Timing of Single-Tooth Implant Rehabilitation and Periapical Inflammation Severity: A Retrospective Study Using the DAIS System
Abstract
1. Introduction
2. Methods
2.1. Study Design
- Age ≥ 18 years
- Indicated for single-tooth extraction with apical pathology
- Underwent socket preservation and subsequent implant placement
- Availability of histopathological evaluation and complete clinical records Exclusion criteria included systemic inflammatory diseases (except for descriptive analysis), immunosuppressive therapy, pregnancy, active periodontitis, and missing procedural data.
- Patient demographic data
- Endodontic status of the extracted tooth (vital vs. previously treated)
- Surgical procedure dates (extraction, socket preservation, implant placement)
- Histopathological results and corresponding DAIS score
- Dates of prosthetic restoration
- BEGO Semados® S/SC/SCX, BEGO Implant Systems GmbH & Co. KG, Bremen, Germany
- Straumann® BLX/BL, Institut Straumann AG, Basel, Switzerland
- 3i T3® and 3i T3® PT, ZimVie Inc., Westminster, CO, USA
- Biomet 3i® Certain®, Boss implant, ZimVie Inc., Westminster, CO, USA
- SDS Zirconia®, Swiss Dental Solutions AG, Kreuzlingen, Switzerland
2.2. Surgical Procedures
2.2.1. Treatment Protocol & Standardization
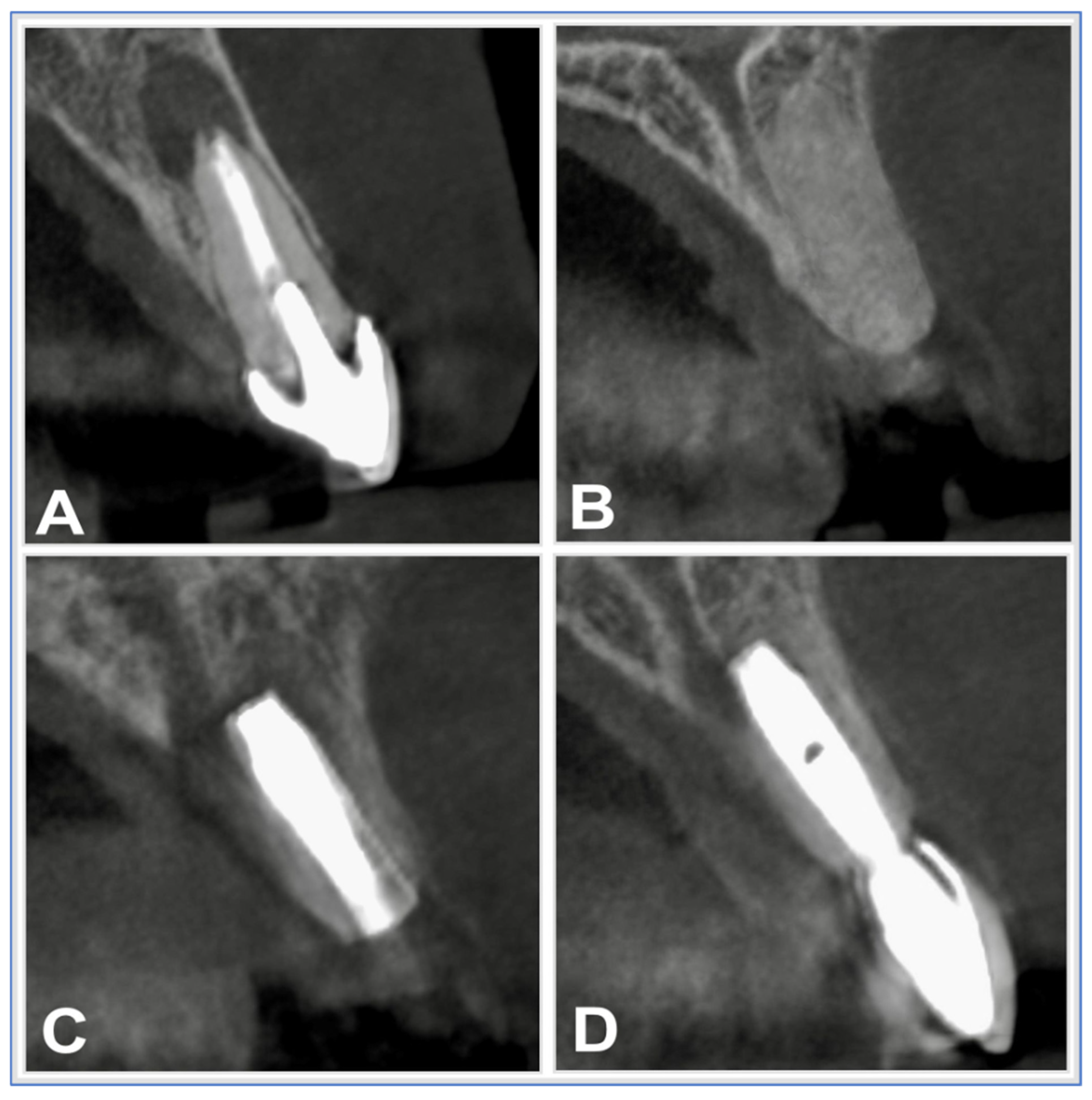
- Preoperative CBCT-based planning
- Atraumatic extraction with socket curettage
- Grafting with Puros® allograft and CopiOs® membrane
- Delayed implant placement based on radiographic healing
- Digital prosthetic workflow using intraoral and facial scans
- Prosthetic restorations were completed by a single prosthodontist (PG) Patients received oral hygiene instructions and were enrolled in a recall program including clinical and radiographic follow-up at 3 and 6 months post-restoration.
2.2.2. Materials Used (Table 1)
| Material | Product Name | Manufacturer | Function/Role |
|---|---|---|---|
