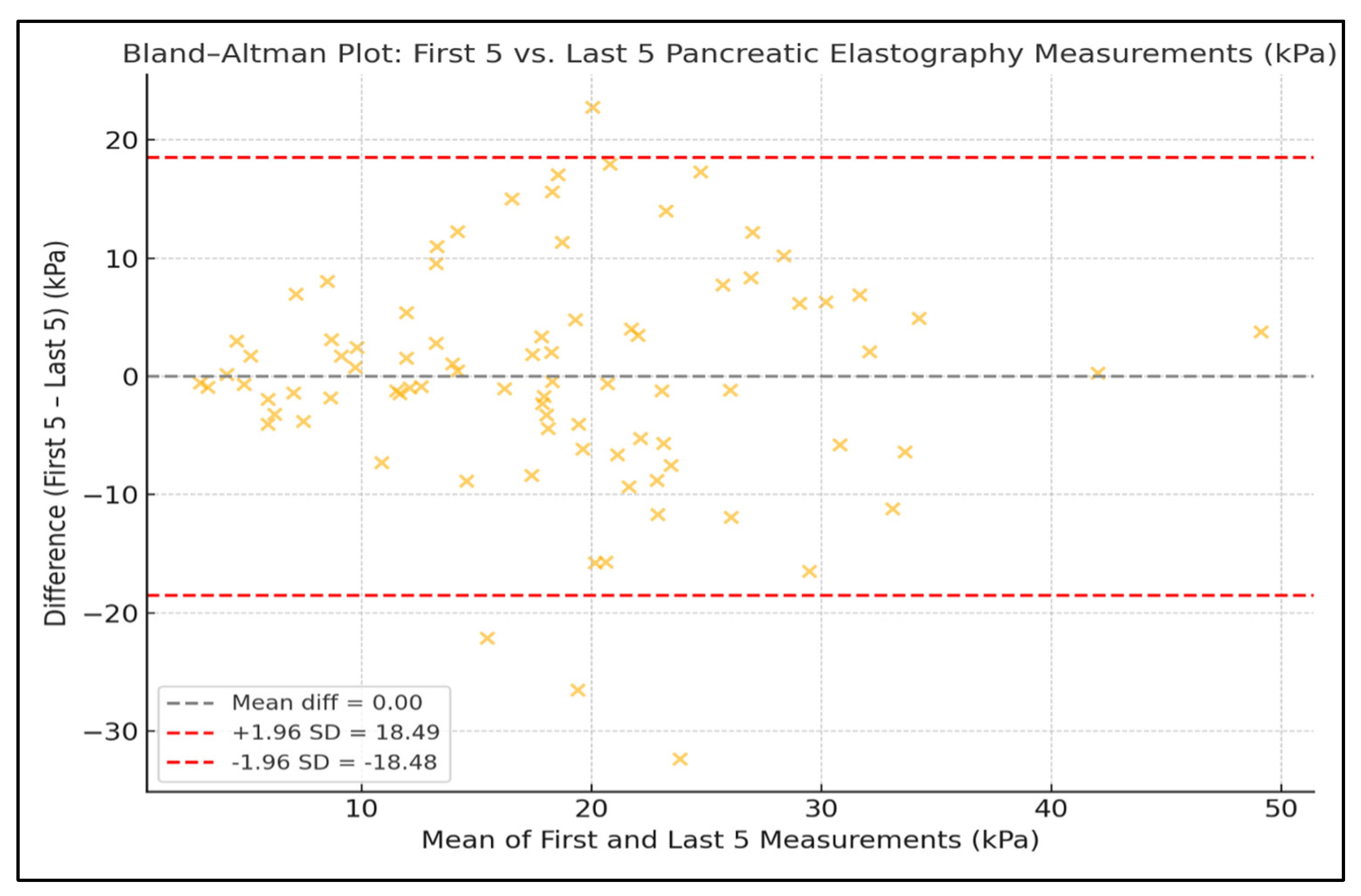
3. Results
4. Discussion
4.1. Assessment of Findings and Additional Literature
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abboud, Y.; Gaddam, S. The Role of Endoscopic Ultrasound-Guided Shear Wave Elastography in Pancreatic Diseases. Diagnostics 2024, 14, 2329. [Google Scholar] [CrossRef]
- Giovannini, M.; Hookey, L.C.; Bories, E.; Pesenti, C.; Monges, G.; Delpero, J.R. Endoscopic Ultrasound Elastography: The First Step towards Virtual Biopsy? Preliminary Results in 49 Patients. Endoscopy 2006, 38, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Săftoiu, A.; Vilmann, P. Endoscopic Ultrasound Elastography—A New Imaging Technique for the Visualization of Tissue Elasticity Distribution. J. Gastrointest. Liver Dis. 2006, 15, 161–165. [Google Scholar]
- Janssen, J.; Schlörer, E.; Greiner, L. EUS Elastography of the Pancreas: Feasibility and Pattern Description of the Normal Pancreas, Chronic Pancreatitis, and Focal Pancreatic Lesions. Gastrointest. Endosc. 2007, 65, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Botelberge, T.; Bories, E.; Pesenti, C.; Caillol, F.; Esterni, B.; Monges, G.; Arcidiacono, P.; Deprez, P.; Yeung, R.; et al. Endoscopic Ultrasound Elastography for Evaluation of Lymph Nodes and Pancreatic Masses: A Multicenter Study. World J. Gastroenterol. 2009, 15, 1587–1593. [Google Scholar] [CrossRef]
- Iglesias-Garcia, J.; Lariño-Noia, J.; Abdulkader, I.; Forteza, J.; Domínguez-Muñoz, J.E. EUS Elastography for the Characterization of Solid Pancreatic Masses. Gastrointest. Endosc. 2009, 70, 1101–1108. [Google Scholar] [CrossRef]
- Hirche, T.O.; Ignee, A.; Barreiros, A.P.; Schreiber-Dietrich, D.; Jungblut, S.; Ott, M.; Hirche, H.; Dietrich, C.F. Indications and Limitations of Endoscopic Ultrasound Elastography for Evaluation of Focal Pancreatic Lesions. Endoscopy 2008, 40, 910–917. [Google Scholar] [CrossRef]
- Iglesias-Garcia, J.; Lariño-Noia, J.; Abdulkader, I.; Forteza, J.; Domínguez-Muñoz, J.E. Quantitative Endoscopic Ultrasound Elastography: An Accurate Method for the Differentiation of Solid Pancreatic Masses. Gastroenterology 2010, 139, 1172–1180. [Google Scholar] [CrossRef]
- Byrne, M.F.; Jowell, P.S. Gastrointestinal Imaging: Endoscopic Ultrasound. Gastroenterology 2002, 122, 1631–1648. [Google Scholar] [CrossRef]
- Kaufman, A.R.; Sivak, M.V., Jr. Endoscopic Ultrasonography in the Differential Diagnosis of Pancreatic Disease. Gastrointest. Endosc. 1989, 35, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Guilabert, L.; Nikolìc, S.; de-Madaria, E.; Vanella, G.; Capurso, G.; Tacelli, M.; Maida, M.; Vladut, C.; Knoph, C.S.; Quintini, D.; et al. Endoscopic ultrasound for pancreatic cystic lesions: A narrative review. BMJ Open Gastroenterol. 2025, 12, e001893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giovannini, M. Contrast-Enhanced Endoscopic Ultrasound and Elastosonoendoscopy. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Vidili, G.; Arru, M.; Meloni, P.; Solinas, G.; Atzori, S.; Maida, I. Comparison of 2D Shear Wave Elastography and Transient Elastography in Non-Invasive Evaluation of Liver Fibrosis in Hepatitis C Virus-Related Chronic Liver Disease. J. Clin. Med. 2024, 13, 4061. [Google Scholar] [CrossRef] [PubMed]
