Neuroimaging and Genetic Markers of Cerebral Small Vessel Disease and Cognitive Outcomes: A Systematic Review and Meta-Analysis (NEUROGEN-SVD Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Definitions of CSVD Imaging Markers, Genetic Risk Factors, and CSVD
2.4. Definitions of Cognitive Outcomes
2.5. Data Extraction
- Baseline study demographics: author, country, publication year, cohort size, and study design.
- Patient demographics: age and sex.
- CSVD neuroimaging marker: WMHs, CMBs, and lacunes.
- Genetic risk factor: APOE ε4 allele carrier status.
- CSVD neuroimaging marker and genetic risk factor characteristics: imaging marker score, CSVD diagnostic criteria, and MRI sequence.
- Cognitive outcome: MCI, ACD, VaD, AD, and GCI.
- Cognitive outcome characteristics: cognitive diagnostic criteria.
2.6. Methodological Quality Assessment of Included Studies
| Study ID | Author | Year | Country | Study Design | CSVD Criteria | Cognitive Outcome | GCI Criteria | MCI Criteria | Age (Mean +/− SD) | Female (n) | Number of Patients with Cognitive Outcome | Cohort Size | Prevalence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | Dobrynina et al. [19] (a) | 2024 | Russia | Cross-sectional | STRIVE | GCI | MOCA | - | 59.9 (7.6) | - | 111 | 166 | 66.9% |
| 4 | Dobrynina et al. [19] (b) | 2024 | Russia | Cross-sectional | STRIVE | MCI | - | DSM-IV | 59.9 (7.6) | 73 | 71 | 166 | 42.8% |
| 6 | Ferro et al. [20] | 2017 | Netherlands | Cross-sectional | WMHs Fazekas score 2–3, or lacunar infarcts, non-lacunar infarcts, CMBs, intracranial hemorrhage, or WMHs Fazekas 1 and 2 vascular risk factors | MCI | - | AHA/ASA VCID | - | - | 61 | 131 | 46.6% |
| 7 | Han et al. [21] | 2024 | China | Prospective | STRIVE | MCI | - | AHA/ASA VCID | 66.2 (6.7) | - | 36 | 69 | 52.2% |
| 12 | Ke et al. [22] | 2022 | China | Cross-sectional | WMHs Fazekas score 2–3 and/or lacunar infarcts, with or without PVS, CMBs, brain atrophy | GCI | AHA/ASA VCID | - | 54 | 81 | 137 | 59.1% | |
| 14 | Lee et al. [23] | 2017 | Korea | Prospective | Moderate-severe periventricular WMHs, severe deep WMHs | MCI | Study-specific protocol | Study-specific protocol | 74.0 (6.9) | 45 | 33 | 72 | 45.8% |
| 17 | Liao et al. [24] | 2024 | China | Cross-sectional | 2 or more of WMHs Fazekas score 2–3, lacunes, moderate–severe PVS, CMBs | GCI | MMSE | - | 65.9 (10.9) | 29 | 39 | 94 | 41.5% |
| 18 | Liu et al. [25] | 2021 | China | Cross-sectional | STRIVE | GCI | Study-specific protocol | - | 69.0 (7.8) | 92 | 112 | 199 | 56.3% |
| 25 | Song et al. [26] | 2022 | China | Cross-sectional | STRIVE | GCI | NINDS-CSN | - | 61.1 (5.0) | 45 | 79 | 156 | 50.6% |
| 26 | Sun et al. [27] | 2022 | China | Cross-sectional | WMHs Fazekas score 2–3 and/or lacunar infarct with or without PVS, CMBs, brain atrophy | GCI | MMSE, MOCA | - | - | 108 | 135 | 242 | 55.8% |
| 27 | Tang et al. [28] | 2022 | China | Cross-sectional prospective | WMHs Fazekas score 2–3 and at least one of CMBs, lacunes, PVS | GCI | MOCA | - | 65.5 (7.7) | 48 | 83 | 133 | 62.4% |
| 31 | Wang et al. [29] | 2023 | China | Cross-sectional | WMHs Fazekas score 2–3 OR Fazekas score 1 and vascular risk factors | GCI | MMSE | - | - | 14 | 31 | 51 | 60.8% |
| 32 | Wei et al. [30] | 2019 | China | Cross-sectional | WMHs | GCI | MOCA, CDR | - | 62.7 (9.1) | 53 | 78 | 113 | 69.0% |
| 34 | Xing et al. [31] | 2021 | China | Cross-sectional | WMHs Fazekas score 2–3 | MCI | - | DSM-IV | 66.4 (6.9) | 37 | 44 | 77 | 57.1% |
| 35 | Xu et al. [32] | 2024 | China | Cross-sectional | STRIVE | MCI | - | Study-specific protocol | 61.7 (9.2) | 76 | 87 | 185 | 47.0% |
| 37 | Zhu et al. [33] | 2024 | China | Cross-sectional | WMHs, lacunes, CMBs, PVS | GCI | MOCA | 67.0 (7.0) | 71 | 100 | 227 | 44.1% | |
| 38 | Zhu et al. [34] | 2021 | China | Cross-sectional | WMHs Fazekas score 3–6 (sum of PVWMHs and DWMHs) | MCI | - | Study-specific protocol | 65.5 (6.2) | 24 | 23 | 66 | 34.8% |
| Study ID | Author | Year | Country | Study Design | MRI Sequence | Cohort Size | Cognitive Outcome | MCI Criteria | GCI Criteria | VaD Criteria | AD Criteria | ACD Criteria | Age (Mean +/− SD) | Female (n) | Imaging Marker (n) | Imaging Marker (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMBs | ||||||||||||||||
| 2 | Chen et al. [35] | 2018 | China | Prospective | SWI | 82 | ACD | - | - | - | - | CDR | 68.2 (10.1) | 20 | 14 | 17.1% |
| 3 | Ding et al. [36] (a) | 2017 | Iceland | Prospective | T2 * GRE | 2601 | ACD | - | - | - | - | DSM-IV | 74.6 (4.8) | 1532 | 20 | 0.8% |
| 3 | Ding et al. [36] (b) | 2017 | Iceland | Prospective | T2 * GRE | 2601 | VaD | - | - | ADDTC | - | - | 74.6 (4.8) | 1532 | 6 | 0.2% |
| 3 | Ding et al. [36] (c) | 2017 | Iceland | Prospective | T2 * GRE | 2601 | AD | - | - | - | NINCDS-ADRDA | - | 74.6 (4.8) | 1532 | 12 | 0.5% |
| 5 | Fan et al. [37] | 2021 | China | Cross-sectional | SWI | 293 | GCI | - | MOCA | - | - | - | 174 | 39 | 13.3% | |
| 8 | Hilal et al. [38] (a) | 2015 | Singapore | Cross-sectional | - | 572 | GCI | - | Study-specific protocol | - | - | - | 70.5 (6.8) | 313 | 152 | 26.6% |
| 8 | Hilal et al. [38] (b) | 2015 | Singapore | Cross-sectional | - | 347 | MCI | Study-specific protocol | - | - | - | - | 68.6 (5.8) | 157 | 62 | 17.9% |
