OCTA Biomarkers Underlying Structure–Function Correlations in Idiopathic Epiretinal Membrane: A Systematic Review
Abstract
1. Introduction
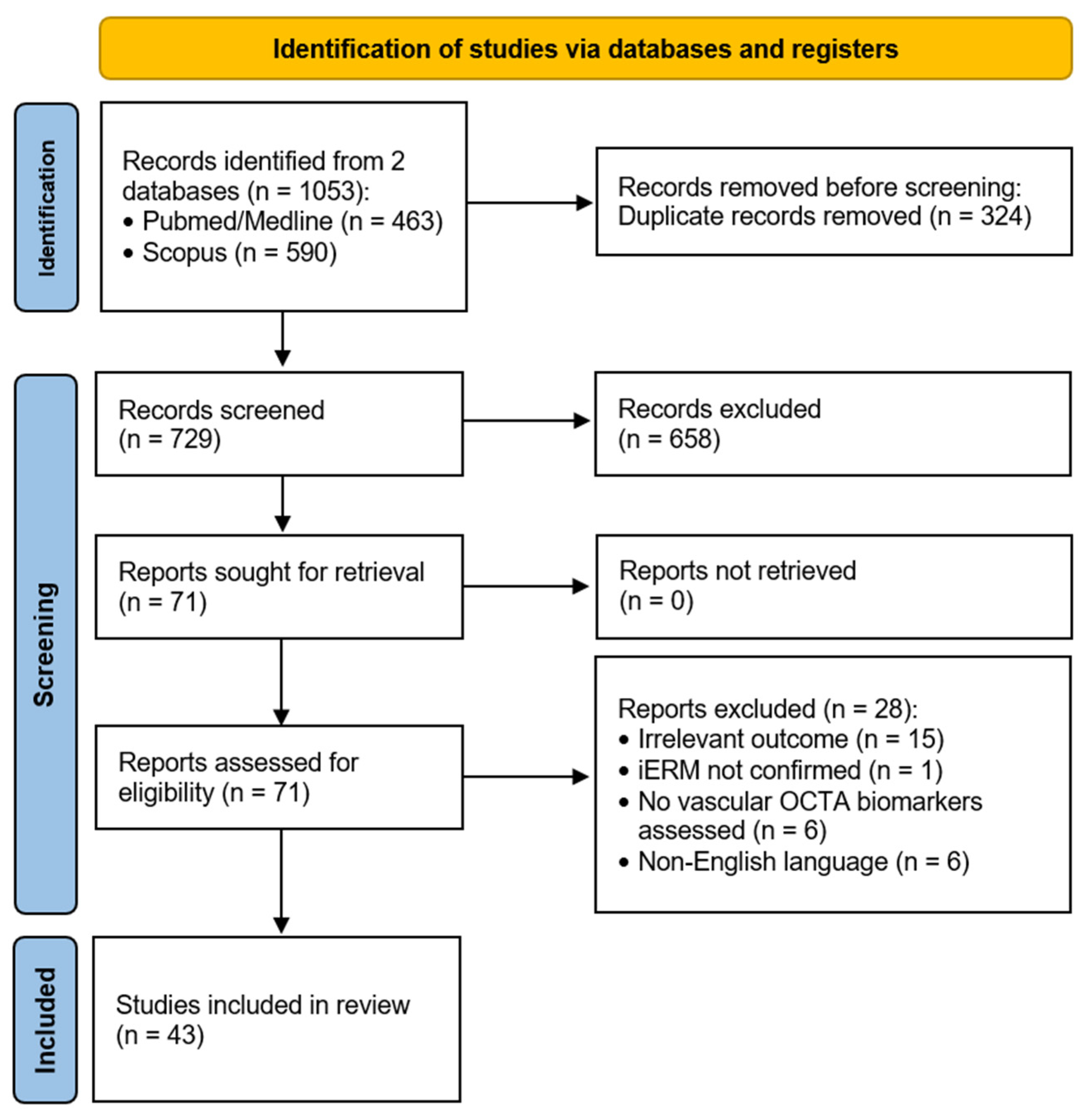
2. Materials and Methods
2.1. Literature Search Methodology
2.2. Risk of Bias Assessment Methodology
3. Results
3.1. Risk of Bias Assessment
3.2. Metrics and Terminology
4. Discussion
4.1. FAZ-Related Biomarkers
4.2. Vessel Density (VD)
4.3. Foveal Density-300 (FD-300)
4.4. Average Vessel Length (VL)
4.5. Blood Flow Area
4.6. Other Indices
4.7. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERM | Epiretinal Membrane |
| iERM | Idiopathic Epiretinal Membrane |
| PPV | Pars Plana Vitrectomy |
| OCT | Optical Coherence Tomography |
| OCTA | Optical Coherence Tomography Angiography |
| VA | Visual Acuity |
| FAZ | Foveal Avascular Zone |
| AI | Acircularity Index |
| ILM | Internal Limiting Membrane |
| VD | Vessel Density |
| VL | Vessel Length |
| VLD | Vessel Length Density |
| FD-300 | Foveal Vessel Density 300 |
| VT | Vessel Tortuosity |
| SCP | Superficial Capillary Plexus |
| DCP | Deep Capillary Plexus |
| BCVA | Best-Corrected Visual Acuity |
| IPL | Inner Plexiform Layer |
| OPL | Outer Plexiform Layer |
| INL | Inner Nuclear Layer |
| EZ | Ellipsoid Zone |
| RS | Retinal Sensitivity |
| MS | Macular Sensitivity |
| CC | Choriocapillaris |
| CH | Choroid |
References
- Bu, S.-C.; Kuijer, R.; Li, X.-R.; Hooymans, J.M.M.; Los, L.I. IDIOPATHIC EPIRETINAL MEMBRANE. Retina 2014, 34, 2317–2335. [Google Scholar] [CrossRef]
- Fraser-Bell, S.; Guzowski, M.; Rochtchina, E.; Wang, J.J.; Mitchell, P. Five-Year Cumulative Incidence and Progression of Epiretinal Membranes: The Blue Mountains Eye Study. Ophthalmology 2003, 110, 34–40. [Google Scholar] [CrossRef]
- Klein, R.; Wang, Q.; Moss, S.E. The epidemiology of epiretinal membranes. Trans. Am. Ophthalmol. Soc. 1994, 92, 403–425. [Google Scholar] [PubMed]
- Govetto, A.; Lalane, R.A.; Sarraf, D.; Figueroa, M.S.; Hubschman, J.P. Insights Into Epiretinal Membranes: Presence of Ectopic Inner Foveal Layers and a New Optical Coherence Tomography Staging Scheme. Am. J. Ophthalmol. 2017, 175, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Mathews, N.R.; Tarima, S.; Kim, D.-G.; Kim, J.E. Foveal Contour Changes Following Surgery for Idiopathic Epiretinal Membrane. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7754–7760. [Google Scholar] [CrossRef]
- Sambhav, K.; Grover, S.; Chalam, K.V. The application of optical coherence tomography angiography in retinal diseases. Surv. Ophthalmol. 2017, 62, 838–866. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Shimada, H.; Shinojima, A.; Nakashizuka, H. FOVEAL AVASCULAR ZONE AREA ANALYSIS USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY BEFORE AND AFTER IDIOPATHIC EPIRETINAL MEMBRANE SURGERY. Retina 2019, 39, 339–346. [Google Scholar] [CrossRef]
- Xu, Z.; Mao, J.; Lao, J.; Deng, X.; Liu, C.; Xu, J.; Wu, S.; Chen, Y.; Shen, L. Macular Retinal Sensitivity and Microvasculature Changes before and after Vitrectomy in Idiopathic Macular Epiretinal Membrane with Classification. Ophthalmologica 2021, 244, 569–580. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Mao, J.; Ye, X.; Chen, H.; Xu, Z.; Shen, L. Long-Term Quantitative Analysis of Inner Retinal Dimples and Visual Function Post Internal Limiting Membrane Peeling in Macular Diseases. Ophthalmol. Ther. 2024, 13, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kawaguchi, A.; Arakawa, H.; Maruko, I.; Iida, T. MISALIGNMENT BETWEEN CENTER OF FOVEAL AVASCULAR ZONE AND CENTER OF FOVEAL PHOTORECEPTORS IN EYES WITH IDIOPATHIC EPIRETINAL MEMBRANE. Retina 2021, 41, 1635–1643. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.; Lee, J.Y.; Kim, J.-G.; Yoon, Y.H. Macular capillary plexuses after epiretinal membrane surgery: An optical coherence tomography angiography study. Br. J. Ophthalmol. 2018, 102, 1086–1091. [Google Scholar] [CrossRef]
- Bacherini, D.; Dragotto, F.; Caporossi, T.; Lenzetti, C.; Finocchio, L.; Savastano, A.; Savastano, M.C.; Barca, F.; Dragotto, M.; Vannozzi, L.; et al. The Role of OCT Angiography in the Assessment of Epiretinal Macular Membrane. J. Ophthalmol. 2021, 2021, 8866407. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, J.; Lao, J.; Deng, X.; Fang, Y.; Chen, N.; Liu, C.; Chen, Y.; Shen, L. A classification of idiopathic epiretinal membrane based on foveal avascular zone area using optical coherence tomography angiography. Ann. Med. 2024, 56, 2316008. [Google Scholar] [CrossRef]
- Isik-Ericek, P.; Sizmaz, S.; Esen, E.; Demircan, N. The effect of epiretinal membrane surgery on macular microvasculature: An optical coherence tomography angiography study. Int. Ophthalmol. 2021, 41, 777–786. [Google Scholar] [CrossRef]
- Shen, Y.; Ye, X.; Tao, J.; Zhao, C.; Xu, Z.; Mao, J.; Chen, Y.; Shen, L. Quantitative assessment of retinal microvascular remodeling in eyes that underwent idiopathic epiretinal membrane surgery. Front. Cell Dev. Biol. 2023, 11, 1164529. [Google Scholar] [CrossRef] [PubMed]
- Okawa, Y.; Maruko, I.; Kawai, M.; Hasegawa, T.; Arakawa, H.; Iida, T. Foveal structure and vasculature in eyes with idiopathic epiretinal membrane. PLoS ONE 2019, 14, e0214881. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, X.; Xu, M.; Wang, Y.; Shi, X.; Zhang, Y.; Huang, P. Association of Microvasculature and Macular Sensitivity in Idiopathic Macular Epiretinal Membrane: Using OCT Angiography and Microperimetry. Front. Med. 2021, 8, 655013. [Google Scholar] [CrossRef]