- Marín-Serrano, E.; Barbado Cano, A.; Fernández Martos, R.; Abadía Barno, M.; Olveira Martín, A.; Martín Arranz, M.D. Protocol for acquisition of images and measurement of transabdominal ultrasound pancreatic two-dimensional shear wave elastography (2D-SWE). Gastroenterol. Hepatol. 2024, 47, 502198. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43(4), 773–785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163, Erratum in J. Chiropr. Med. 2017, 16, 346. https://doi.org/10.1016/j.jcm.2017.10.001. [Google Scholar] [CrossRef]
- Colli, A.; Fraquelli, M.; Casazza, G. Fraquelli, M., Ed.; Elastographic Measures: A Methodological Approach. In Elastography of the Liver and Beyond; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Itoh, A.; Ueno, E.; Tohno, E.; Kamma, H.; Takahashi, H.; Shiina, T.; Yamakawa, M.; Matsumura, T. Breast Disease: Clinical Application of US Elastography for Diagnosis. Radiology 2006, 239, 341–350. [Google Scholar] [CrossRef]
- Krouskop, T.A.; Wheeler, T.M.; Kallel, F.; Garra, B.S.; Hall, T. Elastic Moduli of Breast and Prostate Tissues under Compression. Ultrason. Imaging 1998, 20, 260–274. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Kawai, M.; Suhara, H.; Takeyama, T.; Hashizume, K.; Koya, T.; et al. Quantitative Diagnosis of Chronic Pancreatitis Using EUS Elastography. J. Gastroenterol. 2017, 52, 868–874. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Yamamura, T.; Furukawa, K.; Funasaka, K.; Nakamura, M.; Miyahara, R.; et al. Usefulness of Shear Wave Elastography as a Quantitative Diagnosis of Chronic Pancreatitis. J. Gastroenterol. Hepatol. 2018, 33, 756–761. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanioka, K.; Kawaji, Y.; Tamura, T.; Nuta, J.; Hatamaru, K.; Itonaga, M.; Ida, Y.; Maekita, T.; Iguchi, M.; et al. Endoscopic ultrasonography shear wave as a predictive factor of endocrine/exocrine dysfunction in chronic pancreatitis. J. Gastroenterol. Hepatol. 2021, 36, 391–396. [Google Scholar] [CrossRef]
- Youk, J.H.; Son, E.J.; Park, A.Y.; Kim, J.A. Shear-wave elastography for breast masses: Local shear wave speed (m/sec) versus Young modulus (kPa). Ultrasonography 2014, 33, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Abboud, Y.; Kim, K.; Samaan, J.S.; Chen, C.; Lew, D.; Ghaith, J.; Caldera, W.; El Helou, M.O.; Park, K.H.; Liu, Q.; et al. Endoscopic ultrasound guided shear wave elastography is safe with high feasibility and reproducibility when used in the pancreas: Findings from a prospective cohort. Pancreas 2023, 52, e115–e120. