Hypertension Resistant to RAAS Inhibitors as a Prognostic Indicator for Rapid Progression to ESRD in ADPKD: A Ten-Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Outcomes and Follow-Up
2.4. Statistical Analysis
2.5. Software and Ethics
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Treatment and Therapeutic Patterns
3.3. Biochemical Parameters and eGFR Progression
3.4. Identification of Predictive Factors for CKD Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spithoven, E.M.; Kramer, A.; Meijer, E.; Orskov, B.; Wanner, C.; Abad, J.M.; Aresté, N.; Alonso de la Torre, R.; Caskey, F.; Couchoud, C.; et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: Prevalence and survival—An analysis of data from the ERA-EDTA Registry. Nephrol. Dial. Transplant. 2014, 29, iv15–iv25. [Google Scholar] [CrossRef] [PubMed]
- Hoefele, J.; Mayer, K.; Scholz, M.; Klein, H.-G. Novel PKD1 and PKD2 mutations in autosomal dominant polycystic kidney disease (ADPKD). Nephrol. Dial. Transplant. 2011, 26, 2181–2188. [Google Scholar] [PubMed]
- Lanktree, M.B.; Haghighi, A.; di Bari, I.; Song, X.; Pei, Y. Insights into Autosomal Dominant Polycystic Kidney Disease from Genetic Studies. Clin. J. Am. Soc. Nephrol. 2021, 16, 790. [Google Scholar] [CrossRef] [PubMed]
- Agborbesong, E.; Li, L.X.; Li, L.; Li, X. Molecular Mechanisms of Epigenetic Regulation, Inflammation, and Cell Death in ADPKD. Front. Mol. Biosci. 2022, 9, 922428. [Google Scholar] [CrossRef] [PubMed]
- Cornec-Le Gall, E.; Audrézet, M.P.; Rousseau, A.; Hourmant, M.; Renaudineau, E.; Charasse, C.; Morin, M.P.; Moal, M.C.; Dantal, J.; Wehbe, B.; et al. The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. JASN 2016, 27, 942–951. [Google Scholar] [PubMed]
- Chen, E.W.; Chong, J.; Valluru, M.K.; Durkie, M.; Simms, R.J.; Harris, P.C.; Ong, A.C. Combining genotype with height-adjusted kidney length predicts rapid progression of ADPKD. Nephrol. Dial. Transplant. 2024, 39, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Wigerinck, S.; Schellekens, P.; Smith, B.H.; Hanna, C.; Dachy, A.; Chedid, M.; Borghol, A.H.; Senum, S.R.; Bockenhauer, D.; Harris, P.C.; et al. Characteristics of patients with autosomal polycystic kidney disease reaching kidney failure by age 40. Pediatr. Nephrol. 