- Miyazawa, K.; Sakimoto, S.; Kanai, M.; Shiraki, A.; Takahashi, S.; Shiraki, N.; Maruyama, K.; Sakaguchi, H.; Nishida, K. Vascular tortuosity analysis in eyes with epiretinal membrane imaged by optical coherence tomography angiography. BMC Ophthalmol. 2022, 22, 198. [Google Scholar] [CrossRef] [PubMed]
- Yuce, B.; Cinar, E.; Aslan, F.; Kucukerdonmez, C. Evaluation of retinal vascular structure after epiretinal membrane surgery by optical coherence tomography angiography. Int. Ophthalmol. 2021, 41, 621–627. [Google Scholar] [CrossRef]
- Bae, B.-J.; Ryoo, N.-K. Effect of Foveal Pit Restoration in Foveal Avascular Zone after Surgery for Idiopathic Epiretinal Membrane. Korean J. Ophthalmol. 2022, 36, 44–53. [Google Scholar] [CrossRef]
- D’Aloisio, R.; Carpineto, P.; Aharrh-Gnama, A.; Iafigliola, C.; Cerino, L.; Di Nicola, M.; Porreca, A.; Toto, L.; Mastropasqua, R. Early Vascular and Functional Changes after Vitreoretinal Surgery: A Comparison between the Macular Hole and Epiretinal Membrane. Diagnostics 2021, 11, 1031. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lin, T.; Peng, W.; Lu, L.; Hu, J. Macular vascular circulation and retinal oxygen saturation changes for idiopathic macular epiretinal membrane after vitrectomy. Acta Ophthalmol. 2019, 97, 296–302. [Google Scholar] [CrossRef]
- Nicolai, M.; Franceschi, A.; De Turris, S.; Rosati, A.; Carpenè, M.J.; Danieli, L.; Lassandro, N.V.; Pelliccioni, P.; Lupidi, M.; Mariotti, C. Correlation between retinal sensitivity and retinal vascular perfusion after idiopathic epiretinal membrane peeling. Eur. J. Ophthalmol. 2024, 34, 1228–1238. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Li, H.; Yang, S.; Liu, Y.; Wang, F. Effects of disorganization of retinal inner layers for Idiopathic epiretinal membrane surgery: The surgical status and prognosis. BMC Ophthalmol. 2023, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chi, W.; Cai, X.; Deng, Y.; Jiang, X.; Wei, Y.; Zhang, S. Macular microvasculature features before and after vitrectomy in idiopathic macular epiretinal membrane: An OCT angiography analysis. Eye 2019, 33, 619–628. [Google Scholar] [CrossRef]
- Yanık, Ö.; Aydın Ellialtıoğlu, P.; Demirel, S.; Batıoğlu, F.; Özmert, E. Retinal Vascular Tortuosity Index Change after Idiopathic Epiretinal Membrane Surgery: Does Internal Limiting Membrane Peeling Affect Retinal Vascular Tortuosity? Diagnostics 2023, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Caretti, L.; Pillon, G.; Verzola, G.; Angelini, E.; Monterosso, C.; Bonfiglio, V.; Longo, A.; Formisano, M. Idiopathic epiretinal membrane surgery with internal limiting membrane peeling: An optical coherence tomography angiography analysis of macular capillary plexus changes. Eur. J. Ophthalmol. 2025, 35, 1394–1401. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.H. TEMPORAL CHANGES OF PARAFOVEAL MICROVASCULATURE AFTER EPIRETINAL MEMBRANE SURGERY: An Optical Coherence Tomography Angiography Study. Retina 2021, 41, 1839–1850. [Google Scholar] [CrossRef]
- Ersoz, M.G.; Hocaoglu, M.; Sayman Muslubas, I.; Arf, S.; Karacorlu, M. QUANTITATIVE ASSESSMENT OF THE FOVEAL AVASCULAR ZONE USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY BEFORE AND AFTER SURGERY FOR IDIOPATHIC EPIRETINAL MEMBRANE. Retina 2021, 41, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Xu, Z.; Lao, J.; Chen, Y.; Xu, X.; Wu, S.; Zheng, Z.; Liu, B.; Shen, L. Assessment of macular microvasculature features before and after vitrectomy in the idiopathic macular epiretinal membrane using a grading system: An optical coherence tomography angiography study. Acta Ophthalmol. 2021, 99, e1168–e1175. [Google Scholar] [CrossRef]
- Chatzistergiou, V.; Papasavvas, I.; Ambresin, A.; Pournaras, J.-A.C. Prediction of Postoperative Visual Outcome in Patients with Idiopathic Epiretinal Membrane. Ophthalmologica 2021, 244, 535–542. [Google Scholar] [CrossRef]
- Henry, M.; Ndiaye, N.C.; Angioi-Duprez, K.; Berrod, J.-P.; Conart, J.-B. Association of Microvasculature Changes with Visual Outcomes in Idiopathic Epiretinal Membrane Surgery: A Clinical Trial. J. Clin. Med. 2024, 13, 4748. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Zou, B.; Liu, M.; Dai, C.; Zheng, Y.; Ding, X. Relationship between macular microvasculature and visual function in idiopathic macular epiretinal membrane by using OCT angiography and multifocal electroretinogram. Photodiagn. Photodyn. Ther. 2024, 50, 104403. [Google Scholar] [CrossRef]
- Hondur, A.M.; Aribas, Y.K. Choroidal macrovascular and capillary alterations in eyes with idiopathic epiretinal membranes. Arq. Bras. Oftalmol. 2024, 87, e20220369. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Li, Z.; Hou, Q.; Wang, C.; Li, X. Insights into the underlying choroid in different stages of idiopathic epiretinal membranes after Viteromacular surgery. Acta Ophthalmol. 2023, 101, 403–412. [Google Scholar] [CrossRef]
- Told, R.; Georgopoulos, M.; Reiter, G.S.; Wassermann, L.; Aliyeva, L.; Baumann, L.; Abela-Formanek, C.; Pollreisz, A.; Schmidt-Erfurth, U.; Sacu, S. Intraretinal microvascular changes after ERM and ILM peeling using SSOCTA. PLoS ONE 2020, 15, e0242667. [Google Scholar] [CrossRef]