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Ryou, M. Defining the optimal technique for endoscopic ultrasound shear wave elastography: A combined benchtop and animal model study with comparison to transabdominal shear wave elastography. Clin. Endosc. 2023, 56, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ohno, E.; Hirooka, Y.; Kawashima, H.; Ishikawa, T. Feasibility of EUS-guided shear-wave measurement: A preliminary clinical study. Endosc. Ultrasound 2019, 8, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yamazaki, H.; Shimokawa, T.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Kitano, M. Shear-wave versus strain elastography in endoscopic ultrasound for the diagnosis of chronic pancreatitis. Pancreatology 2023, 23, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ohno, E.; Hirooka, Y.; Kawashima, H.; Ishikawa, T.; Tanaka, H.; Sakai, D.; Ishizu, Y.; Kuzuya, T.; Nakamura, M.; Honda, T. Feasibility and usefulness of endoscopic ultrasonography-guided shear-wave measurement for assessment of autoimmune pancreatitis activity: A prospective exploratory study. J. Med. Ultrason. 2019, 46, 425–433. [Google Scholar] [CrossRef]
- El Helou, M.O.; Abboud, Y.; Kim, K.; Chhoda, A.; Jeon, C.; Smith, Z.; Mubarak, M.; Advani, R.; Rafique, Y.; Meza, J.; et al. Utility of endoscopic ultrasound (EUS) guided shear wave elastography (SWE) in fatty pancreas: A large prospective study. Gastroenterology 2023, 164, S-185. [Google Scholar] [CrossRef]
- Mohamed, G.; Zalomek, C.; El Helou, M.; Li, D.; Perrotta, G.; Rastegar, R.; Mathur, K.; Abboud, Y.; Kim, K.; Chhoda, A.; et al. Endoscopic ultrasound guided shearwave elastography (EUS-SWE) predicts fat in the pancreas and correlates with fat-fraction on magnetic resonance imaging (MRI): Results from a prospective study. Gastrointest. Endosc. 2024, 99, AB804. [Google Scholar] [CrossRef]
- Huang, J.; Peng, J.; Long, H.; Ruan, S.; Yao, L.; Xie, X.; Lin, M.; Zhang, X. Feasibility and measurement value of pancreatic 2-D shear wave elastography in healthy adults: Evaluation, influencing factors, reference range, measurement stability and reproducibility. Ultrasound Med. Biol. 2024, 50, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Zhang, X.; Tang, Y.; Yan, Y.; Chen, Z. Two-dimensional shear wave elastography of the pancreas: Measurement success rate, repeatability, and factors affecting measurement values. J. Med. Ultrason. 2022, 49, 261–268. [Google Scholar] [CrossRef]
- Hristov, B.; Andonov, V.; Doykov, D.; Tsvetkova, S.; Doykova, K.; Doykov, M. Evaluation of ultrasound-based point shear wave elastography for differential diagnosis of pancreatic diseases. Diagnostics 2022, 12, 841. [Google Scholar] [CrossRef]


| Variables | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Gender comparison | |||
| Measurements unit | Females | Males | |
| kPa | 20.31 ± 9.49 | 17.63 ± 6.62 | 0.215 |
| m/s | 2.38 ± 0.62 | 2.28 ± 0.47 | 0.481 |
| Age group comparison | |||
| Measurements unit | <65 years (n = 30) | ≥65 years (n = 56) | |