| 8 | Hilal et al. [38] (c) | 2015 | Singapore | Cross-sectional | - | 204 | ACD | - | - | - | - | DSM-IV | 68.8 (6.5) | 98 | 12 | 5.9% |
| 11 | Jacob et al. [39] (a) | 2023 | Netherlands | Prospective | T2 * | 498 | ACD | - | - | - | - | DSM-IV | 65.7 (8.8) | 217 | 22 | 4.4% |
| 11 | Jacob et al. [39] (b) | 2023 | Netherlands | Prospective | T2 * | 424 | VaD | - | - | NINDS-AIREN | - | - | 64.5 (8.6) | 217 | 14 | 3.3% |
| 11 | Jacob et al. [39] (c) | 2023 | Netherlands | Prospective | T2 * | 428 | AD | - | - | - | NIA-AA | - | 64.6 (8.7) | 217 | 4 | 0.9% |
| 15 | Li et al. [40] | 2021 | China | Retrospective | SWI | 270 | MCI | NIA-AA | - | - | - | - | 66.5 (8.7) | 118 | 48 | 17.8% |
| 17 | Liao et al. [24] | 2024 | China | Cross-sectional | - | 94 | GCI | - | MMSE | - | - | - | 65.9 (10.9) | 29 | 25 | 26.6% |
| 16 | Li et al. [41] (a) | 2020 | China | Prospective | T2 * GRE | 792 | MCI | Study-specific protocol | - | - | - | - | 72.6 (7.1) | 366 | 124 | 15.7% |
| 16 | Li et al. [41] (b) | 2020 | China | Prospective | T2 * GRE | 792 | AD | - | - | - | NINCDS-ADRDA | - | 72.6 (7.1) | 366 | 39 | 4.9% |
| 18 | Liu et al. [25] | 2021 | China | Cross-sectional | - | 199 | GCI | - | Other | - | - | - | 69.0 (7.8) | 92 | 34 | 17.1% |
| 21 | Paradise et al. [42] | 2019 | Australia | Prospective | SWI | 267 | ACD | - | - | - | - | DSM-IV/DSM-V | - | - | 8 | 3.0% |
| 23 | Romero et al. [43] (a) | 2018 | US | Prospective | T2 * GRE | 1296 | ACD | - | - | - | - | DSM-IV | 72 (8) | 585 | 17 | 1.3% |
| 23 | Romero et al. [43] (b) | 2018 | US | Prospective | T2 * GRE | 1296 | VaD | - | - | NINDS-AIREN | - | - | 72 (8) | 586 | 4 | 0.3% |
| 23 | Romero et al. [43] (c) | 2018 | US | Prospective | T2 * GRE | 1296 | AD | - | - | - | NINCDS-ADRDA | - | 72 (8) | 587 | 13 | 1.0% |
| 24 | Shaikh et al. [44] (a) | 2022 | US | Cross-sectional | - | 94 | MCI | NIA-AA | - | - | - | - | 69.6 (7.5) | 52 | 7 | 7.4% |
| 24 | Shaikh et al. [44] (b) | 2022 | US | Cross-sectional | - | 89 | AD | - | - | - | NIA-AA | - | 68.6 (6.9) | 52 | 1 | 1.1% |
| 28 | Uetani et al. [45] (a) | 2013 | Japan | Cross-sectional | SWI | 347 | GCI | International Working Group on MCI | - | NINDS-AIREN | NINCDS-ADRDA | - | 74.3 (8.8) | 217 | 153 | 44.1% |
| 28 | Uetani et al. [45] (b) | 2013 | Japan | Cross-sectional | SWI | 83 | MCI | International Working Group on MCI | - | - | - | - | 74.1 (9.5) | 55 | 21 | 25.3% |
| 28 | Uetani et al. [45] (c) | 2013 | Japan | Cross-sectional | SWI | 296 | ACD | - | - | NINDS-AIREN | NINCDS-ADRDA | - | - | 188 | 132 | 44.6% |
| 28 | Uetani et al. [45] (d) | 2013 | Japan | Cross-sectional | SWI | 60 | VaD | - | - | NINDS-AIREN | - | - | 73.3 (10.0) | 40 | 24 | 40.0% |
| 28 | Uetani et al. [45] (e) | 2013 | Japan | Cross-sectional | SWI | 194 | AD | - | - | NINDS-AIREN | NINCDS-ADRDA | - | 74.3 (9.5) | 134 | 77 | 39.7% |
| 33 | Wrigley et al. [46] (a) | 2024 | Australia | Retrospective | SWI | 219 | MCI | Study-specific protocol | - | - | - | - | - | 112 | 37 | 16.9% |
| 33 | Wrigley et al. [46] (b) | 2024 | Australia | Retrospective | SWI | 234 | ACD | - | - | - | - | Study-specific protocol | - | 115 | 50 | 21.4% |
| 35 | Xu et al. [32] | 2024 | China | Retrospective | 185 | MCI | Study-specific protocol | - | - | - | - | 61.7 (9.2) | 76 | 18 | 9.7% | |
| 39 | Zonneveld et al. [47] (a) | 2014 | Netherlands | Cross-sectional | SWI | 311 | MCI | Petersen’s criteria | - | - | - | - | 64.2 (10.2) | 125 | 40 | 12.9% |
| 39 | Zonneveld et al. [47] (b) | 2014 | Netherlands | Cross-sectional | SWI | 509 | ACD | - | - | - | - | Other | - | - | 107 | 21.0% |
| 39 | Zonneveld et al. [47] (c) | 2014 | Netherlands | Cross-sectional | SWI | 180 | VaD | - | - | NINDS-AIREN | - | - | 61.5 (10.0) | 80 | 9 | 5.0% |
| 39 | Zonneveld et al. [47] (d) | 2014 | Netherlands | Cross-sectional | SWI | 417 | AD | - | - | - | NINCDS-ADRDA | 65.2 (10.0) | 204 | 79 | 18.9% | |
| Moderate–severe WMHs | ||||||||||||||||
| 4 | Dobrynina et al. [19] | 2024 | Russia | Cross-sectional | - | 126 | MCI | DSM-V | - | - | - | - | 59.9 (7.6) | 73 | 64 | 50.8% |
| 22 | Rennie et al. [48] (a) | 2024 | Sweden | Cross-sectional | - | 2994 | MCI | ICD-10 | - | - | - | - | 70.2 (9.2) | 1592 | 388 | 13.0% |
| 22 | Rennie et al. [48] (b) | 2024 | Sweden | Cross-sectional | - | 2213 | AD | - | - | - | ICD-10 | - | 69.0 (8.6) | 1299 | 188 | 8.5% |
| 22 | Rennie et al. [48] (c) | 2024 | Sweden | Cross-sectional | - | 1653 | VaD | - | - | ICD-10 | - | - | 68.1 (8.4) | 942 | 92 | 5.6% |
| 28 | Uetani et al. [45] (a) | 2013 | Japan | Cross-sectional | - | 83 | MCI | International Working Group on MCI | - | - | - | - | 74.1 (9.5) | 55 | 20 | 24.1% |
| 28 | Uetani et al. [45] (b) | 2013 | Japan | Cross-sectional | - | 194 | AD | - | - | - | NINCDS-ADRDA | - | 74.3 (9.5) | 134 | 64 | 33.0% |
| 28 | Uetani et al. [45] (c) | 2013 | Japan | Cross-sectional | - | 60 | VaD | - | - | NINDS-AIREN | - | - | 73.3 (10.0) | 40 | 24 | 40.0% |
| 39 | Zonneveld et al. [47] (a) | 2014 | Netherlands | Cross-sectional | - | 311 | MCI | Petersen’s criteria | - | - | - | - | 64.2 (10.2) | 125 | 44 | 14.1% |
| 39 | Zonneveld et al. [47] (b) | 2014 | Netherlands | Cross-sectional | - | 417 | AD | - | - | - | NINCDS-ADRDA | - | 65.2 (10.0) | 204 | 81 | 19.4% |