- Osada, U.; Kunikata, H.; Yasuda, M.; Hashimoto, K.; Nishiguchi, K.M.; Nakazawa, T. Association of retinal vessel density with retinal sensitivity in surgery for idiopathic epiretinal membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1911–1920. [Google Scholar] [CrossRef]
- Hirata, A.; Nakada, H.; Mine, K.; Masumoto, M.; Sato, T.; Hayashi, K. Relationship between the morphology of the foveal avascular zone and the degree of aniseikonia before and after vitrectomy in patients with unilateral epiretinal membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 507–515. [Google Scholar] [CrossRef]
- Honzawa, K.; Horiguchi, H.; Terauchi, R.; Iida, Y.; Katagiri, S.; Gunji, H.; Nakano, T. Rhombus Deformation Of Retinal Lateral Displacement After Epiretinal Membrane Removal Revealed By Diffeomorphic Image Registration. Retina 2023, 43, 1132–1142. [Google Scholar] [CrossRef]
- Mao, J.; Lao, J.; Liu, C.; Zhang, C.; Chen, Y.; Tao, J.; Shen, L. A study analyzing macular microvasculature features after vitrectomy using OCT angiography in patients with idiopathic macular epiretinal membrane. BMC Ophthalmol. 2020, 20, 165. [Google Scholar] [CrossRef]
- Frisina, R.; De Salvo, G.; Tozzi, L.; Gius, I.; Sahyoun, J.-Y.; Parolini, B.; Meduri, A. Effects of physiological fluctuations on the estimation of vascular flow in eyes with idiopathic macular pucker. Eye 2023, 37, 1470–1478. [Google Scholar] [CrossRef]
- Kim, G.-H.; Hwang, B.-E.; Chun, H.; Kim, J.Y.; Kim, R.Y.; Kim, M.; Park, Y.-G.; Park, Y.-H. Morphologic analysis of the foveal avascular zone for prediction of postoperative visual acuity in advanced idiopathic epiretinal membrane. Sci. Rep. 2023, 13, 10400. [Google Scholar] [CrossRef]
- Liao, X.; Keyal, K.; Li, H.; Wang, F. One-year outcomes of 27G core-pars plana vitrectomy of idiopathic epiretinal membrane. Exp. Ther. Med. 2020, 20, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Terashima, H.; Ueda, E.; Hasebe, H.; Matsuoka, N.; Nakano, H.; Fukuchi, T. Relationship between morphological changes in the foveal avascular zone of the epiretinal membrane and postoperative visual function. BMJ Open Ophthalmol. 2020, 5, e000636. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiuseppe, E.; Visioli, G.; Albanese, G.M.; Iannetti, L.; Romano, E.; Guillot, A.; Lucchino, L.; Gharbiya, M. Peripapillary and Macular Optical Coherence Tomography Angiography Predictors of Visual Improvement in Patients Treated with Vitrectomy for Idiopathic Epiretinal Membrane. Ophthalmologica 2025, 248, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H.; Lee, J.; Park, Y.-H. Exploratory analysis of choriocapillaris vasculature as a biomarker of idiopathic epiretinal membrane. PLoS ONE 2024, 19, e0306735. [Google Scholar] [CrossRef]
- Lin, G.-C.; Lin, H.-S.; Horng, Y.-H.; Chu, H.-C.; Sheu, S.-J. Intraocular pressure might play a role in the surgical management of patients with epiretinal membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2691–2699. [Google Scholar] [CrossRef]
- Li, J.; Cheng, F.; Li, Z.; Wang, L. Assessment of clinical outcomes and prognostic factors following membrane peeling in idiopathic epiretinal membrane using EIFL staging system: An optical coherence tomography angiography analysis. BMC Ophthalmol. 2025, 25, 54. [Google Scholar] [CrossRef]
- Rommel, F.; Brinkmann, M.P.; Sochurek, J.A.M.; Prasuhn, M.; Grisanti, S.; Ranjbar, M. Ocular Blood Flow Changes Impact Visual Acuity Gain after Surgical Treatment for Idiopathic Epiretinal Membrane. J. Clin. Med. 2020, 9, 1768. [Google Scholar] [CrossRef]
- Mavi Yildiz, A.; Avci, R.; Yilmaz, S. The predictive value of ectopic inner retinal layer staging scheme for idiopathic epiretinal membrane: Surgical results at 12 months. Eye 2021, 35, 2164–2172. [Google Scholar] [CrossRef]
- Zhan, J.; Chen, C.; Wang, T.; Zhang, Q.; Huang, X.; Lu, L.; Zhao, X. Perfusion Capacity as a Predictive Index for Assessing Visual Functional Recovery in Patients With Idiopathic Epiretinal Membrane. Transl. Vis. Sci. Technol. 2025, 14, 19. [Google Scholar] [CrossRef]
- Baba, T.; Kakisu, M.; Nizawa, T.; Oshitari, T.; Yamamoto, S. Study of foveal avascular zone by OCTA before and after idiopathic epiretinal membrane removal. Spektrum Augenheilkd 2018, 32, 31–38. [Google Scholar] [CrossRef]
- Munk, M.R.; Kashani, A.H.; Tadayoni, R.; Korobelnik, J.-F.; Wolf, S.; Pichi, F.; Tian, M. Standardization of OCT Angiography Nomenclature in Retinal Vascular Diseases: First Survey Results. Ophthalmol. Retina 2021, 5, 981–990. [Google Scholar] [CrossRef]
- Kadonosono, K.; Itoh, N.; Nomura, E.; Ohno, S. Capillary blood flow velocity in patients with idiopathic epiretinal membranes. Retina 1999, 19, 536–539. [Google Scholar] [CrossRef]
- Jonas, J.B.; Schneider, U.; Naumann, G.O.H. Count and density of human retinal photoreceptors. Graefes Arch. Clin. Exp. Ophthalmol. 1992, 230, 505–510. [Google Scholar] [CrossRef]