| Allograft | Puros® Demineralized Bone Matrix (DBM) | ZimVie Inc., Westminster, CO, USA | Osteoconductive & osteoinductive scaffold |
| Membrane | CopiOs® Pericardium Membrane | ZimVie Inc., Westminster, CO, USA | Barrier membrane for guided bone regeneration |
| Sutures | Prolene® 6-0 | Johnson & Johnson, NJ, USA | Wound closure |
| Anesthetic | Articaine 4% with 1:200,000 epinephrine | 3M ESPE, St. Paul, MN, USA | Local anesthesia |
| Mouth rinse | Chlorhexidine 0.2% | GlaxoSmithKline, Münchenbuchsee, Switzerland | Antiseptic oral rinse |
| Antibiotic | Amoxicillin/Clavulanate 1 g BID, 5 days | Ratiopharm, Ulm, Germany | Postoperative infection prophylaxis |
| Analgesic | Mefenamic acid 100 mg | Pfizer, Austria | Postoperative pain management |
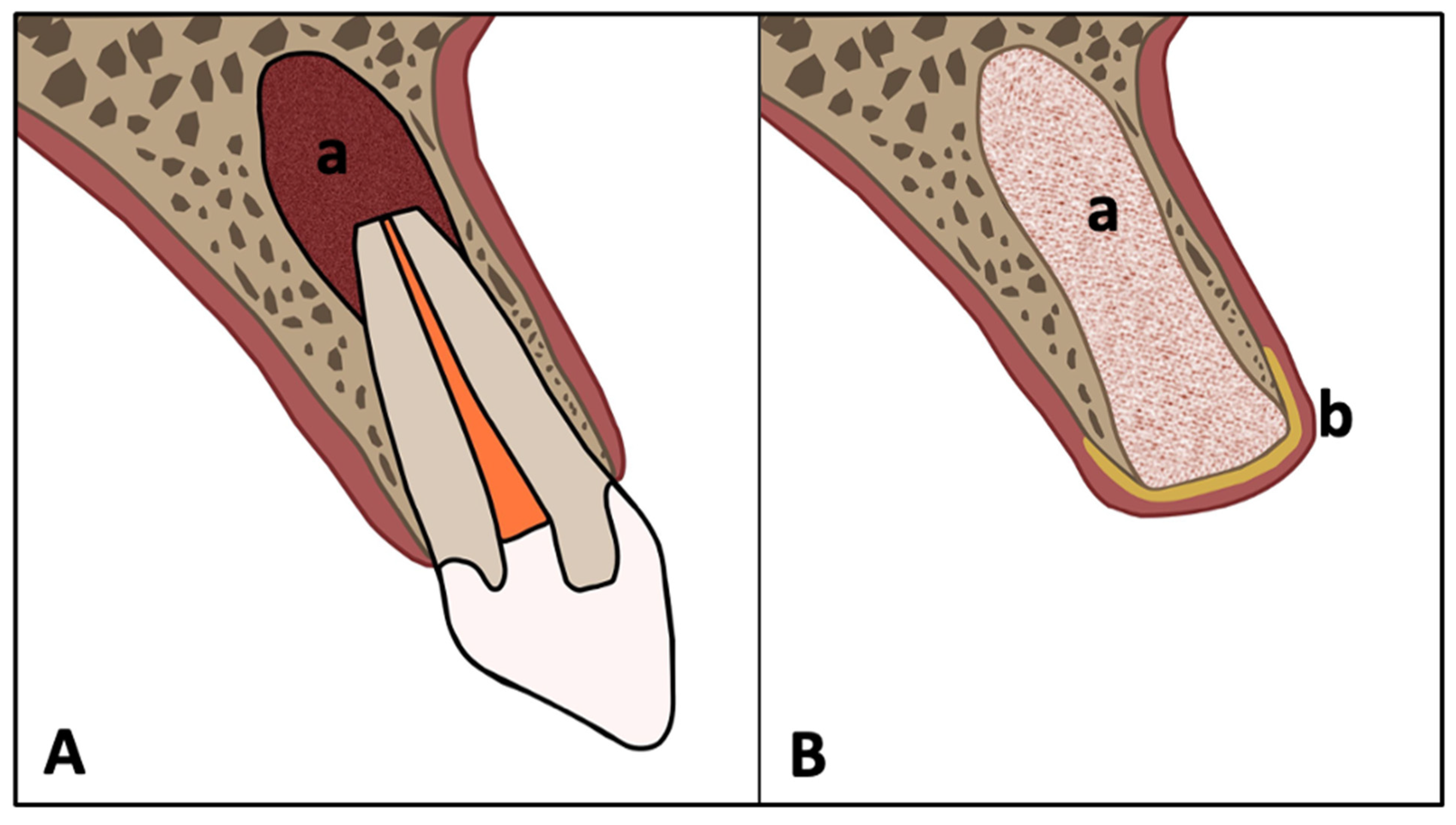
2.2.3. Tooth Extraction
2.2.4. Socket Preservation
2.2.5. Implant Placement
2.2.6. Prosthetic Rehabilitation
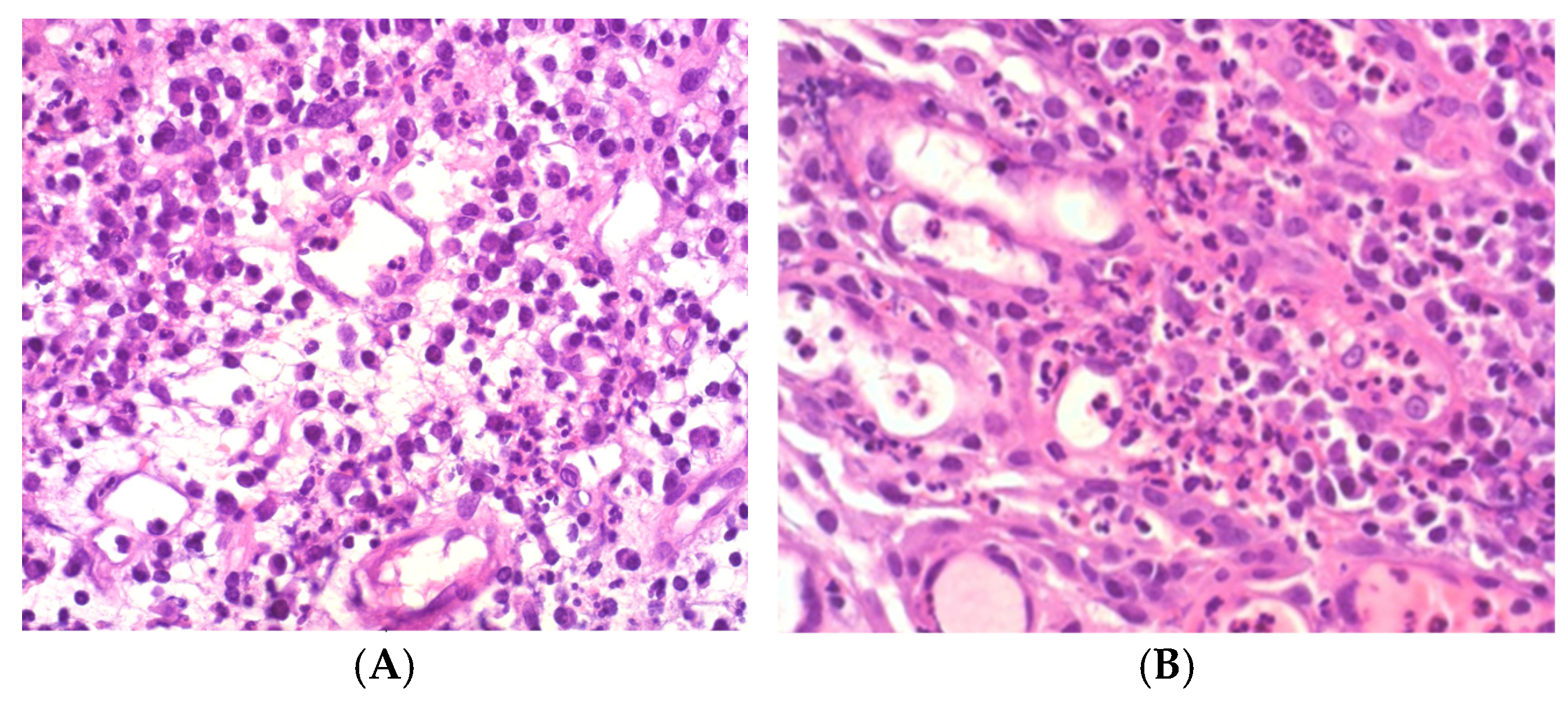
2.3. Histopathological Processing and Evaluation