| 39 | Zonneveld et al. [47] (c) | 2014 | Netherlands | Cross-sectional | - | 180 | VaD | - | - | NINDS-AIREN | - | - | 61.5 (10.0) | 80 | 10 | 5.6% |
| Lacunes | ||||||||||||||||
| 5 | Fan et al. [37] | 2021 | China | Retrospective | T1, T2, FLAIR, DWI | 293 | GCI | - | MOCA | - | - | - | - | 174 | 130 | 44.4% |
| 8 | Hilal et al. [38] | 2015 | Singapore | Cross-sectional | T2, FLAIR | 204 | ACD | - | - | - | - | DSM-IV | 68.8 (6.5) | 98 | 13 | 2.3% |
| 8 | Hilal et al. [38] | 2015 | Singapore | Cross-sectional | T2, FLAIR | 572 | GCI | - | Study-specific protocol | - | - | DSM-IV | 70.5 (6.8) | 313 | 99 | 17.3% |
| 9 | Hilal et al. [49] | 2021 | Singapore | Cross-sectional | T1, T2, FLAIR | 253 | MCI | Study-specific protocol | - | - | - | - | 70.2 (6.1) | 129 | 36 | 14.2% |
| 11 | Jacob et al. [39] | 2023 | Netherlands | Prospective | T1, FLAIR | 498 | ACD | - | - | - | - | DSM-V | 65.7 (8.8) | 217 | 42 | 8.4% |
| 15 | Li et al. [40] | 2021 | China | Retrospective | - | 270 | MCI | NIA-AA | MMSE | - | - | - | 66.5 (8.7) | - | 118 | 15.2% |
| 17 | Liao et al. [24] | 2024 | China | Cross-sectional | - | 94 | GCI | - | MMSE | - | - | - | 65.9 (10.9) | - | 29 | 37.2% |
| 18 | Liu et al. [25] | 2021 | China | Cross-sectional | - | 199 | GCI | - | Study-specific protocol | - | - | - | 69.0 (7.8) | - | 85 | 42.7% |
| 28 | Uetani et al. [45] (a) | 2013 | Japan | Cross-sectional | T2WI GRE FLAIR | 296 | ACD | - | - | NINDS-AIREN | NINCDS-ADRDA | - | - | 188 | 58 | 19.6% |
| 28 | Uetani et al. [45] (b) | 2013 | Japan | Cross-sectional | T2WI GRE FLAIR | 83 | MCI | International Working Group on MCI | - | - | - | - | 74.1 (9.5) | - | 13 | 15.7% |
| 28 | Uetani et al. [45] (c) | 2013 | Japan | Cross-sectional | T2WI GRE FLAIR | 347 | GCI | International Working Group on MCI | - | NINDS-AIREN | NINCDS-ADRDA | - | 74.3 (8.8) | - | 217 | 20.5% |
| 29 | Wang et al. [50] | 2022 | China | Cross-sectional | - | 442 | ACD | - | - | - | - | DSM-IV | 71.6 (11.3) | - | 205 | 10.9% |
| 30 | Wang et al. [51] | 2024 | China | Cross-sectional | FLAIR | 1230 | MCI | Petersen’s criteria | - | - | - | - | 69.4 (4.3) | - | 720 | 8.0% |
| Study ID | Author | Year | Country | Study Design | Cohort Size | Cognitive Outcome | GCI Criteria | Age (Mean +/− SD) | Female (n) | APOE ε4 Carrier (n) | APOE ε4 Carrier (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Brickman et al. [52] | 2015 | USA | Cross-sectional | 694 | GCI | DSM-IV | 80.4 (5.7) | 462 | 14 | 2.0% |
| 10 | Hong et al. [53] | 2011 | Korea | Cross-sectional | 216 | GCI | DSM-IV | 68.4 (9.3) | 161 | 17 | 7.9% |
| 13 | Kim et al. [54] | 2013 | Korea | Cross-sectional | 364 | GCI | DSM-IV, Petersen’s criteria | 68.3 (8.5) | 212 | 35 | 9.6% |
| 19 | Nicoll et al. [13] | 2010 | UK | Prospective | 310 | GCI | Neuropathological | - | 188 | 56 | 18.1% |
| 20 | Paradela et al. [14] | 2023 | Brazil | Prospective | 648 | GCI | CDR | 74.7 (12.0) | 339 | 76 | 11.7% |
| 36 | Yu et al. [55] | 2023 | China | Cross-sectional | 166 | GCI | CDR | 76.5 (7.7) | 101 | 19 | 11.4% |
2.7. Certainty of Evidence Assessment (NEUROGEN-SVD)
2.8. Statistical Analyses
3. Results
3.1. Description of Included Studies
3.2. Prevalence of Cognitive Outcomes in CSVD Patients
3.2.1. Prevalence of GCI in CSVD Patients
3.2.2. Prevalence of MCI in CSVD Patients
3.2.3. Geographical Subgroup Analysis
3.3. Association Between Neuroimaging Markers of CSVD and the APOE ε4 Allele with Cognitive Outcomes
3.3.1. Analysis by Imaging Marker
Cerebral Microbleeds (CMBs)
White Matter Hyperintensities (WMHs)
Lacunes
APOE ε4 Allele
3.3.2. Analysis by Cognitive Outcome
3.3.3. Diagnostic Performance of Neuroimaging Markers of CSVD and the APOE ε4 Allele for Cognitive Outcomes
3.3.4. Certainty of Evidence (NEUROGEN-SVD)
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Jin, C.; Bhaskar, S. Unifying Vascular Injury and Neurodegeneration: A Mechanistic Continuum in Cerebral Small Vessel Disease and Dementia. Eur. J. Neurosci. 2025, 62, e70246. [Google Scholar] [CrossRef]
- World Health Organisation. Dementia; WHO: Geneva, Switzerland, 2025; Volume 2025. [Google Scholar]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Tan, R.; Spring, K.J.; Killingsworth, M.; Bhaskar, S. Comparative Diagnostic and Prognostic Performance of SWI and T2-Weighted MRI in Cerebral Microbleed Detection Following Acute Ischemic Stroke: A Meta-Analysis and SPOT-CMB Study. Medicina 2025, 61, 1566. [Google Scholar] [CrossRef]
- Rastogi, A.; Weissert, R.; Bhaskar, S.M.M. Emerging role of white matter lesions in cerebrovascular disease. Eur. J. Neurosci. 2021, 54, 5531–5559. [Google Scholar] [CrossRef]
- Tipirneni, S.; Stanwell, P.; Weissert, R.; Bhaskar, S.M.M. Prevalence and Impact of Cerebral Microbleeds on Clinical and Safety Outcomes in Acute Ischaemic Stroke Patients Receiving Reperfusion Therapy: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 2865. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.M.; Heinsinger, N.M.; Rebeck, G.W. Alzheimer’s Disease Genetic Risk Factor APOE-ε4 Also Affects Normal Brain Function. Curr. Alzheimer Res. 2016, 13, 1200–1207. [Google Scholar] [CrossRef]
- Fongang, B.; Sargurupremraj, M.; Jian, X.; Mishra, A.; Damotte, V.; de Rojas, I.; Skrobot, O.; Bis, J.C.; Fan, K.-H. A genome-wide association meta-analysis of all-cause and vascular dementia. Alzheimer’s Dement. 2024, 20, 5973–5995. [Google Scholar]