- Mansour, A.M.; Schachat, A.; Bodiford, G.; Haymond, R. Foveal avascular zone in diabetes mellitus. Retina 1993, 13, 125–128. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Inoue, M.; Ahn, S.; McCann, J.; Dhrami-Gavazi, E.; Yannuzzi, L.A.; Freund, K.B. Visual Acuity Is Correlated with the Area of the Foveal Avascular Zone in Diabetic Retinopathy and Retinal Vein Occlusion. Ophthalmology 2016, 123, 2352–2367. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Morita, S.; Watanabe, Y.; Kaneko, T.; Yamane, S.; Kobayashi, S.; Arakawa, A.; Kadonosono, K. Inner Segment/Outer Segment Junction Assessed by Spectral-Domain Optical Coherence Tomography in Patients with Idiopathic Epiretinal Membrane. Am. J. Ophthalmol. 2010, 150, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T.; et al. Determinants of Quantitative Optical Coherence Tomography Angiography Metrics in Patients with Diabetes. Sci. Rep. 2017, 7, 2575. [Google Scholar] [CrossRef] [PubMed]
- Sampson, D.M.; Dubis, A.M.; Chen, F.K.; Zawadzki, R.J.; Sampson, D.D. Towards standardizing retinal optical coherence tomography angiography: A review. Light Sci. Appl. 2022, 11, 63. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, S.J.; Woo, S.J.; Park, K.H. CORRELATION BETWEEN INNER-RETINAL CHANGES AND OUTER-RETINAL DAMAGE IN PATIENTS WITH IDIOPATHIC EPIRETINAL MEMBRANE. Retina 2018, 38, 2327–2335. [Google Scholar] [CrossRef]
- Okamoto, F.; Sugiura, Y.; Okamoto, Y.; Hiraoka, T.; Oshika, T. INNER NUCLEAR LAYER THICKNESS AS A PROGNOSTIC FACTOR FOR METAMORPHOPSIA AFTER EPIRETINAL MEMBRANE SURGERY. Retina 2015, 35, 2107–2114. [Google Scholar] [CrossRef]
- Spaide, R.F. Volume-Rendered Optical Coherence Tomography of Diabetic Retinopathy Pilot Study. Am. J. Ophthalmol. 2015, 160, 1200–1210. [Google Scholar] [CrossRef]
- Jeon, S.; Jung, B.; Lee, W.K. LONG-TERM PROGNOSTIC FACTORS FOR VISUAL IMPROVEMENT AFTER EPIRETINAL MEMBRANE REMOVAL. Retina 2019, 39, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Rommel, F.; Siegfried, F.; Sochurek, J.A.M.; Rothe, M.; Brinkmann, M.P.; Kurz, M.; Prasuhn, M.; Grisanti, S.; Ranjbar, M. Mapping diurnal variations in choroidal sublayer perfusion in patients with idiopathic epiretinal membrane: An optical coherence tomography angiography study. Int. J. Retina Vitr. 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Laíns, I.; Silverman, R.F.; Sobrin, L.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Visualization of Choriocapillaris and Choroidal Vasculature in Healthy Eyes With En Face Swept-Source Optical Coherence Tomography Versus Angiography. Transl. Vis. Sci. Technol. 2018, 7, 25. [Google Scholar] [CrossRef]
- Inanc, M.; Tekin, K.; Kiziltoprak, H.; Ozalkak, S.; Doguizi, S.; Aycan, Z. Changes in Retinal Microcirculation Precede the Clinical Onset of Diabetic Retinopathy in Children With Type 1 Diabetes Mellitus. Am. J. Ophthalmol. 2019, 207, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Koustenis, A.; Harris, A.; Gross, J.; Januleviciene, I.; Shah, A.; Siesky, B. Optical coherence tomography angiography: An overview of the technology and an assessment of applications for clinical research. Br. J. Ophthalmol. 2017, 101, 16–20. [Google Scholar] [CrossRef]
- Abdolahi, F.; Zhou, X.; Ashimatey, B.S.; Chu, Z.; Jiang, X.; Wang, R.K.; Kashani, A.H. Optical Coherence Tomography Angiography–Derived Flux As a Measure of Physiological Changes in Retinal Capillary Blood Flow. Transl. Vis. Sci. Technol. 2021, 10, 5. [Google Scholar] [CrossRef]
- Chu, Z.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.; Chen, C.-L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Opt. 2016, 21, 066008. [Google Scholar] [CrossRef] [PubMed]
- Kofod, M.; La Cour, M. Quantification of Retinal Tangential Movement in Epiretinal Membranes. Ophthalmology 2012, 119, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Dolz-Marco, R.; Freund, K.B.; Balaratnasingam, C.; Dansingani, K.; Gilani, F.; Mehta, N.; Young, E.; Klifto, M.R.; Chae, B.; et al. Fractal Dimensional Analysis of Optical Coherence Tomography Angiography in Eyes With Diabetic Retinopathy. Investig. Opthalmology Vis. Sci. 2016, 57, 4940. [Google Scholar] [CrossRef]
- Dave, V.P.; Pappuru, R.R.; Gindra, R.; Ananthakrishnan, A.; Modi, S.; Trivedi, M.; Harikumar, P. OCT angiography fractal analysis-based quantification of macular vascular density in branch retinal vein occlusion eyes. Can. J. Ophthalmol. 2019, 54, 297–300. [Google Scholar] [CrossRef]
- Avakian, A.; Kalina, R.E.; Sage, E.H.; Rambhia, A.H.; Elliott, K.E.; Chuang, E.L.; Clark, J.I.; Hwang, J.-N.; Parsons-Wingerter, P. Fractal analysis of region-based vascular change in the normal and non-proliferative diabetic retina. Curr. Eye Res. 2002, 24, 274–280. [Google Scholar] [CrossRef] [PubMed]
| Database | Strategy | Results |
|---|---|---|
| Pubmed | (“membrane”[all fields] AND “epimacular”[all fields]) OR (“membrane”[all fields] AND “epiretinal”[all fields]) OR (“cellophane”[all fields] AND “maculopathy”[all fields]) OR (“macular”[all fields] AND “pucker”[all fields]) AND (“peeling”[all fields] OR “surgery”[all fields]) AND (“OCTA”[all fields] OR “angiography”[all fields]) | 463 |
| Scopus | ((membrane AND epimacular) OR (membrane AND epiretinal) OR (cellophane AND maculopathy) OR (macular AND pucker) AND (peeling OR surgery) AND (octa OR angiography)) | 590 |
| Study | Study Design | No. of iERM Eyes Included | Control Group | Type of Surgery | Follow-Up OCTA Regimen | Analyzed OCTA Biomarkers | OCTA Biomarkers Correlated with Visual Function |
|---|---|---|---|---|---|---|---|
| Kim et al., 2018 [12] | Retrospective | 43 | Fellow healthy eye | PPV with ERM and ILM peeling + Phacoemulsification and foldable IOL implantation in phakic eyes | 6 months | FAZ area in the SCP and DCP, Parafoveal VD in the SCP and DCP | 6 months: Greater decreases in FAZ area and parafoveal VD both in the SCP and DCP compared to the fellow eye correlated with worse BCVA |