- DAIS 1: Low acute, low chronic inflammation
- DAIS 2: Low acute, high chronic
- DAIS 3: High acute, low chronic
- DAIS 4: High acute, high chronic (Figure 3)
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
- DAIS 1: 8 patients
- DAIS 2: 14 patients
- DAIS 3: 1 patient
- DAIS 4: 64 patients
- 3iT3 (n = 28)
- 3iT3PT (n = 17)
- Straumann BLX (n = 14)
- BEGO S (n = 12)
- SDS (n = 3)
- Biomet Boss 3i (n = 2)
- BEGO SCX (n = 1)
- BEGO SC (n = 1)
- Straumann BL (n = 1)
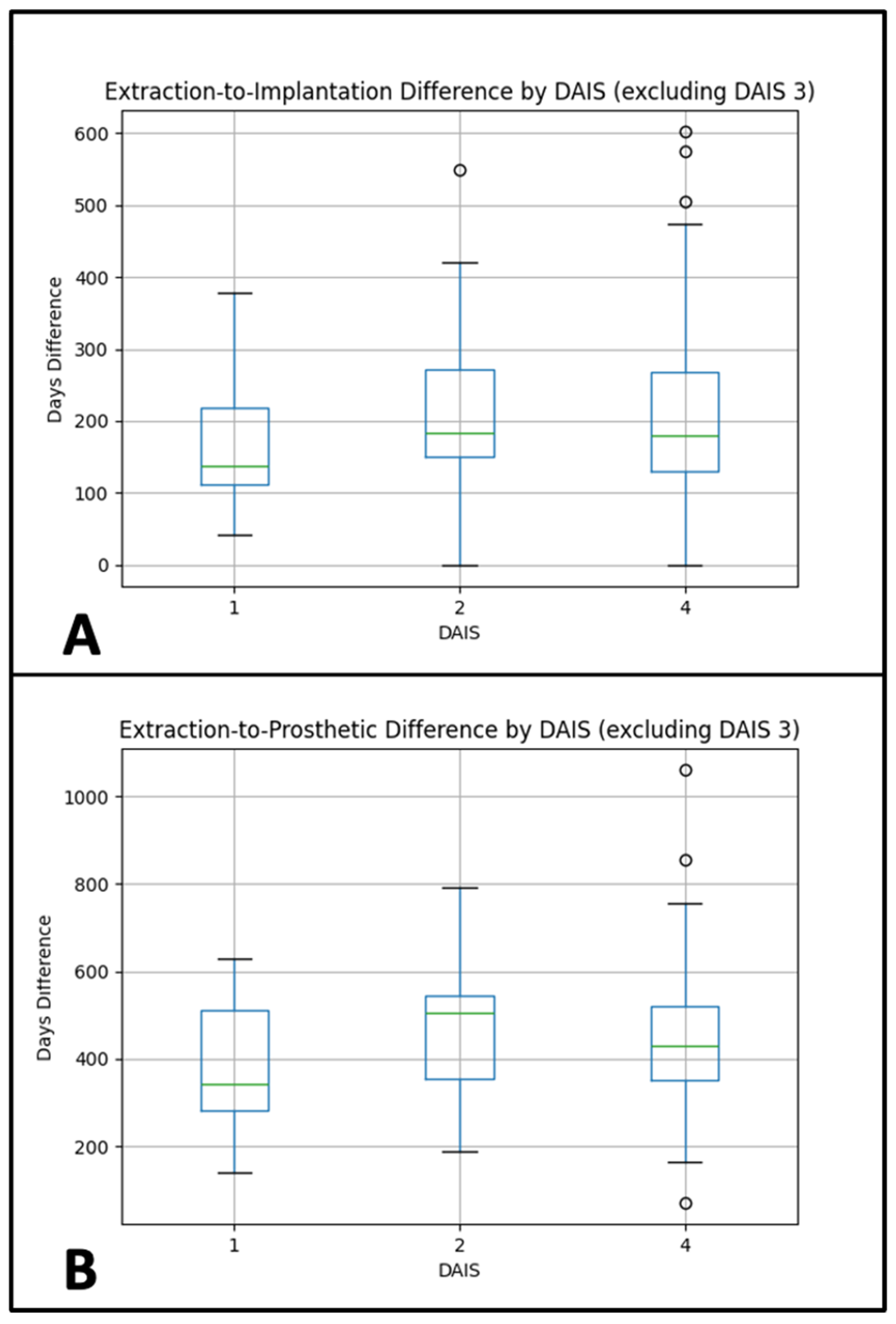
3.2. Extraction-to-Implantation Interval
- DAIS 1: 174.38 days
- DAIS 2: 224.43 days
- DAIS 4: 207.67 days
- Normality: Shapiro–Wilk p-values: DAIS 1 = 0.331; DAIS 2 = 0.589; DAIS 4 = 0.002
- Homogeneity of variances: Levene’s test, p = 0.791
- Interpretation: The mean interval between extraction and implant placement did not differ significantly across DAIS groups. Post-hoc pairwise comparisons with Tukey’s HSD also showed no significant differences between any groups.
- Sensitivity analyses. Additional testing with Welch’s ANOVA and the non-parametric Kruskal–Wallis test confirmed the ANOVA results, indicating no significant group differences in extraction-to-implantation time. See Figure 4A.
3.3. Extraction-to-Prosthetic Interval
- DAIS 1: 375.38 days
- DAIS 2: 480.07 days
- DAIS 4: 452.03 days
- Normality: Shapiro–Wilk p-values: DAIS 1 = 0.382; DAIS 2 = 0.899; DAIS 4 = 0.005
- Homogeneity: Levene’s test, p = 0.894
- Post hoc: Tukey’s HSD test showed no significant pairwise differences.
- Interpretation: The mean interval between extraction and final prosthetic restoration also showed no significant variation across DAIS categories. Post-hoc comparisons again revealed no significant group differences.
- Sensitivity analyses. Welch’s ANOVA and Kruskal–Wallis testing supported the main findings, likewise indicating no significant differences across DAIS groups for extraction-to-prosthetic time. See Figure 4B.