- Song, X.; Jin, C.; Luan, M.; Zheng, X. Roles of neuroimaging markers and biomarkers in cerebral small vessel disease and their associations with cognitive function. Front. Neurol. 2025, 16, 1483842. [Google Scholar] [CrossRef]
- Nicoll, J.A.; Savva, G.M.; Stewart, J.; Matthews, F.E.; Brayne, C.; Ince, P.; Medical Research Council Cognitive Function and Ageing Study. Association between APOE genotype, neuropathology and dementia in the older population of England and Wales. Neuropathol. Appl. Neurobiol. 2011, 37, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Paradela, R.S.; Justo, A.F.O.; Paes, V.R.; Leite, R.E.P.; Pasqualucci, C.A.; Grinberg, L.T.; Naslavsky, M.S.; Zatz, M.; Nitrini, R.; Jacob-Filho, W.; et al. Association between APOE-ε4 allele and cognitive function is mediated by Alzheimer’s disease pathology: A population-based autopsy study in an admixed sample. Acta Neuropathol. Commun. 2023, 11, 205. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J. Mol. Med. 2016, 94, 739–746. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S.; et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 2023, 22, 602–618. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Dobrynina, L.A.; Kremneva, E.I.; Shamtieva, K.V.; Geints, A.A.; Filatov, A.S.; Gadzhieva, Z.S.; Gnedovskaya, E.V.; Krotenkova, M.V.; Maximov, I.I. Cognitive Impairment in Cerebral Small Vessel Disease Is Associated with Corpus Callosum Microstructure Changes Based on Diffusion MRI. Diagnostics 2024, 14, 1838. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.A.; van Veluw, S.J.; Koek, H.L.; Exalto, L.G.; Biessels, G.J.; Utrecht Vascular Cognitive Impairment (VCI) Study Group. Cortical Cerebral Microinfarcts on 3 Tesla MRI in Patients with Vascular Cognitive Impairment. J. Alzheimer’s Dis. 2017, 60, 1443–1450. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Chen, Y.; Qiu, Y.; Gu, X.; Dai, Y.; Xu, Q.; Sun, Y.; Zhou, Y. Predicting white-matter hyperintensity progression and cognitive decline in patients with cerebral small-vessel disease: A magnetic resonance-based habitat analysis. Quant. Imaging Med. Surg. 2024, 14, 6621–6634. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Mo, Y.; Li, J.; Yang, D.; Huang, L.; Yang, Z.; Qin, R.; Mao, C.; Lv, W.; Huang, Y.; et al. Glymphatic Dysfunction Mediates the Influence of White Matter Hyperintensities on Episodic Memory in Cerebral Small Vessel Disease. Brain Sci. 2022, 12, 1611. [Google Scholar] [CrossRef]
- Lee, J.; Seo, S.W.; Yang, J.J.; Jang, Y.K.; Lee, J.S.; Kim, Y.J.; Chin, J.; Lee, J.M.; Kim, S.T.; Lee, K.H.; et al. Longitudinal cortical thinning and cognitive decline in patients with early- versus late-stage subcortical vascular mild cognitive impairment. Eur. J. Neurol. 2018, 25, 326–333. [Google Scholar] [CrossRef]
- Liao, M.; Wang, M.; Li, H.; Li, J.; Yi, M.; Lan, L.; Ouyang, F.; Shi, L.; Fan, Y. Discontinuity of deep medullary veins in SWI is associated with deep white matter hyperintensity volume and cognitive impairment in cerebral small vessel disease. J. Affect. Disord. 2024, 350, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, W.; Gui, Q.; Zhang, Y.; Guo, Z.; Liu, W. Addition of Abeta(42) to Total Cerebral Small Vessel Disease Score Improves the Prediction for Cognitive Impairment in Cerebral Small Vessel Disease Patients. Neuropsychiatr. Dis. Treat. 2021, 17, 195–201. [Google Scholar] [CrossRef]
- Song, H.; Ruan, Z.; Gao, L.; Lv, D.; Sun, D.; Li, Z.; Zhang, R.; Zhou, X.; Xu, H.; Zhang, J. Structural network efficiency mediates the association between glymphatic function and cognition in mild VCI: A DTI-ALPS study. Front. Aging Neurosci. 2022, 14, 974114. [Google Scholar] [CrossRef]
- Sun, W.; Huang, L.; Cheng, Y.; Qin, R.; Xu, H.; Shao, P.; Ma, J.; Yao, Z.; Shi, L.; Xu, Y. Medial Temporal Atrophy Contributes to Cognitive Impairment in Cerebral Small Vessel Disease. Front. Neurol. 2022, 13, 858171. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, M.; Liu, N.; Xue, Y.; Ren, X.; Huang, Q.; Shi, L.; Fu, J. The Association Between Glymphatic System Dysfunction and Cognitive Impairment in Cerebral Small Vessel Disease. Front. Aging Neurosci. 2022, 14, 916633. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Chen, Y.; Gao, Y.; Wang, T.; Li, Z.; Wang, Y. Blood-Brain Barrier Breakdown is a Sensitive Biomarker of Cognitive and Language Impairment in Patients with White Matter Hyperintensities. Neurol. Ther. 2023, 12, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Deng, Y.; Yao, L.; Jia, W.; Wang, J.; Shi, Q.; Chen, H.; Pan, Y.; Yan, H.; Zhang, Y.; et al. A neuroimaging marker based on diffusion tensor imaging and cognitive impairment due to cerebral white matter lesions. Front. Neurol. 2019, 10, 81. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, J.; Zhou, A.; Wang, F.; Wei, C.; Tang, Y.; Jia, J. White Matter Fractional Anisotropy Is a Superior Predictor for Cognitive Impairment Than Brain Volumes in Older Adults with Confluent White Matter Hyperintensities. Front. Psychiatry 2021, 12, 633811. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, H.; Wu, L. Magnetic resonance diffusion tensor imaging for detecting the cerebral microstructure changes in patients with CSVD-induced mild cognitive impairment. J. Neurophysiol. 2024, 132, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yao, J.; Feng, M.; Sun, Z. Establishment and evaluation of a clinical prediction model for cognitive impairment in patients with cerebral small vessel disease. BMC Neurosci. 2024, 25, 35. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, H.; Yang, S.; Luo, X.; Zhu, W.; Xu, S.; Meng, Q.; Zuo, C.; Liu, Y.; Wang, W.; et al. Cortical and Subcortical Grey Matter Abnormalities in White Matter Hyperintensities and Subsequent Cognitive Impairment. Neurosci. Bull. 2021, 37, 789–803. [Google Scholar] [CrossRef]