| Bacherini et al., 2021 [13] | Prospective | 23 | None | PPV 25G with ERM and ILM peeling + Gas tamponade + Phacoemulsification and IOL implantation | Baseline, 1, 3, 6 months | FAZ area, FAZ perimeter, FAZ circularity, VD and PD in the SCP, DCP, CC and CH | Baseline: Lower SCPPD, DCPPD, DCPVD, CHPD, CHVD, FAZ area and FAZ perimeter correlated with lower BCVA 1 month: BCVA negatively correlated with FAZ area and FAZ perimeter 3 months: BCVA negatively correlated with CHPD, CHVD, FAZ circularity 6 months: BCVA correlated with SCPVD (inverse finding—higher SCPVD associated with worse BCVA) Predictive: Baseline FAZ circularity and CHPD negatively correlated with BCVA at 3 months |
| Zhang et al., 2024 [14] | Retrospective | 162 (105 had surgery and the 12-month follow-up) | Fellow healthy eye | PPV 23G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1, 3, 6, 12 months | FAZ area, FAZ perimeter, FAZ AI, FD-300 on the retina slab | Baseline: FAZ AI positively correlated with BCVA across all stages; FAZ area, FAZ perimeter and FD-300 negatively correlated with BCVA generally, across all stages, and at stage 1 iERM (FAZ-based classification) Predictive: Baseline FAZ area (over all stages) and FD-300 (overall stages and stages 2 and 3) negatively correlated with final BCVA |
| Isik-Ericek et al., 2021 [15] | Prospective | 24 | Age- and sex-matched group | PPV 23G with ERM ± ILM peeling + Fluid/air exchange ± Gas tamponade + Phacoemulsification and IOL implantation in case of advanced lens opacities | Baseline, 1, 3, 6 months | FAZ area, Parafoveal VD in the SCP and DCP, flow area in the SCP and DCP | 6 months: Parafoveal VD and flow area, both in DCP negatively correlated with BCVA |
| Shen et al., 2023 [16] | Retrospective | 41 | Age-matched group | PPV 23G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes over 55 years of age | Baseline, 1, 3 months | FAZ area, FAZ perimeter, FAZ AI, VD in the SCP and DCP, VT | 3 months: SCP VD negatively correlated with BCVA and positively correlated with MS Predictive: Reduction in VT in the temporal, superior, and inferior quadrants over a 3-month period correlated with improvement of retinal MS in multivariate linear regression |
| Okawa et al., 2019 [17] | Retrospective | 49 (20 had surgery) | Age-matched group | PPV with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline & final (mean 147 d); follow-up <6 vs. ≥6 months | FAZ area in the SCP | None identified |
| Feng et al., 2021 [18] | Retrospective observational | 25 | None | PPV 23G with ERM and ILM peeling + Fluid/air exchange + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 3 months | FAZ area, VD in the SCP and DCP in the fovea and parafovea | Baseline: VD of foveal and parafoveal SCP negatively correlated with foveal sensitivity Predictive: Higher baseline foveal VD of the DCP predicts better BCVA and higher foveal and parafoveal sensitivity at 3 months postoperatively (confirmed by a multiple linear regression model) |
| Miyazawa et al., 2022 [19] | Prospective | 22 | None | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1, 3, 6 months | FAZ area in the SCP and DCP, Distortion of vessels (VL/BD) | 1, 3, 6 months: VL/BD in the superior and inferior quadrants was positively correlated with postoperative BCVA Predictive: Change in VL in the nasal quadrant was positively associated with change in BCVA at 3 and 6 months |
| Yuce et al., 2021 [20] | Retrospective | 22 | Fellow healthy eye | PPV 25G with ERM and ILM peeling ± Phacoemulsification and IOL implantation | Baseline, 6 months | FAZ area in SCP and DCP, VD in the SCP and DCP in the fovea and parafovea | Baseline: BCVA negatively correlates with FAZ area in SCP and DCP 6 months: BCVA positively correlates with FAZ area in SCP and DCP Baseline and 6 months: VD DCP in the fovea and parafovea positively correlated with BCVA |
| Bae and Ryoo, 2022 [21] | Retrospective | 43 | Fellow healthy eye | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | 3 months before, baseline, 1, 3, 6, 12 months | FAZ area in the SCP | Baseline: FAZ area was negatively correlated with BCVA |
| D’Aloisio et al., 2021 [22] | Observational | 23 | None | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1, 3 months | PD in the SCP, DCP and CC, VLD in the SCP and DCP | 3 months: macular SCP PD positively correlated with MS at 10° and 2°, macular DCP PD with MS at 2°, and peripheral DCP PD with MS at 10°. Similarly, macular SCP VLD positively correlated with MS at both 10° and 2°, and peripheral DCP VLD with MS at 10° and 2° |
| Li et al., 2019 [23] | Prospective | 24 | None | PPV with ERM and ILM peeling + Air or Gas tamponade | Baseline, 3 months | VD of the CCP, flow area in the SCP, DCP and CCP | None identified |
| Nicolai et al., 2024 [24] | Prospective, observational | 29 | Fellow healthy eye | PPV 27G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 6 months | FAZ area, VPD in the SCP, DCP and CC | 6 months: BCVA negatively correlated with postoperative VPD in the SCP and CC plexus Predictive: Patients with improved RS postoperatively showed significantly greater increases in VPD in the SCP, DCP, and CC within both the foveal and parafoveal regions |