2025, 40, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Capelli, I.; Aiello, V.; Carretta, E.; Graziano, C.; Sciascia, N.; Corsi, C.; Mantovani, V.; Monteduro, F.; Seri, M.; La Manna, G. P0085MAYO and PRO-PKD score concordance for progression of renal failure evaluation in ADPKD patients. Nephrol. Dial. Transplant. 2020, 35, gfaa142.P0085. [Google Scholar] [CrossRef]
- Pei, Y.; Obaji, J.; Dupuis, A.; Paterson, A.D.; Magistroni, R.; Dicks, E.; Parfrey, P.; Cramer, B.; Coto, E.; Torra, R.; et al. Unified Criteria for Ultrasonographic Diagnosis of ADPKD. J. Am. Soc. Nephrol. 2009, 20, 205. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.B.; Devuyst, O.; Eckardt, K.U.; Gansevoort, R.T.; Harris, T.; Horie, S.; Kasiske, B.L.; Odland, D.; Pei, Y.; Perrone, R.D.; et al. Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015, 88, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Rangel, L.J.; Bergstralh, E.J.; Osborn, S.L.; Harmon, A.J.; Sundsbak, J.L.; Bae, K.T.; Chapman, A.B.; Grantham, J.J.; Mrug, M.; et al. Imaging Classification of Autosomal Dominant Polycystic Kidney Disease: A Simple Model for Selecting Patients for Clinical Trials. J. Am. Soc. Nephrol. 2015, 26, 160. [Google Scholar] [CrossRef] [PubMed]
- Sans-Atxer, L.; Torra, R.; Fernández-Llama, P. Hypertension in autosomal-dominant polycystic kidney disease (ADPKD). Clin. Kidney J. 2013, 6, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Iversen, J.; Wilcox, C.S.; Strandgaard, S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003, 64, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Chu, Y.-C.; Lu, T.; Lin, H.Y.-H.; Chan, T.-C. Cardiometabolic comorbidities in autosomal dominant polycystic kidney disease: A 16-year retrospective cohort study. BMC Nephrol. 2023, 24, 333. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Kim, Y.J.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Ahn, C.; Oh, K.H.; Park, S.K.; Lee, K.B.; Sung, S.; et al. Persistent Resistant Hypertension Has Worse Renal Outcomes in Chronic Kidney Disease than that Resolved in Two Years: Results from the KNOW-CKD Study. J. Clin. Med. 2021, 10, 3998. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
| Variable | No. (%) |
|---|---|
| Male Sex | 59 (44.36) |
| Family History | 113 (85.00) |
| PKD Gene | |
| PKD1 | 98 (73.68) |
| PKD2 | 14 (10.53) |