| kPa | 20.68 ± 9.13 | 17.03 ± 8.70 | 0.078 |
| m/s | 2.43 ± 0.57 | 2.18 ± 0.61 | 0.067 |
| Diagnostic | n | % |
|---|---|---|
| Pancreatic neoplasm | 30 | 34.9 |
| Suspected choledocholithiasis (not confirmed) | 30 | 34.9 |
| Choledocholithiasis | 12 | 14.0 |
| Acute pancreatitis | 4 | 4.7 |
| Chronic pancreatitis | 3 | 3.5 |
| Pancreatic cyst | 3 | 3.5 |
| Neuroendocrine tumor | 2 | 2.3 |
| Malignant ampullary tumor | 1 | 1.2 |
| Benign ampullary tumor | 1 | 1.2 |
| Total | 86 | 100 |
| Sub-Group | n | Mean Stiffness ± SD (kPa) | CV (kPa) ± SD | ICC (First 5 vs. Last 5, kPa) | Mean Velocity ± SD (m/s) | CV (m/s) ± SD | ICC (First 5 vs. Last 5, m/s) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Females | 51 | 20.3 ± 9.5 | 0.64 ± 0.14 | 0.99 | 2.38 ± 0.62 | 0.33 ± 0.10 | 0.61 |
| Males | 35 | 17.6 ± 6.6 | 0.63 ± 0.13 | 0.98 | 2.28 ± 0.47 | 0.32 ± 0.09 | 0.60 |
| Age (years) | |||||||
| < 65 | 30 | 20.7 ± 9.1 | 0.65 ± 0.15 | 0.99 | 2.43 ± 0.57 | 0.34 ± 0.11 | 0.62 |
| ≥ 65 | 56 | 17.0 ± 8.7 | 0.63 ± 0.14 | 0.98 | 2.18 ± 0.61 | 0.32 ± 0.10 | 0.60 |
| BMI Category (kg m−2) | n (Patients) | Mean Stiffness kPa ± SD | Mean CV (kPa) ± SD | ICC (First 5 vs. Last 5, kPa) | Mean Velocity m s−1 ± SD | Mean CV (m/s) ± SD | ICC (First 5 vs. Last 5, m/s) |
|---|---|---|---|---|---|---|---|
| Normal (<25) | 24 | 17.8 ± 8.2 | 0.62 ± 0.14 | 0.98 | 2.25 ± 0.48 | 0.31 ± 0.09 | 0.6 |
| Overweight (25–29.9) | 32 | 18.9 ± 8.9 | 0.64 ± 0.13 | 0.99 | 2.30 ± 0.54 | 0.33 ± 0.10 | 0.62 |
| Obese (≥30) | 30 | 19.7 ± 9.6 | 0.65 ± 0.15 | 0.99 | 2.34 ± 0.61 | 0.34 ± 0.11 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miutescu, B.; Bende, R.; Bende, F.; Burdan, A.; Gadour, E.; Ghiuchici, A.M.; Alomar, M.; Burciu, C.; AlQahtani, M.S.; Sirli, R.; et al. Assessment of Intra-Individual Variability and Reproducibility in Pancreatic EUS-Guided Elastography. Diagnostics 2025, 15, 2601. https://doi.org/10.3390/diagnostics15202601
Miutescu B, Bende R, Bende F, Burdan A, Gadour E, Ghiuchici AM, Alomar M, Burciu C, AlQahtani MS, Sirli R, et al. Assessment of Intra-Individual Variability and Reproducibility in Pancreatic EUS-Guided Elastography. Diagnostics. 2025; 15(20):2601. https://doi.org/10.3390/diagnostics15202601
Chicago/Turabian StyleMiutescu, Bogdan, Renata Bende, Felix Bende, Adrian Burdan, Eyad Gadour, Ana Maria Ghiuchici, Mohammed Alomar, Calin Burciu, Mohammed Saad AlQahtani, Roxana Sirli, and et al. 2025. "Assessment of Intra-Individual Variability and Reproducibility in Pancreatic EUS-Guided Elastography" Diagnostics 15, no. 20: 2601. https://doi.org/10.3390/diagnostics15202601
APA StyleMiutescu, B., Bende, R., Bende, F., Burdan, A., Gadour, E., Ghiuchici, A. M., Alomar, M., Burciu, C., AlQahtani, M. S., Sirli, R., Popescu, A., & Ratiu, I. (2025). Assessment of Intra-Individual Variability and Reproducibility in Pancreatic EUS-Guided Elastography. Diagnostics, 15(20), 2601. https://doi.org/10.3390/diagnostics15202601