3.4. Histological Diagnosis vs. DAIS Classification
4. Additional Analyses
4.1. Tooth Location (Maxilla vs. Mandible) vs. DAIS
4.2. Endodontic Treatment vs. DAIS
4.3. Age vs. DAIS
4.4. Sex vs. DAIS
5. Discussion
5.1. Clinical Relevance and Impact on Implant Success
5.2. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Calabró, D.G.; Portigliatti, R.P.; Stolbizer, F. Treatment of vital teeth involved in the extension of inflammatory radicular cysts: A systematic review. Acta Odontol. Latinoam. 2024, 37, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Chisci, G.; Chisci, D.; Chisci, E.; Chisci, V.; Stumpo, M.; Chisci, E. The Management of a Geriatric Patient Using Dabigatran Therapy on Dentigerous Cyst with Oral Bleeding. J. Clin. Med. 2024, 13, 1499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Odendaal, A.; Kassan, A.; van Rensburg, L.J.; Afrogheh, A.H. BRAF p.V600E-Negative Langerhans Cell Histiocytosis Associated with a Periapical Cyst: A Case Presentation with Broad Review of the Differential Diagnosis and Disease Pathophysiology. Head Neck Pathol. 2025, 19, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tewari, N.; Rajeswary, A.; Wikström, A.; Tsilingaridis, G. Non-Surgical Endodontic Management of Large Periapical Lesions After Traumatic Dental Injuries. Dent. Traumatol. 2025, 41 (Suppl. S1), 43–52. [Google Scholar] [CrossRef] [PubMed]
- Fontenele, R.C.; Jacobs, R. Unveiling the power of artificial intelligence for image-based diagnosis and treatment in endodontics: An ally or adversary? Int. Endod. J. 2025, 58, 155–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes, P.G.; Novaes, A.B.; de Queiroz, A.C.; de Souza, S.L.S.; Taba, M.; Palioto, D.B.; Grisi, M.F.d.M. Ridge preservation with acellular dermal matrix and anorganic bone matrix cell-binding peptide P-15 after tooth extraction in humans. J. Periodontol. 2011, 82, 72–79. [Google Scholar] [CrossRef]
- Sculean, A.; Stavropoulos, A.; Bosshardt, D. Self-regenerative capacity of intra-oral bone defects. J. Clin. Periodontol. 2019, 46, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 22–38. [Google Scholar] [CrossRef] [PubMed]
- Morjaria, K.R.; Wilson, R.; Palmer, R.M. Bone healing after tooth extraction with or without an intervention: A systematic review of randomized controlled trials. Clin. Implant. Dent. Relat. Res. 2014, 16, 1–20. [Google Scholar] [CrossRef]
- Lang, N.P.; Pun, L.; Lau, K.Y.; Li, K.Y.; Wong, M.C. A systematic review on survival and success rates of implants placed immediately into fresh extraction sockets after at least 1 year. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 39–66. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chiu, T.-S.; Chuang, S.-K.; Tarnow, D.; Stoupel, J. Alterations of the bone dimension following immediate implant placement into extraction socket: Systematic review and meta-analysis. J. Clin. Periodontol. 2014, 41, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Scoggin, Z.D.; Yu, Q. Assessment of bone width for implants in the posterior mandible. J. Oral Maxillofac. Surg. 2015, 73, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Horváth, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.G.; Donos, N. Alveolar ridge preservation. A systematic review. Clin. Oral Investig. 2013, 17, 341–363. [Google Scholar] [CrossRef]