| No Mutation Identified | 21 (15.79) |
| Extrarenal Manifestations | |
| Cerebral Aneurysm | 2 (1.50) |
| Pulmonary Disease | 3 (2.26) |
| Nephrolithiasis (Kidney Stones) | 11 (8.27) |
| IPMN * | 23 (17.29) |
| Cardiac Hypertrophy | 50 (37.59) |
| Cardiac Valve Defect | 8 (6.02) |
| Diabetes Mellitus | 3 (2.26) |
| Diverticulosis | 5 (3.76) |
| Kidney Pain (>2 episodes/year) | 70 (52.63) |
| Hernia | 9 (6.77) |
| Risk Factors for End-Stage Kidney Disease | |
| Smoking | 14 (10.53) |
| Cyst Infection (per year) | 19 (14.29) |
| Hypertension | 74 (55.63) |
| Urinary Tract Infection | 39 (29.32) |
| Hematuria | 38 (28.57) |
| Nephrectomy | 2 (1.50) |
| Mayo Classification for ADPKD | |
| Mayo 1A | 33 (24.81) |
| Mayo 1B | 46 (34.59) |
| Mayo 1C | 27 (20.30) |
| Mayo 1D | 16 (12.03) |
| Mayo 1E | 11 (8.27) |
| Treatments | |
| ACE Inhibitors | 64 (48.12) |
| ARBs | 23 (17.29) |
| Other Antihypertensive Drugs | 10 (7.5) |
| No Antihypertensive | 36 (27.1) |
| RAAS + Other Antihypertensives | 37 (27.8) |
| Tolvaptan | 17 (12.80) |
| Proteinuria > 300 mg/24 h, Baseline | 33 (24.81) |
| Proteinuria > 300 mg/24 h, Last Visit | 49 (36.84) |
| Variable | Median (IQR) |
|---|---|
| Age (years) | 52.1 (13.2) |
| Height (cm) | 165.89 (7.95) |
| Weight (kg) | 65.2 (10.9) |
| Body Mass Index (kg/m2) | 23.74 (7.41) |
| Body Surface Area (m2) | 1.7268 (0.1497) |
| Kidney Volume on MRI (mL) | 1379.95 (881.8) |
| Annual eGFR slope (mL/min/1.73 m2) | 3.8 (2.38) |
| eGFR (first visit) (mL/min/1.73 m2) | 67.9 (31.0) |
| eGFR (last visit) (mL/min/1.73 m2) | 48.9 (32.9) |
| eGFR slope (mL/min/1.73 m2) in 5 years | 19.0 (11.89) |
| PRO-PKD score, median (IQR) | 4 (2–6) |
| Covariate | OR | Lower CI (95%) | Upper CI (95%) | p-Value |
|---|---|---|---|---|
| ACEI | 1.475 | 0.604 | 3.603 | 0.39346 |
| Age | 1.029 | 0.994 | 1.065 | 0.10244 |
| Antihypertensive Drugs, Other† | 3.03 | 1.16 | 7.914 | 0.02360 |
| ARBS | 1.247 | 0.415 | 3.749 | 0.69460 |
| Body Mass Index | 1.026 | 0.933 | 1.129 | 0.59939 |
| Body Surface Area | 1.099 | 0.059 | 20.584 | 0.94980 |
| Cyst Infection | 1.458 | 0.379 | 5.614 | 0.58329 |
| Diabetes | 2.923 | 0.291 | 29.356 | 0.36211 |
| Diverticulosis | 6.333 | 0.726 | 55.221 | 0.09480 |
| Estimated Heart Risk | 1.029 | 0.994 | 1.065 | 0.10365 |