| Li et al., 2023 [25] | Retrospective | 74 (36 with OCTA at 12-month follow-up) | None | PPV 23G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | 12 months | FAZ area, FAZ perimeter, FAZ AI, FD-300 | 12 months: FD-300 negatively correlated with BCVA (in a multiple linear regression analysis) |
| Chen et al., 2019 [26] | Observational | 33 | Fellow healthy eye | PPV with ERM peeling | Baseline, 6 months | FAZ area in the SCP, VD in the fovea and parafovea in the SCP, DCP, OCP and CCP, flow area in the OCP and CCP | 6 months: FAZ area negatively correlated with BCVA and larger interocular differences in FAZ area correlated with worse BCVA |
| Xu et al., 2021 [9] | Retrospective | 53 (35 had surgery) | 22 eyes with mild cataract only | PPV 23G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes age over 50 | Baseline, 3 months | VD in the SCP and DCP in the fovea, parafovea, perifovea and whole VD (6 × 6 mm2 area) | 3 months: whole RS positively correlated with whole VD, parafoveal RS with parafoveal VD, and perifoveal RS with perifoveal VD, all within the SCP, only in eyes classified as grade 2 iERM according to the Mathews OCT classification |
| Yanık et al., 2023 [27] | Retrospective | 25 | None | PPV 25G with ERM ± ILM peeling (15 had ERM + ILM peeling) | Baseline, 1 month | FAZ area, FAZ perimeter, FAZ AI, FD-300, RVTI in the SCP | 1 month: BCVA correlated with RVTI |
| Caretti et al., 2025 [28] | Retrospective | 39 | None | PPV 27G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes (all patients) | Baseline, 1, 6 months | FAZ area, FAZ perimeter, FD-300 on the retina slab, VD in the SCP and DCP in the fovea and parafovea | None identified |
| Kim and Park, 2021 [29] | Retrospective | 71 | None | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in eyes with visually significant cataract | Baseline, 1 week, 1, 3, 6 months | FD and lacunarity in the parafoveal DCP, FBP length and difference in the SCP | Baseline: BCVA negatively correlated with FD and positively with lacunarity in the DCP and FBP difference in the SCP; Metamorphopsia negatively correlated with FD and positively with lacunarity in the DCP Predictive: Baseline FD in the DCP negatively correlated with BCVA at 1 week and 1 and 4 months after surgery (significant correlation at 10 months in the pseudophakic group that remained significant in multivariate analysis) |
| Ersoz et al., 2021 [30] | Retrospective | 28 (included patients with intact EZ only) | None | PPV 23G with ERM and ILM peeling | Baseline, 6 months | FAZ area, FAZ perimeter, FAZ AI, FD-300 on the retina slab | Predictive: Postoperative letter score gain correlated negatively with baseline FAZ area and FAZ perimeter (FAZ perimeter confirmed by multivariable linear regression analysis) |
| Mao et al., 2021 [31] | Retrospective | 100 (62 had surgery) | Fellow healthy eye | PPV with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes over 50 age | Baseline, 3 months | FAZ area, FAZ perimeter, FAZ AI, FD-300, SCP VD and DCP VD in the fovea and parafovea, MVR (FVD/PRVD) in the SCP and DCP | Baseline: BCVA negatively correlated with FAZ area and FAZ perimeter and positively correlated with the MVR in the SCP Predictive: Postoperative BCVA positively correlated with baseline foveal VD in the DCP |
| Chatzistergiou et al., 2021 [32] | Retrospective | 54 | None | PPV with ERM peeling | Baseline, 3 months | VD in the SCP and DCP of the whole image (6 × 6 mm2 area) and of the fovea | None identified |
| Henry et al., 2024 [33] | Retrospective observational | 47 | Fellow healthy eye | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes over 60 age or younger in case of cataract | Baseline, 1 week, 1, 6 months | FAZ area in the SCP, VD in the SCP and DCP in the whole macular region (3 × 3 mm2), fovea, parafovea, and perifovea | Baseline: BCVA negatively correlated with FAZ area and macular VD in the DCP Predictive: BCVA at 6 months was negatively correlated with baseline macular VD in both the DCP and SCP; neither association remained significant in multivariate regression analysis |
| Xu et al., 2024 [34] | Retrospective | 30 | None | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1, 3 months | FAZ area, VD in the SCP and DCP in the fovea and parafovea | Predictive: Baseline parafoveal VD in DCP negatively correlated with postoperative BCVA and baseline FAZ area positively correlated with postoperative P1 amplitude (ring 1); both correlations persisted as independent predictors in the multivariate linear regression analysis |
| Hondur and Aribas, 2024 [35] | Retrospective | 33 | Fellow healthy eye | PPV 25G with ERM and ILM peeling + Fluid/air exchange (air/ gas SF6 exchange performed in 1 eye with retinal tear) | Baseline, 6 months | CC flow density | None identified |
| Wang et al., 2023 [36] | Prospective | 102 | Fellow healthy eye | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1 week, 1, 3, 6, 12 months | Choroidal capillary perfusion | 1, 3, 6, 12 months: CC perfusion and ΔCC perfusion negatively correlated with BCVA |
| Told et al., 2020 [37] | Prospective | 32 | Fellow healthy eye | PPV 23G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes with cataracts | Baseline, 1 day, 1 week, 1, 3 months | FAZ area in the SCP and DCP, VD in the fovea and parafovea | Baseline: BCVA was negatively correlated with foveal VD in stage 1-2 iERM Week 1: BCVA was positively correlated with FAZ area in SCP in stage 3–4 iERM |