- Danesh-Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: A systematic review and meta-analysis. J. Periodontal Res. 2017, 52, 301–312. [Google Scholar] [CrossRef]
- Le, B.T.; Borzabadi-Farahani, A. Simultaneous implant placement and bone grafting with particulate mineralized allograft in sites with buccal wall defects, a three-year follow-up and review of literature. J. Cranio-Maxillofac. Surg. 2014, 42, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Al-Sebeih, K.H.; Albazee, E.; Alsakka, M.A. Safety of Using Tutoplast-Processed Fascia Lata in Rhinoplasty: A Systematic Review and Meta-Analysis. Aesthetic Plast. Surg. 2025, 49, 4224–4237. [Google Scholar] [CrossRef] [PubMed]
- Gapski, R.; Neiva, R.; Oh, T.-J.; Wang, H.-L. Histologic analyses of human mineralized bone grafting material in sinus elevation procedures: A case series. Int. J. Periodontics Restor. Dent. 2006, 26, 59–69. [Google Scholar]
- Froum, S.J.; Tarnow, D.P.; Wallace, S.S.; Jalbout, Z.; Cho, S.C.; Rohrer, M.D.; Prasad, H.S. The use of a mineralized allograft for sinus augmentation: An interim histological case report from a prospective clinical study. Compend. Contin. Educ. Dent. 2005, 26, 259–260. [Google Scholar]
- Solakoglu, Ö.; Götz, W.; Heydecke, G. Histological and immunohistochemical comparison of two different allogeneic bone grafting materials for alveolar ridge reconstruction: A prospective randomized trial in humans. Clin. Implant. Dent. Relat. Res. 2019, 21, 1002–1016. [Google Scholar] [CrossRef]
- Perelman-Karmon, M.; Kozlovsky, A.; Liloy, R.; Artzi, Z. Socket site preservation using bovine bone mineral with and without a bioresorbable collagen membrane. Int. J. Periodontics Restor. Dent. 2012, 32, 459–465. [Google Scholar]
- Krenn, S.; Gutwald, R.; Bönigk, M.; Krenn, V. Dental Apical Inflammation Score (DAIS): Histopathological scoring for the evaluation of the apical inflammatory activity and local bone destruction. Pathol.-Res. Pract. 2020, 216, 153223. [Google Scholar] [CrossRef]
- Ganapathy, V.; Balaji, A.; Shanmugam, M.; Farjana, N.; Dheraj; Krishnan, M. Survival Rate of Immediate Implants in Periodontally Compromised Patients: A Systematic Review. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S2), S1038–S1042. [Google Scholar] [CrossRef]
- Grün, P.; Hatamikia, S.; Jadadic, R.; Gjergjindreaja, A.; Jansen, L.; Pfaffeneder-Mantai, F.; Fitzek, S.; Mostegel, M.; Choi, K.-E.A.; Turhani, D. Volumetric measurements from manually drawn segmentations of periapical lesions in cone-beam computed tomography scans correlate with the inflammatory activity classified using the dental apical inflammation score. Adv. Oral Maxillofac. Surg. 2025, 17, 100510. [Google Scholar] [CrossRef]