| eGFR, first visit | 0.969 | 0.952 | 0.987 | 0.00059 |
| Hernia | 4.0 | 0.427 | 37.459 | 0.22451 |
| Hypertension | 1.96 | 0.767 | 5.01 | 0.15963 |
| htTKV | 1.0 | 0.999 | 1.001 | 0.66333 |
| Hypertension on drugs | 1.552 | 0.622 | 3.87 | 0.34593 |
| Kidney Pain | 2.336 | 0.958 | 5.697 | 0.06218 |
| Kidney Volume to Body Surface Area | 1.0 | 0.999 | 1.001 | 0.65432 |
| MRI kidney volume | 1.0 | 0.999 | 1.0 | 0.67345 |
| Proteinuria, Time 0 | 0.682 | 0.187 | 2.48 | 0.56100 |
| Macrohematuria, recurrent | 0.655 | 0.227 | 1.889 | 0.43313 |
| Smoking | 0.893 | 0.357 | 2.232 | 0.80847 |
| Tolvaptan | 1.1 | 0.335 | 3.614 | 0.87522 |
| Urinary Tract Infection | 1.603 | 0.574 | 4.482 | 0.36799 |
| Weight | 1.005 | 0.966 | 1.046 | 0.79594 |
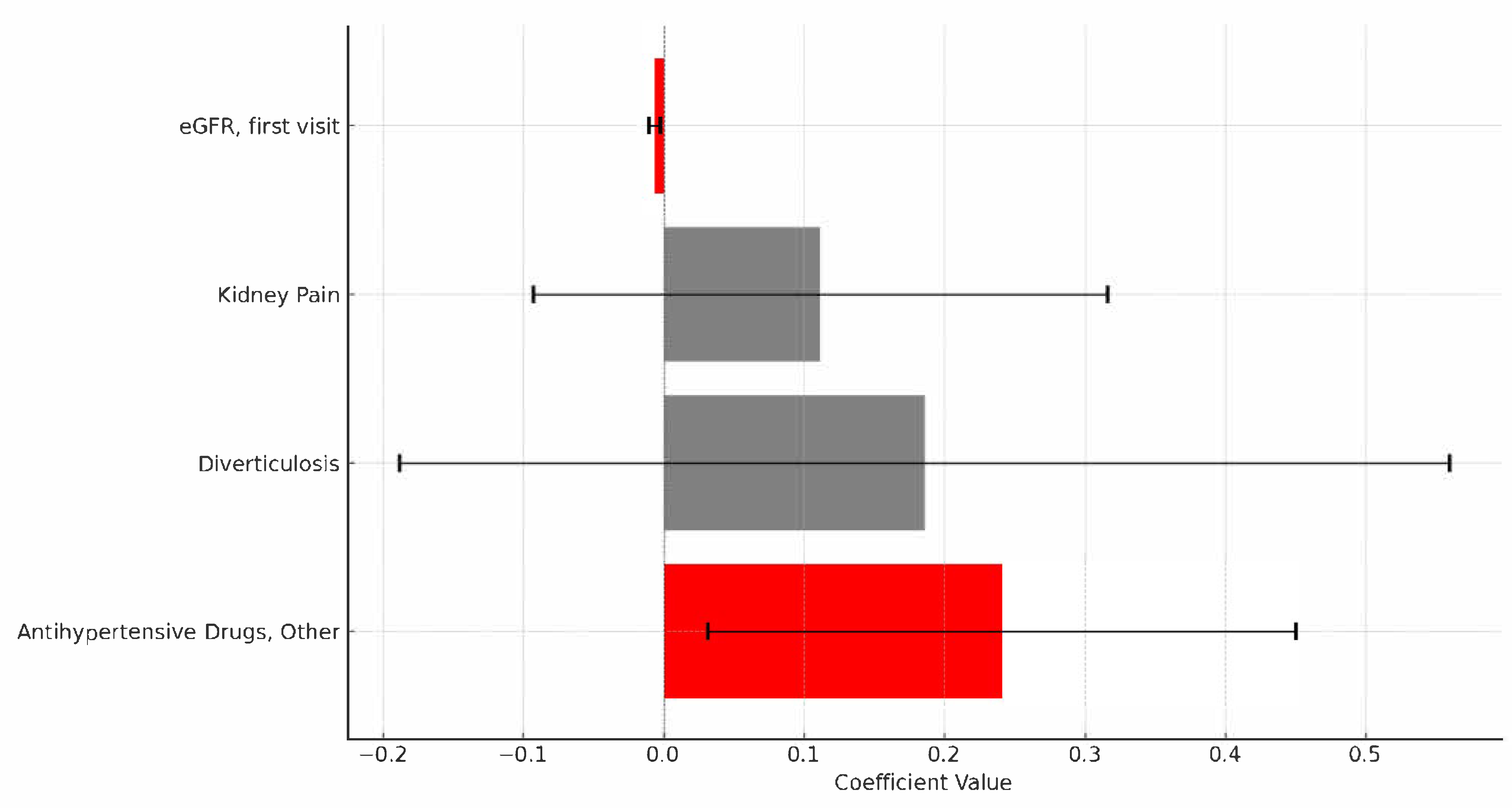
| Variable | OR | 95% CI (Upper) | 95% CI (Lower) | p-Value |
|---|---|---|---|---|
| eGFR, first visit | 0.993 | 0.989 | 0.997 | 0.0012 |
| Antihypertensive Drugs, Other † | 1.272 | 1.032 | 1.569 | 0.0248 |
| Diverticulosis | 1.204 | 829 | 1.75 | 0.3255 |
| Kidney Pain | 1.118 | 911 | 1.372 | 0.2810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angioi, A.; Piras, D.; Lepori, N.; Floris, M.; Cabiddu, G.; Pani, A. Hypertension Resistant to RAAS Inhibitors as a Prognostic Indicator for Rapid Progression to ESRD in ADPKD: A Ten-Year Follow-Up. Diagnostics 2025, 15, 2583. https://doi.org/10.3390/diagnostics15202583
Angioi A, Piras D, Lepori N, Floris M, Cabiddu G, Pani A. Hypertension Resistant to RAAS Inhibitors as a Prognostic Indicator for Rapid Progression to ESRD in ADPKD: A Ten-Year Follow-Up. Diagnostics. 2025; 15(20):2583. https://doi.org/10.3390/diagnostics15202583
Chicago/Turabian StyleAngioi, Andrea, Doloretta Piras, Nicola Lepori, Matteo Floris, Gianfranca Cabiddu, and Antonello Pani. 2025. "Hypertension Resistant to RAAS Inhibitors as a Prognostic Indicator for Rapid Progression to ESRD in ADPKD: A Ten-Year Follow-Up" Diagnostics 15, no. 20: 2583. https://doi.org/10.3390/diagnostics15202583
APA StyleAngioi, A., Piras, D., Lepori, N., Floris, M., Cabiddu, G., & Pani, A. (2025). Hypertension Resistant to RAAS Inhibitors as a Prognostic Indicator for Rapid Progression to ESRD in ADPKD: A Ten-Year Follow-Up. Diagnostics, 15(20), 2583. https://doi.org/10.3390/diagnostics15202583