| Osada et al., 2020 [38] | Retrospective | 25 | Fellow healthy eye | PPV 27G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1, 3, 6, 12 months | FAZ area in the SCP, VD in the fovea in the SCP and DCP | 6 months: BCVA and RS positively correlated with foveal VD in the DCP Predictive: BCVA and RS at 6 months were positively correlated with foveal DCP VD at 1 and 3 months; higher foveal DCP VD at 1 month was an independent predictor of better RS at 6 months in the multivariate analysis |
| Hirata et al., 2019 [39] | Prospective | 30 | Fellow healthy eye | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1, 3, 6, 12 months | FAZ area, FAZ perimeter, FAZ circularity in the SCP, FAZ area ratio and FAZ perimeter ratio (interocular ratios) | 12 months: Aniseikonia negatively correlated with FAZa, FAZa ratio, FAZp, and FAZp ratio Predictive: Aniseikonia at 12 months negatively correlated with baseline FAZa, FAZa ratio, FAZp, and FAZp ratio; baseline FAZa ratio was identified as an independent predictor in multivariate analysis |
| Honzawa et al., 2023 [40] | Retrospective | 37 | Fellow healthy eye and 26 healthy eyes | PPV with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes (33) | 6 months | FAZ area in the SCP | Predictive: Measured FAZ area changes were negatively correlated with baseline BCVA |
| Mao et al., 2020 [41] | Prospective | 35 | Fellow healthy eye | PPV 23G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1, 3, 6 months | FAZ area, FAZ perimeter, FAZ AI, FD-300 on the retina slab, VD in the SCP and DCP in 5 regions (fovea, S, I, N, T) | Predictive: D-value BCVA (degree of visual improvement postoperatively) positively correlated with foveal VD in SCP and negatively correlated with FAZ area and FAZ perimeter |
| Frisina et al., 2023 [42] | Prospective | 40 | Fellow healthy eye | PPV 27G with ERM and ILM peeling (all eyes were pseudophakic) | Baseline, 1, 3, 6 months | FAZ area in the SCP, VAD, VLF, VD index in the SCP and DCP, CC flow | 6 months: Inverse correlation between FAZ area and BCVA |
| Kim et al., 2023 [43] | Retrospective observational | 28 | Fellow healthy eye | PPV 25G with ERM and ILM peeling + Gas C3F8 tamponade + Phacoemulsification and IOL implantation in phakic eyes | Baseline, minimum 6 months | FAZ area, FAZ perimeter and FAZ circularity in the SCP | Baseline: BCVA negatively correlated with FAZ area and FAZ perimeter Predictive: BCVA improvement and postoperative final BCVA was positively correlated with baseline FAZ area and FAZ perimeter |
| Liao et al., 2020 [44] | Retrospective | 38 | Fellow healthy eye | PPV 27G with ERM and ILM peeling | Baseline, 6 months | FAZ area, VD in the SCP and DCP | 6 months: FAZ area negatively correlated with BCVA (univariate logistic analysis) |
| Yoshida et al., 2020 [45] | Retrospective | 36 | Fellow healthy eye | PPV 25G or 27G with ERM and ILM peeling ± Fluid/gas exchange in case of retinal breaks + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 6 months | FAZ area ratio in the SCP (interocular ratio) | Baseline: FAZ area ratio negatively correlated with BCVA Predictive: FAZ area ratio negatively correlated with changes in the ETDRS letter score |
| Mastrogiuseppe et al., 2025 [46] | Prospective | 57 | Fellow healthy eye | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 1 week, 1, 3, 6, 12 months | ONH: Whole VD of RPC, Inside-disc RPC VD, Peripapillary RPC VD Macula: FAZ area, FAZ perimeter, FD, VD in the SCP and DCP of the whole macular region (3 × 3 mm2), fovea, parafovea, flow area in the outer retina and CC | Predictive: Predictors of ΔBCVA in the multivariate linear regression analysis: wsVD, wRPC (higher wRPC VD and lower wsVD at baseline were considered as predictive factors for ΔBCVA) |
| Kim et al., 2024 [47] | Retrospective observational | 28 | Fellow healthy eye | PPV 25G with ERM and ILM peeling ± Phacoemulsification and IOL implantation (27) | Baseline and at least 6 months | FAZ area, FAZ perimeter, FAZ circularity in the SCP, CC perfusion measured at baseline only | Baseline: FAZ area negatively correlated with BCVA Predictive: Baseline FAZ area and perimeter positively correlated with BCVA improvement; baseline FAZ circularity negatively correlated with postoperative BCVA; baseline CCP positively correlated with BCVA improvement and postoperative BCVA, each confirmed by multivariate regression analysis |
| Lin et al., 2020 [48] | Retrospective | 85 | None | PPV 25G with ERM and ILM peeling ± Phacoemulsification and IOL implantation (4) | Baseline, 1 month, repeated every 1 or 2 months (minimum 1 year follow-up required) | FAZ area in the SCP and DCP, VD in the SCP and DCP in the parafoveal region | None identified |
| Li et al., 2025 [49] | Retrospective observational | 46 | Fellow healthy eye | PPV 23G with ERM and ILM peeling + Gas/ liquid exchange + Phacoemulsification and IOL implantation in case of cataract or refractive error | Baseline, 1, 3 months | FAZ area, FAZ perimeter, FD-300, VT, VD in the SCP and DCP in the fovea and parafovea | Predictive: BCVA-d correlated positively with baseline foveal VD in the SCP and VT, and negatively with FAZ area and foveal VD in the DCP; BCVA at 3-month correlated positively with baseline foveal VD in the SCP and VT, and negatively with FAZ area and foveal VD in the DCP |