- Chen, Y.K.; Xiao, W.M.; Li, W.; Ni, Z.X.; Liu, Y.L.; Xu, L.; Qu, J.F.; Ng, C.; Xiang, Y.T. Microbleeds in fronto-subcortical circuits are predictive of dementia conversion in patients with vascular cognitive impairment but no dementia. Neural Regen. Res. 2018, 13, 1913–1918. [Google Scholar] [CrossRef]
- Ding, J.; Sigurethsson, S.; Jonsson, P.V.; Eiriksdottir, G.; Meirelles, O.; Kjartansson, O.; Lopez, O.L.; van Buchem, M.A.; Gudnason, V.; Launer, L.J. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology 2017, 88, 2089–2097. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Y.; Shen, M.; Guo, H.; Zhang, Z. Total Cerebral Small Vessel Disease Burden on MRI Correlates with Cognitive Impairment in Outpatients with Amnestic Disorders. Front. Neurol. 2021, 12, 747115. [Google Scholar] [CrossRef]
- Hilal, S.; Xin, X.; Ang, S.L.; Tan, C.S.; Venketasubramanian, N.; Niessen, W.J.; Vrooman, H.; Wong, T.Y.; Chen, C.; Ikram, M.K. Risk Factors and Consequences of Cortical Thickness in an Asian Population. Medicine 2015, 94, e852. [Google Scholar] [CrossRef]
- Jacob, M.A.; Cai, M.; van de Donk, V.; Bergkamp, M.; Marques, J.; Norris, D.G.; Kessels, R.P.C.; Claassen, J.; Duering, M.; Tuladhar, A.M.; et al. Cerebral Small Vessel Disease Progression and the Risk of Dementia: A 14-Year Follow-Up Study. Am. J. Psychiatry 2023, 180, 508–518. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Qin, W.; Yang, L.; Yang, S.; Li, Y.; Hu, W. Higher Total Cerebral Small Vessel Disease Burden Was Associated with Mild Cognitive Impairment and Overall Cognitive Dysfunction: A Propensity Score-Matched Case-Control Study. Front. Aging Neurosci. 2021, 13, 695732. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, D.H.; Li, H.Q.; Tan, L.; Xu, W.; Dong, Q.; Tan, L.; Yu, J.T.; Alzheimer’s Disease Neuroimaging Initiative. Association of Cerebral Microbleeds with Cognitive Decline: A Longitudinal Study. J. Alzheimer’s Dis. 2020, 75, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Paradise, M.; Seruga, A.; Crawford, J.D.; Chaganti, J.; Thalamuthu, A.; Kochan, N.A.; Brodaty, H.; Wen, W.; Sachdev, P.S. The relationship of cerebral microbleeds to cognition and incident dementia in non-demented older individuals. Brain Imaging Behav. 2019, 13, 750–761. [Google Scholar] [CrossRef]
- Romero, J.R.; Beiser, A.; Himali, J.J.; Shoamanesh, A.; DeCarli, C.; Seshadri, S. Cerebral microbleeds and risk of incident dementia: The Framingham Heart Study. Neurobiol. Aging 2017, 54, 94–99. [Google Scholar] [CrossRef]
- Shaikh, I.; Beaulieu, C.; Gee, M.; McCreary, C.R.; Beaudin, A.E.; Valdes-Cabrera, D.; Smith, E.E.; Camicioli, R. Diffusion tensor tractography of the fornix in cerebral amyloid angiopathy, mild cognitive impairment and Alzheimer’s disease. Neuroimage Clin. 2022, 34, 103002. [Google Scholar] [CrossRef]
- Uetani, H.; Hirai, T.; Hashimoto, M.; Ikeda, M.; Kitajima, M.; Sakamoto, F.; Utsunomiya, D.; Oda, S.; Sugiyama, S.; Matsubara, J.; et al. Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am. J. Neuroradiol. 2013, 34, 984–989. [Google Scholar] [CrossRef]
- Wrigley, S.; Cody, R.; Amadoru, S.; Huynh, A.; Galante, O.; Mandrawa, C.; Yassi, N.; Yates, P. Prevalence and associations of cerebral microbleeds in an Australian Memory clinic cohort. Intern. Med. J. 2025, 55, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, H.I.; Goos, J.D.; Wattjes, M.P.; Prins, N.D.; Scheltens, P.; van der Flier, W.M.; Kuijer, J.P.; Muller, M.; Barkhof, F. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology 2014, 82, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Rennie, A.; Ekman, U.; Shams, S.; Ryden, L.; Samuelsson, J.; Zettergren, A.; Kern, S.; Oppedal, K.; Blanc, F.; Hort, J.; et al. Cerebrovascular and Alzheimer’s disease biomarkers in dementia with Lewy bodies and other dementias. Brain Commun. 2024, 6, fcae290. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Liu, S.; Wong, T.Y.; Vrooman, H.; Cheng, C.Y.; Venketasubramanian, N.; Chen, C.L.H.; Zhou, J.H. White matter network damage mediates association between cerebrovascular disease and cognition. J. Cereb. Blood Flow Metab. 2021, 41, 1858–1872. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; He, Y.; Li, Q.; Zhang, W.; Luo, Z.; Xue, R.; Lou, M. Impact of different white matter hyperintensities patterns on cognition: A cross-sectional and longitudinal study. Neuroimage Clin. 2022, 34, 102978. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Li, Y.; Fa, W.; Zhao, M.; Li, C.; Mao, M.; Hou, T.; Wang, Y.; Cong, L.; et al. Strategic Lacunes Associated with Mild Cognitive Impairment in Rural Chinese Older Adults: A Population-Based Study. Stroke 2024, 55, 1288–1298. [Google Scholar] [CrossRef]