- Al-Juboori, H.; Petronis, Z.; Razukevicius, D. The interrelation between cortical bone thickness and primary and secondary dental implant stability: A systematic review. J. Oral Maxillofac. Res. 2024, 15, e2. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.R. An update on contemporary implant dentistry for general dental practice. Prim. Dent. J. 2024, 13, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 106–134. [Google Scholar] [CrossRef]
- Scheyer, E.T.; Richardson, C.; Mandelaris, G.; Pickering, S.; Nevins, M.; Pope, B.; Janakievski, J.; Toback, G.; Heard, R.H. Retrospective study to determine patient satisfaction of immediately placed and provisionalized implants in the esthetic zone from a US private-practice research network. Compend. Contin. Educ. Dent. 2017, 38, 9–12. [Google Scholar]
- Rashid, R.; Sohrabi, C.B.; Kerwan, A.; Franchi, T.; Mathew, G.; Nicola, M.; Agha, R.A. The STROCSS 2024 guideline: Strengthening the reporting of cohort, cross-sectional, and case–control studies in surgery. Int. J. Surg. 2024, 110, 3151–3165. [Google Scholar] [CrossRef] [PubMed]
- Berlin-Broner, Y.; Levin, L. Dental implant success and endodontic condition of adjacent teeth: A systematic review. Int. J. Oral Maxillofac. Implant. 2020, 35, e91–e97. [Google Scholar] [CrossRef]
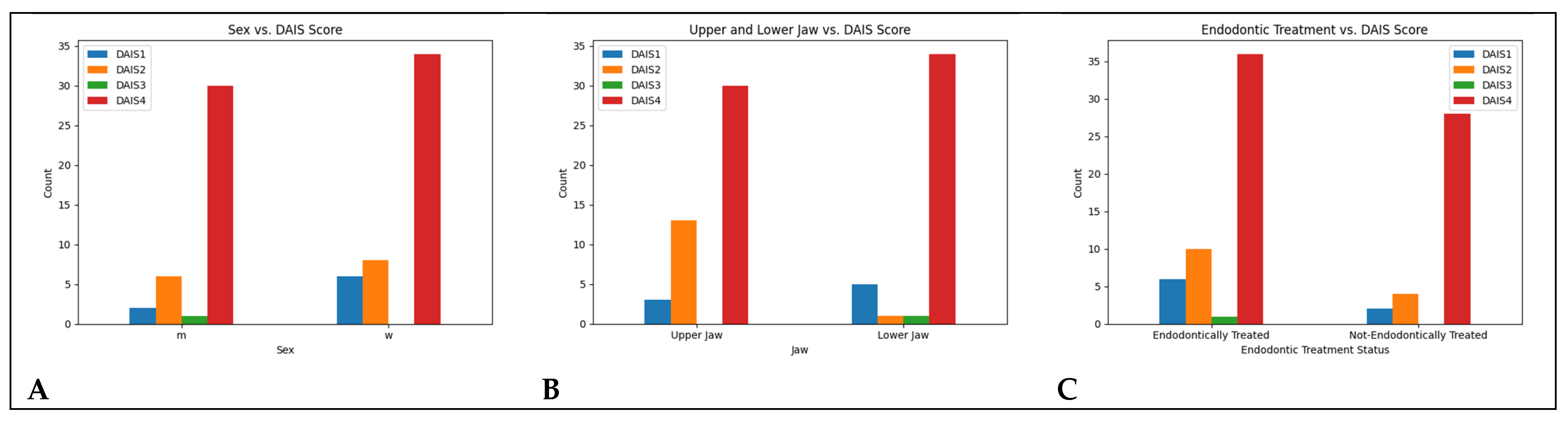
| Sex | DAIS 1 | DAIS 2 | DAIS 3 | DAIS 4 |
|---|---|---|---|---|
| m | 2 | 6 | 1 | 30 |
| w | 6 | 8 | 0 | 34 |
| OK_UK | DAIS 1 | DAIS 2 | DAIS 3 | DAIS 4 |
|---|---|---|---|---|
| OK | 3 | 13 | 0 | 30 |
| UK | 5 | 1 | 1 | 34 |
| Endo_Treated | DAIS 1 | DAIS 2 | DAIS 3 | DAIS 4 |
|---|---|---|---|---|
| yes | 6 | 10 | 1 | 36 |
| no | 2 | 4 | 0 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grün, P.; Meier, M.; Islam, S.M.R.S.; Rödermund, L.; Bytyqi, D.; Turhani, F.; Jung, M.; Fitzek, S.; Mostegel, M.; Turhani, D. Timing of Single-Tooth Implant Rehabilitation and Periapical Inflammation Severity: A Retrospective Study Using the DAIS System. Diagnostics 2025, 15, 2597. https://doi.org/10.3390/diagnostics15202597
Grün P, Meier M, Islam SMRS, Rödermund L, Bytyqi D, Turhani F, Jung M, Fitzek S, Mostegel M, Turhani D. Timing of Single-Tooth Implant Rehabilitation and Periapical Inflammation Severity: A Retrospective Study Using the DAIS System. Diagnostics. 2025; 15(20):2597. https://doi.org/10.3390/diagnostics15202597
Chicago/Turabian StyleGrün, Pascal, Marius Meier, S. M. Ragib Shahriar Islam, Lilli Rödermund, Ditjon Bytyqi, Flora Turhani, Maximilian Jung, Sebastian Fitzek, Margit Mostegel, and Dritan Turhani. 2025. "Timing of Single-Tooth Implant Rehabilitation and Periapical Inflammation Severity: A Retrospective Study Using the DAIS System" Diagnostics 15, no. 20: 2597. https://doi.org/10.3390/diagnostics15202597
APA StyleGrün, P., Meier, M., Islam, S. M. R. S., Rödermund, L., Bytyqi, D., Turhani, F., Jung, M., Fitzek, S., Mostegel, M., & Turhani, D. (2025). Timing of Single-Tooth Implant Rehabilitation and Periapical Inflammation Severity: A Retrospective Study Using the DAIS System. Diagnostics, 15(20), 2597. https://doi.org/10.3390/diagnostics15202597