| Rommel et al., 2020 [50] | Prospective, observational | 63 | Fellow healthy eye | PPV 23G with ERM and ILM peeling + Fluid/air exchange + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 3 months | Full retinal perfusion, CC perfusion, Sattler’s layer perfusion, Haller’s layer perfusion | Predictive: Higher baseline Sattler’s layer perfusion predicts better postoperative BCVA, a finding confirmed in the multiple regression analysis |
| Mavi Yildiz et al., 2021 [51] | Retrospective | 112 (64 performed baseline FAZ assessment) | Fellow healthy eye | PPV 27G with ERM and ILM peeling + Phacoemulsification and IOL implantation in case of cataract (10) | Baseline, 6, 12 months | FAZ in the SCP | None identified |
| Zhan et al., 2025 [52] | Retrospective | 30 | 28 healthy eyes | PPV 25G with ERM and ILM peeling + Phacoemulsification and IOL implantation in patients over 55 years of age with mild cataract | Baseline, 3 months | VD, PA, PC in the SCP and DCP | 3 months: BCVA correlated with PC in the 6 × 6 mm2 SCP region Predictive: Higher baseline PC in the SCP in both 3 × 3 mm2 and 6 × 6 mm2 regions was associated with greater RS improvement; postoperative RS positively correlated with baseline PC in the SCP in the 3 × 3 mm2 region, a finding confirmed in the multiple linear regression analysis |
| Baba et al., 2018 [53] | Retrospective | 17 | Fellow healthy eye | PPV 25G or 27G with ERM and ILM peeling + Phacoemulsification and IOL implantation in phakic eyes | Baseline, 3, 6, 12 months | FAZ in the SCP | 3 and 6 months: FAZ area inversely correlated with the RS |
| OCTA Parameter (Abbreviation) | Unit | Definition |
|---|---|---|
| Foveal avascular zone (FAZ) area | mm2 | Measured area of avascularity in the foveal region circumscribed by the retinal vascular complexes |
| FAZ perimeter | mm | Length of the perimeter of the FAZ |
| FAZ circularity | A metric ranging from 0 to 1 that quantifies how closely the FAZ shape approximates a perfect circle, with 1 indicating perfect circularity | |
| Acircularity index (AI) | % | A metric that quantifies the deviation of the FAZ from a perfect circle by comparing its measured perimeter to that of a circle with the same area, where 1 represents a perfect circle and higher values indicate greater distortion |
| Vessel/vascular density (VD) or Vessel area density (VAD) | % | The percentage of the scanned area occupied by blood vessels, calculated as the ratio of pixels representing vasculature to the total number of pixels in the scan |
| Average vessel length (VL) | mm | Lengths of all identified vessel segments along the centerline of the vessel |
| Vessel length density (VLD) or Vessel skeleton density (VSD) or Vessel length fraction (VLF) | % | Ratio of the total length of blood vessels to the total scanned area, with each vessel represented as a single-pixel-width line along its centerline |
| Foveal VD 300 (FD-300) | % | Vessel density within a 300 µm wide annulus surrounding the FAZ, calculated as the percentage of the area occupied by vessels within this rim |
| Flow area | mm2 | The area or intensity of flow signal within a predefined region of interest |
| Vessel tortuosity (VT) | A metric that quantifies the curvature of vessels in the OCTA scan, calculated as the ratio of the segment length along the vessel centerline to the straight-line distance between its endpoints | |
| Fractal dimension | A metric that quantifies the geometric complexity of the retinal vascular network, reflecting how vessels branch and fill space across different scales; higher values indicate a more complex and dense branching pattern |
| OCTA Device | Studies (n; References) |
|---|---|
| AngioVue Imaging System (RTVue XR Avanti, Optovue Inc., Fremont, CA, USA) | 24 studies; [9,12,14,15,16,17,18,23,25,26,27,28,30,31,32,34,35,36,41,44,46,48,49,53] |
| Carl Zeiss Meditec Inc. (Dublin, CA, USA) | 6 studies; [19,22,39,40,45,50] |
| DRI OCT Triton (Topcon Corporation, Tokyo, Japan) | 6 studies; [20,21,29,37,43,47] |
| NIDEK OCTA systems (NIDEK Co., Ltd., Gamagori, Japan) | 5 studies; [13,24,33,38,42] |
| VG200 (S Vision Imaging, Luoyang, China) | 1 study; [52] |
| Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) with integrated OCTA | 1 study; [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sere, A.M.; Muntean, G.A.; Cristea, A.P.; Nicoară, S.D. OCTA Biomarkers Underlying Structure–Function Correlations in Idiopathic Epiretinal Membrane: A Systematic Review. Diagnostics 2025, 15, 2596. https://doi.org/10.3390/diagnostics15202596
Sere AM, Muntean GA, Cristea AP, Nicoară SD. OCTA Biomarkers Underlying Structure–Function Correlations in Idiopathic Epiretinal Membrane: A Systematic Review. Diagnostics. 2025; 15(20):2596. https://doi.org/10.3390/diagnostics15202596
Chicago/Turabian StyleSere, Anca Mădălina, George Adrian Muntean, Andreea Petra Cristea, and Simona Delia Nicoară. 2025. "OCTA Biomarkers Underlying Structure–Function Correlations in Idiopathic Epiretinal Membrane: A Systematic Review" Diagnostics 15, no. 20: 2596. https://doi.org/10.3390/diagnostics15202596
APA StyleSere, A. M., Muntean, G. A., Cristea, A. P., & Nicoară, S. D. (2025). OCTA Biomarkers Underlying Structure–Function Correlations in Idiopathic Epiretinal Membrane: A Systematic Review. Diagnostics, 15(20), 2596. https://doi.org/10.3390/diagnostics15202596