- Brickman, A.M.; Schupf, N.; Manly, J.J.; Stern, Y.; Luchsinger, J.A.; Provenzano, F.A.; Narkhede, A.; Razlighi, Q.; Collins-Praino, L.; Artero, S.; et al. APOE epsilon4 and risk for Alzheimer’s disease: Do regionally distributed white matter hyperintensities play a role? Alzheimer’s Dement. 2014, 10, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Yoon, B.; Shim, Y.S.; Cho, A.H.; Shin, H.E.; Kim, Y.I.; Kim, S.Y.; Yang, D.W. APOE epsilon4 allele status in korean dementia patients with severe white matter hyperintensities. J. Alzheimer’s Dis. 2011, 24, 519–524. [Google Scholar] [CrossRef]
- Kim, H.J.; Ye, B.S.; Yoon, C.W.; Cho, H.; Noh, Y.; Kim, G.H.; Choi, Y.S.; Kim, J.H.; Jeon, S.; Lee, J.M.; et al. Effects of APOE ε4 on brain amyloid, lacunar infarcts, and white matter lesions: Astudy among patients with subcortical vascular cognitive impairment. Neurobiol. Aging 2013, 34, 2482–2487. [Google Scholar] [CrossRef]
- Yu, M.C.; Chuang, Y.F.; Wu, S.C.; Ho, C.F.; Liu, Y.C.; Chou, C.J. White matter hyperintensities in cholinergic pathways are associated with dementia severity in e4 carriers but not in non-carriers. Front. Neurol. 2023, 14, 1100322. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Elahi, F.M.; Wang, M.A.-O.; Meschia, J.F. Cerebral Small Vessel Disease-Related Dementia: More Questions Than Answers. Stroke 2023, 54, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Geriatric Neurology Group; Chinese Society of Geriatrics; Clinical Practice Guideline for Cognitive Impairment of Cerebral Small Vessel Disease Writing Group. Clinical practice guideline for cognitive impairment of cerebral small vessel disease. Aging Med. 2019, 2, 64–73. [Google Scholar] [CrossRef]
- Ter Telgte, A.; Duering, M. Cerebral Small Vessel Disease: Advancing Knowledge with Neuroimaging. Stroke 2024, 55, 1686–1688. [Google Scholar] [CrossRef]
- Jansma, A.; de Bresser, J.; Schoones, J.W.; van Heemst, D.; Akintola, A.A. Sporadic cerebral small vessel disease and cognitive decline in healthy older adults: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2024, 44, 660–679. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Schilling, S.; Duperron, M.G.; Larsson, S.C.; Markus, H.S. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 81–94. [Google Scholar] [CrossRef]
- Rensma, S.P.; van Sloten, T.T.; Launer, L.J.; Stehouwer, C.D.A. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.; Wolters, F.J.; Darweesh, S.K.L.; Vernooij, M.W.; de Wolf, F.; Ikram, M.A.; Hofman, A. Cerebral small vessel disease and the risk of dementia: A systematic review and meta-analysis of population-based evidence. Alzheimer’s Dement. 2018, 14, 1482–1492. [Google Scholar] [CrossRef]
- Dichgans, M.; Leys, D. Vascular Cognitive Impairment. Circ. Res. 2017, 120, 573–591. [Google Scholar] [CrossRef]
- Hu, H.Y.; Ou, Y.N.; Shen, X.N.; Qu, Y.; Ma, Y.H.; Wang, Z.T.; Dong, Q.; Tan, L.; Yu, J.T. White matter hyperintensities and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 36 prospective studies. Neurosci. Biobehav. Rev. 2021, 120, 16–27. [Google Scholar] [CrossRef]
- Van Veluw, S.J.; Biessels, G.J.; Klijn, C.J.; Rozemuller, A.J. Heterogeneous histopathology of cortical microbleeds in cerebral amyloid angiopathy. Neurology 2016, 86, 867–871. [Google Scholar] [CrossRef]
- Arnaud, L.; Benech, P.; Greetham, L.; Stephan, D.; Jimenez, A.; Jullien, N.; García-González, L.; Tsvetkov, P.O.; Devred, F.; Sancho-Martinez, I.; et al. APOE4 drives inflammation in human astrocytes via TAGLN3 repression and NF-κB activation. Cell Rep. 2022, 40, 111200. [Google Scholar] [CrossRef]
- Ahmad, S.; Yang, W.; Orellana, A.; Frölich, L.; de Rojas, I.; Cano, A.; Boada, M.; Hernández, I.; Hausner, L.; Harms, A.C.; et al. Association of oxidative stress and inflammatory metabolites with Alzheimer’s disease cerebrospinal fluid biomarkers in mild cognitive impairment. Alzheimer’s Res. Ther. 2024, 16, 171. [Google Scholar] [CrossRef]
- Montagne, A.; Nikolakopoulou, A.M.; Huuskonen, M.T.; Sagare, A.P.; Lawson, E.J.; Lazic, D.; Rege, S.V.; Grond, A.; Zuniga, E.; Barnes, S.R.; et al. APOE4 accelerates advanced-stage vascular and neurodegenerative disorder in old Alzheimer’s mice via cyclophilin A independently of amyloid-β. Nat. Aging 2021, 1, 506–520. [Google Scholar] [CrossRef]
- Love, S.; Miners, J.S. White matter hypoperfusion and damage in dementia: Post-mortem assessment. Brain Pathol. 2015, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, J.; Arenaza-Urquijo, E.M.; Knopman, D.S.; Schwarz, C.G.; Brown, R.D., Jr.; Rabinstein, A.A.; Gunter, J.L.; Senjem, M.L.; Przybelski, S.A.; Lesnick, T.; et al. White matter hyperintensities: Relationship to amyloid and tau burden. Brain 2019, 142, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Garnier-Crussard, A.; Bougacha, S.; Wirth, M.; Dautricourt, S.; Sherif, S.; Landeau, B.; Gonneaud, J.; De Flores, R.; de la Sayette, V.; Vivien, D.; et al. White matter hyperintensity topography in Alzheimer’s disease and links to cognition. Alzheimer’s Dement. 2022, 18, 422–433. [Google Scholar] [CrossRef]
- Zare, A.; Salehi, S.; Bader, J.M.; Wiessler, A.L.; Prokesch, M.; Albrecht, V.; Villmann, C.; Mann, M.; Briese, M.; Sendtner, M. Axonal Tau Reduction Ameliorates Tau and Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Transl. Neurodegener. 2025, 14, 39. [Google Scholar] [CrossRef]
- Pescoller, J.; Dewenter, A.; Dehsarvi, A.; Steward, A.; Frontzkowski, L.; Zhu, Z.; Roemer-Cassiano, S.N.; Palleis, C.; Hirsch, F.; Wagner, F.; et al. Cortical tau deposition promotes atrophy in connected white matter regions in Alzheimer’s disease. Brain 2025, awaf339. [Google Scholar] [CrossRef] [PubMed]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef]
- He, Z.; Wang, G.; Wu, J.; Tang, Z.; Luo, M. The molecular mechanism of LRP1 in physiological vascular homeostasis and signal transduction pathways. Biomed. Pharmacother. 2021, 139, 111667. [Google Scholar] [CrossRef]
- Yang, W.; Wei, Z.; Wang, T. Unraveling the Role of LRP1 in Alzheimer’s Disease: A Focus on Aβ Clearance and the Liver-Brain Axis. J. Mol. Neurosci. 2025, 75, 43. [Google Scholar] [CrossRef]
- Uchida, Y.; Kano, Y.; Kan, H.; Sakurai, K.; Morita, H.; Akagawa, Y.; Matsukawa, N.; Oishi, K. Blood-brain barrier water exchange and paramagnetic susceptibility alterations during anti-amyloid therapy: Preliminary MRI findings. J. Prev. Alzheimer’s Dis. 2025, 12, 100256. [Google Scholar] [CrossRef]
- Van den Brink, H.; Doubal, F.N.; Duering, M. Advanced MRI in cerebral small vessel disease. Int. J. Stroke 2023, 18, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Godia, E.; Dwivedi, P.; Sharma, S.; Ois Santiago, A.; Roquer Gonzalez, J.; Balcells, M.; Laird, J.; Turk, M.; Suri, H.S.; Nicolaides, A.; et al. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J. Stroke 2018, 20, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Nyúl-Tóth, Á.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking peripheral atherosclerosis to blood-brain barrier disruption: Elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef]
- Heidari, P.; Taghizadeh, M.; Vakili, O. Signaling pathways and molecular mechanisms involved in the onset and progression of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL); a focus on Notch3 signaling. J. Headache Pain 2025, 26, 96. [Google Scholar] [CrossRef]
- Uchida, Y.; Kan, H.; Sakurai, K.; Arai, N.; Inui, S.; Kobayashi, S.; Kato, D.; Ueki, Y.; Matsukawa, N. Iron leakage owing to blood-brain barrier disruption in small vessel disease CADASIL. Neurology 2020, 95, e1188–e1198. [Google Scholar] [CrossRef]
- Guey, S.; Hervé, D. Main features of COL4A1-COL4A2 related cerebral microangiopathies. Cereb. Circ. Cogn. Behav. 2022, 3, 100140. [Google Scholar] [CrossRef]
- Jolly, A.A.; Nannoni, S.; Edwards, H.; Morris, R.G.; Markus, H.S. Prevalence and Predictors of Vascular Cognitive Impairment in Patients with CADASIL. Neurology 2022, 99, e453–e461. [Google Scholar] [CrossRef]
- Xiao, X.; Guo, L.; Liao, X.; Zhou, Y.; Zhang, W.; Zhou, L.; Wang, X.; Liu, X.; Liu, H.; Xu, T.; et al. The role of vascular dementia associated genes in patients with Alzheimer’s disease: A large case-control study in the Chinese population. CNS Neurosci. Ther. 2021, 27, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
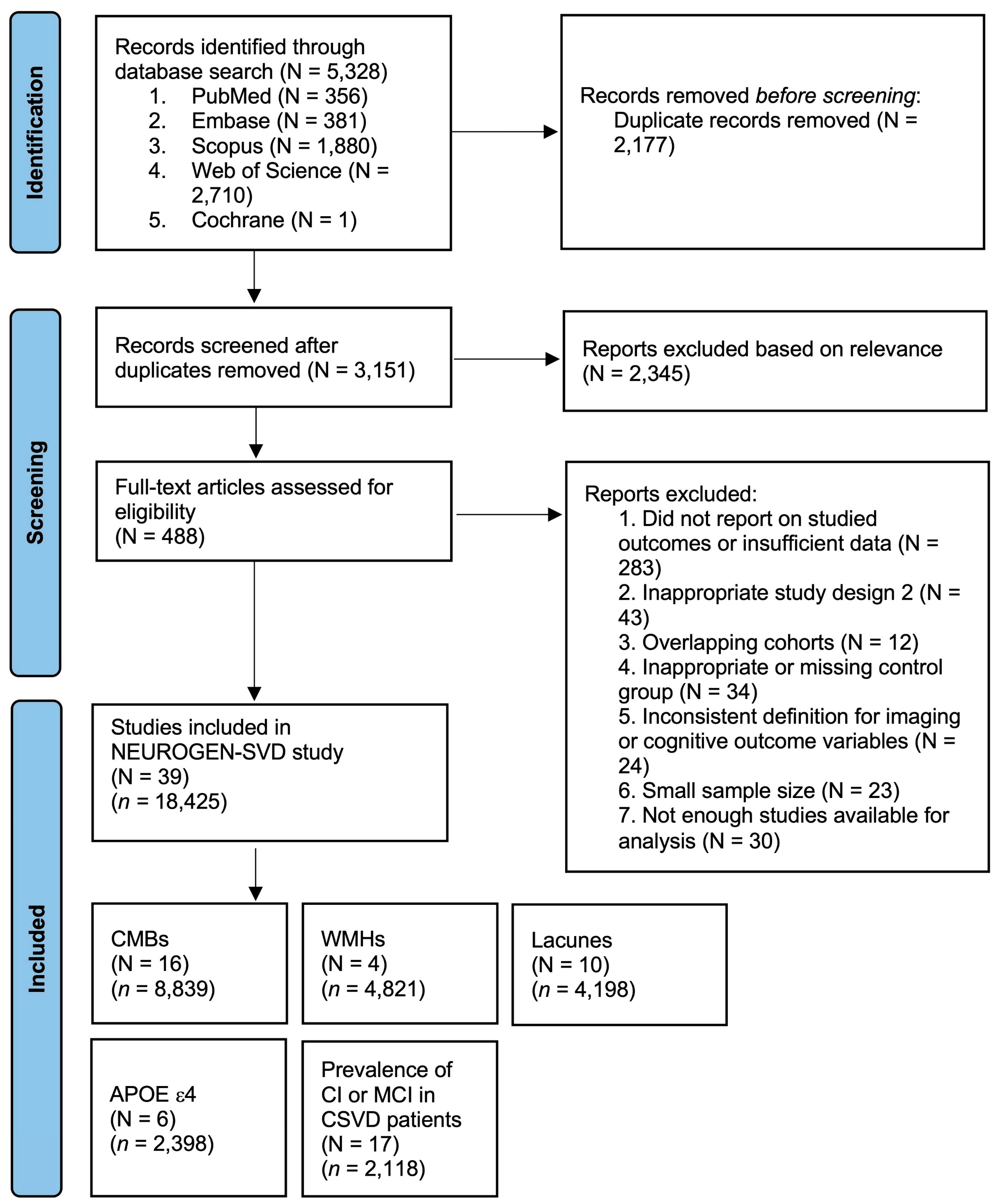
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in Epidemiology. A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]



| Subgroup | Studies (N) | Participants (n) | Summary Effects | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude Prevalence | Pooled Prevalence | 95% CI | z | p | Q | I2 (%) | p (Q) | τ2 | |||
| Overall | 16 | 2118 | 53.5% | 53% | 0.49–0.58 | 33.88 | p < 0.001 | 62.89 | 76.15 | p < 0.001 | 0.02 |
| GCI | 10 | 1518 | 55.9% | 57% | 0.51–0.62 | 28.48 | p < 0.001 | 42.15 | 78.65 | p < 0.001 | 0.02 |
| MCI | 7 | 766 | 46.3% | 46% | 0.42–0.51 | 31.02 | p < 0.001 | 8.91 | 32.67 | p = 0.18 | 0.00 |
| Imaging/Genetic Marker | Cognitive Subgroup | Studies (N) | Participants (n) | Summary Effects DL | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | p, z | Cochran’s Q | p (Q) | H | I2 (%) | τ2 | ||||
| CMBs | MCI | 8 | 2301 | 1.93 [1.48; 2.51] | p < 0.001, z = 4.813 | 11.11 | 0.134 | 1.260 | 37.0 | 0.051 |
| GCI | 5 | 1505 | 1.70 [0.92; 3.16] | p = 0.091, z = 1.689 | 13.45 | 0.009 | 1.834 | 70.3 | 0.310 | |
| ACD | 9 | 5987 | 1.92 [1.41; 2.60] | p < 0.001, z = 4.20 | 15.75 | 0.046 | 1.403 | 49.2 | 0.101 | |
| VaD | 5 | 4399 | 4.70 [2.10; 10.52] | p < 0.001, z = 3.77 | 11.49 | 0.022 | 1.695 | 65.2 | 0.535 | |
| AD | 7 | 5369 | 1.52 [1.04; 2.24] | p = 0.033, z = 2.14 | 12.40 | 0.054 | 1.437 | 80.8 | 2.275 | |
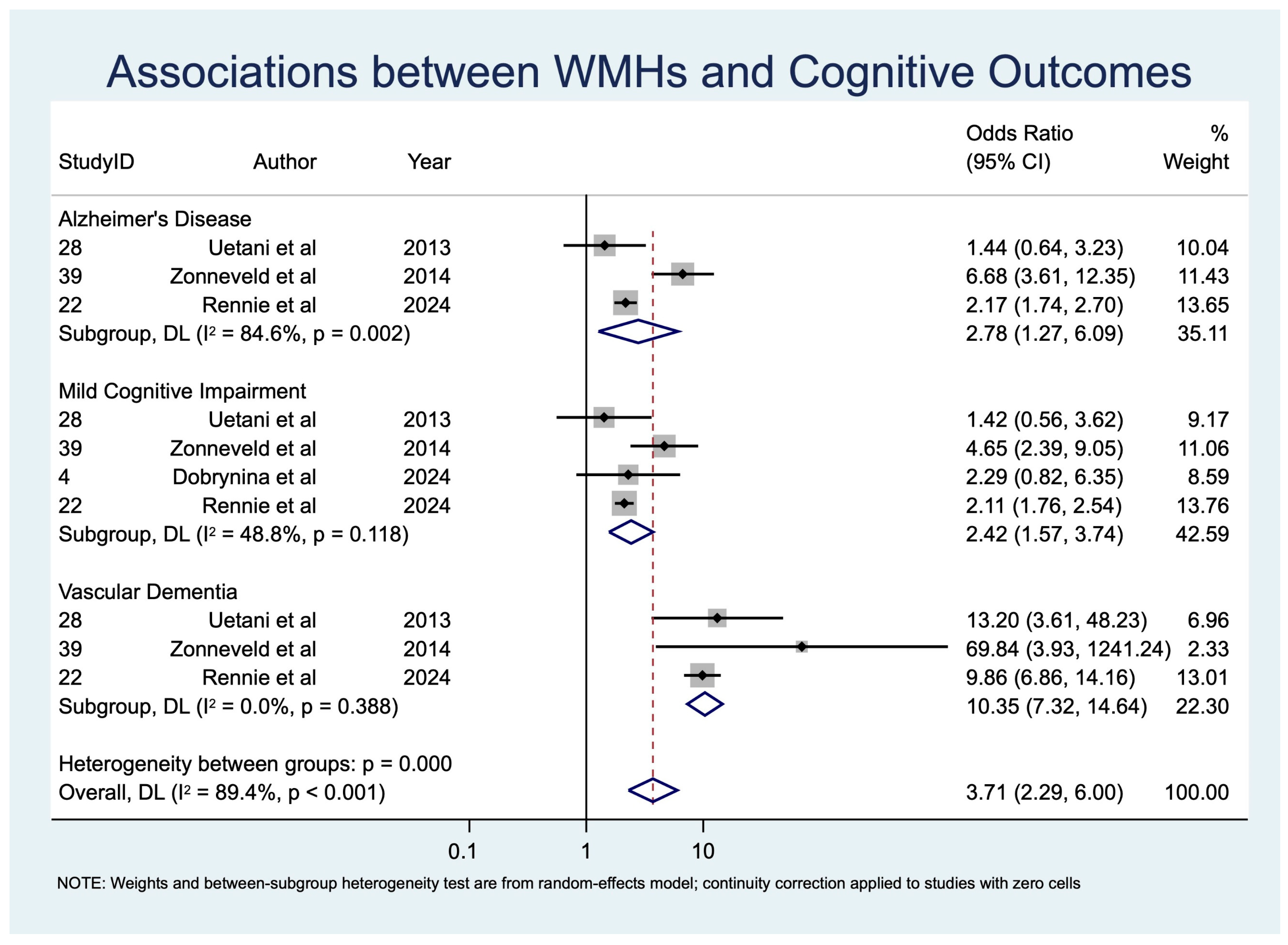
| WMHs | MCI | 4 | 3513 | 2.42 [1.57; 3.74] | p < 0.001, z = 3.980 | 5.86 | 0.118 | 1.398 | 48.8 | 0.094 |
| VaD | 3 | 1815 | 10.35 [7.32; 14.64] | p < 0.001, z = 13.229 | 1.90 | 0.388 | 0.974 | 0.0 | 0.000 | |
| AD | 3 | 2621 | 2.78 [1.27; 6.09] | p = 0.011, z = 2.558 | 12.98 | 0.002 | 2.548 | 84.6 | 0.394 | |
| Lacunes | MCI | 4 | 1836 | 2.70 [1.25; 5.84] | p = 0.011, z = 2.528 | 14.40 | 0.002 | 2.191 | 79.2 | 0.398 |
| GCI | 5 | 1505 | 2.41 [1.33; 4.40] | p = 0.004, z = 2.88 | 10.07 | 0.039 | 1.586 | 60.3 | 0.246 | |
| ACD | 4 | 1440 | 3.18 [1.24; 8.20] | p = 0.017, z = 2.40 | 20.51 | 0.000 | 2.615 | 85.4 | 0.664 | |
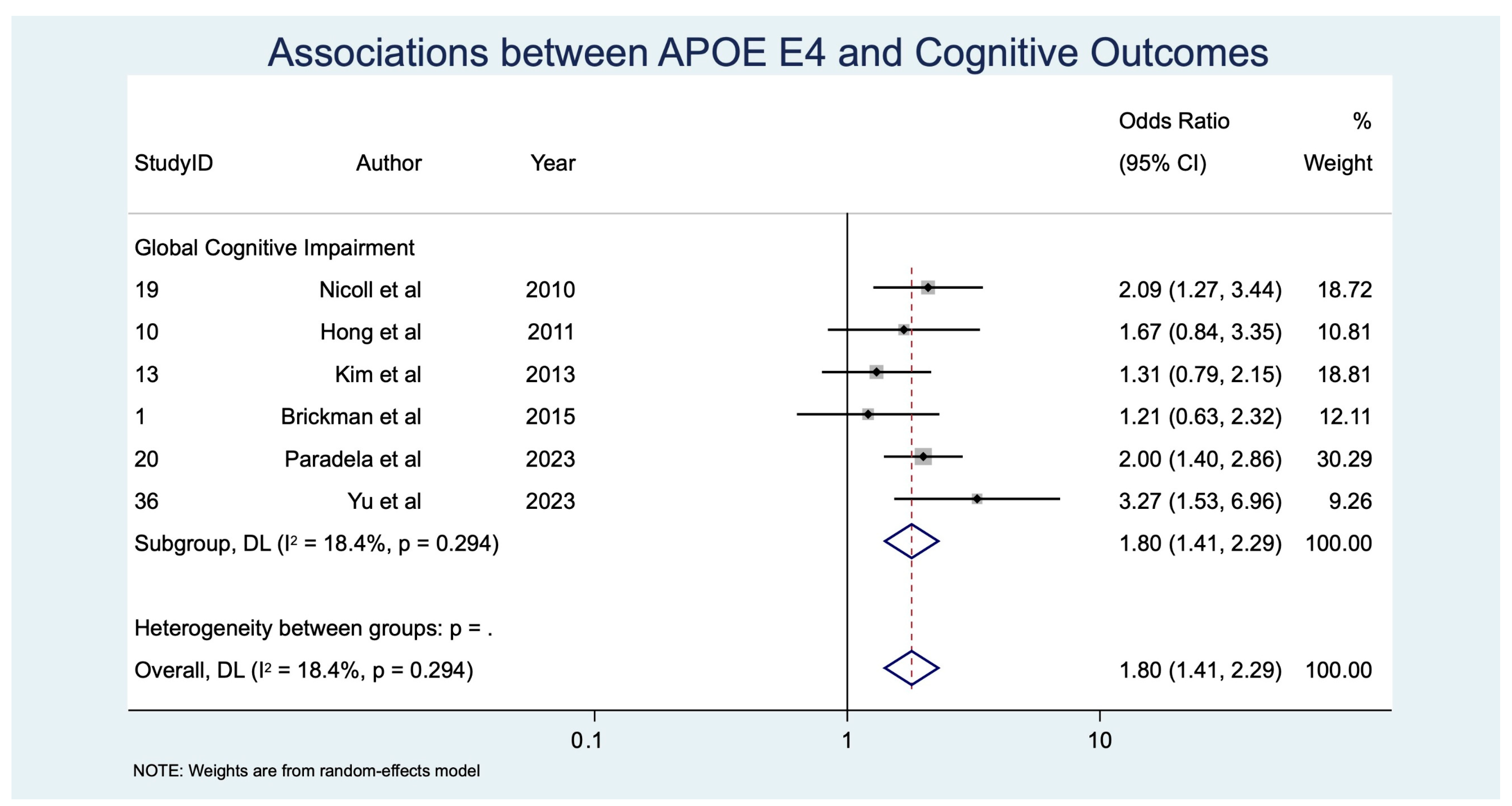
| APOE ε4 | GCI | 6 | 2398 | 1.80 [1.41, 2.29] | p < 0.001, z = 4.729 | 6.13 | 0.294 | 1.107 | 68.3 | 0.017 |
| Cognitive Outcome | Marker | Studies (N) | Participants (n) | Summary Effects DL | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | p, z | Cochran’s Q | p (Q) | H | I2 | τ2 | ||||
| MCI | CMB | 8 | 2301 | 1.93 [1.48; 2.51] | p < 0.001, z = 4.813 | 11.11 | 0.134 | 1.260 | 37.0 | 0.051 |
| WMHs | 4 | 3513 | 2.42 [1.57; 3.74] | p < 0.001, z = 3.980 | 5.86 | 0.118 | 1.398 | 48.8 | 0.094 | |
| Lacunes | 4 | 1836 | 2.70 [1.25; 5.84] | p = 0.011, z = 2.528 | 14.40 | 0.002 | 2.191 | 79.2 | 0.398 | |
| GCI | CMBs | 5 | 1505 | 1.70 [0.92; 3.16] | p = 0.091, z = 1.689 | 13.45 | 0.009 | 1.834 | 70.3 | 0.310 |
| Lacunes | 5 | 1505 | 2.41 [1.33; 4.40] | p = 0.004, z = 2.88 | 10.07 | 0.039 | 1.586 | 60.3 | 0.246 | |
| APOE ε4 allele | 6 | 2398 | 1.80 [1.41, 2.29] | p < 0.001, z = 4.729 | 6.13 | 0.294 | 1.107 | 68.3 | 0.017 | |
| ACD | CMBs | 9 | 5987 | 1.92 [1.41; 2.60] | p < 0.001, z = 4.20 | 15.75 | 0.046 | 1.403 | 49.2 | 0.101 |
| Lacunes | 4 | 1440 | 3.18 [1.24; 8.20] | p = 0.017, z = 2.40 | 20.51 | 0.000 | 2.615 | 85.4 | 0.664 | |
| VaD | CMBs | 5 | 4399 | 4.70 [2.10; 10.52] | p < 0.001, z = 3.77 | 11.49 | 0.022 | 1.695 | 65.2 | 0.535 |
| WMHs | 3 | 1815 | 10.35 [7.32; 14.64] | p < 0.001, z = 13.229 | 1.90 | 0.388 | 0.974 | 0.0 | 0.000 | |
| AD | CMBs | 7 | 5369 | 1.52 [1.04; 2.24] | p = 0.033, z = 2.14 | 12.40 | 0.054 | 1.437 | 80.8 | 2.275 |
| WMHs | 3 | 2621 | 2.78 [1.27; 6.09] | p = 0.011, z = 2.558 | 12.98 | 0.002 | 2.548 | 84.6 | 0.394 | |
| Outcome | No. of Studies (Participants) | Study Design | Relative Effect (95% CI) | Assumed Risk (Control) | Risk with Marker | Absolute Effect (per 1000) | Certainty of Evidence | Reasons |
|---|---|---|---|---|---|---|---|---|
| CMBs → MCI | 8 (~2301) | Observational (meta-analysis, random-effects) | OR 1.93 (1.48–2.51) | 300 per 1000 | 450 per 1000 | 150 more per 1000 | ⊕⊕◯◯ Low | −1 risk of bias, −1 inconsistency, +1 moderate effect |
| CMBs → ACD | 9 (~5987) | Observational (meta-analysis, random-effects) | OR 1.92 (1.41–2.60) | 350 per 1000 | 500 per 1000 | 150 more per 1000 | ⊕⊕⊕◯ Moderate | −1 risk of bias, +1 consistent effect |
| CMBs → VaD | 5 (~4399) | Observational (meta-analysis, random-effects) | OR 4.70 (2.10–10.52) | 200 per 1000 | 560 per 1000 | 360 more per 1000 | ⊕⊕◯◯ Low to Moderate | −1 heterogeneity, +1 strong effect |
| CMBs → AD | 7 (~5369) | Observational (meta-analysis, random-effects) | OR 1.52 (1.04–2.24) | 250 per 1000 | 360 per 1000 | 110 more per 1000 | ⊕⊕◯◯ Low | −1 imprecision, −1 heterogeneity |
| WMHs → MCI | 4 (~3513) | Observational (meta-analysis, random-effects) | OR 2.42 (1.57–3.74) | 300 per 1000 | 520 per 1000 | 220 more per 1000 | ⊕⊕◯◯ Low to Moderate | −1 risk of bias, −1 inconsistency, +1 effect size |
| WMHs → VaD | 4 (~1815) | Observational (meta-analysis, random-effects) | OR 10.35 (7.32–14.64) | 200 per 1000 | 740 per 1000 | 540 more per 1000 | ⊕⊕⊕◯ Moderate | −1 diagnostic variability, +1 very strong effect, +1 low heterogeneity |
| WMHs → AD | 3 (~2621) | Observational (meta-analysis, random-effects) | OR 2.78 (1.27–6.09) | 250 per 1000 | 480 per 1000 | 230 more per 1000 | ⊕⊕◯◯ Low | −1 inconsistency, −1 indirectness, −1 imprecision |
| Lacunes → MCI | 4 (~1836) | Observational (meta-analysis, random-effects) | OR 2.70 (1.25–5.84) | 300 per 1000 | 560 per 1000 | 260 more per 1000 | ⊕⊕◯◯ Low | −1 heterogeneity, −1 indirectness |
| Lacunes → GCI | 5 (~1505) | Observational (meta-analysis, random-effects) | OR 2.41 (1.33–4.40) | 400 per 1000 | 610 per 1000 | 210 more per 1000 | ⊕⊕◯◯ Low | −1 marker misclassification, −1 heterogeneity |
| Lacunes → ACD | 4 (~1440) | Observational (meta-analysis, random-effects) | OR 3.18 (1.24–8.20) | 350 per 1000 | 640 per 1000 | 290 more per 1000 | ⊕⊕◯◯ Low | −1 inconsistency, −1 imprecision |
| APOE ε4 → GCI | 6 (~2398) | Observational (meta-analysis, random-effects) | OR 1.80 (1.41–2.29) | 400 per 1000 | 570 per 1000 | 170 more per 1000 | ⊕⊕◯◯ Low | −1 inconsistency, −1 indirectness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Beran, R.G.; Bhaskar, S.M.M. Neuroimaging and Genetic Markers of Cerebral Small Vessel Disease and Cognitive Outcomes: A Systematic Review and Meta-Analysis (NEUROGEN-SVD Study). Diagnostics 2025, 15, 2585. https://doi.org/10.3390/diagnostics15202585
Jin C, Beran RG, Bhaskar SMM. Neuroimaging and Genetic Markers of Cerebral Small Vessel Disease and Cognitive Outcomes: A Systematic Review and Meta-Analysis (NEUROGEN-SVD Study). Diagnostics. 2025; 15(20):2585. https://doi.org/10.3390/diagnostics15202585
Chicago/Turabian StyleJin, Chelsea, Roy G. Beran, and Sonu M. M. Bhaskar. 2025. "Neuroimaging and Genetic Markers of Cerebral Small Vessel Disease and Cognitive Outcomes: A Systematic Review and Meta-Analysis (NEUROGEN-SVD Study)" Diagnostics 15, no. 20: 2585. https://doi.org/10.3390/diagnostics15202585
APA StyleJin, C., Beran, R. G., & Bhaskar, S. M. M. (2025). Neuroimaging and Genetic Markers of Cerebral Small Vessel Disease and Cognitive Outcomes: A Systematic Review and Meta-Analysis (NEUROGEN-SVD Study). Diagnostics, 15(20), 2585. https://doi.org/10.3390/diagnostics15202585









