Abstract
Background/Objectives: This study aimed to evaluate the effectiveness of core needle biopsy (CNB) by comparing its diagnostic yield to fine needle aspiration cytology (FNAC) across primary and secondary examinations. Methods: This retrospective review analyzed medical records of patients who visited Soonchunhyang University Cheonan Hospital between January 2021 and August 2023 for thyroid nodule evaluation. Demographic data and the malignancy risk of thyroid nodules were collected based on the 2021 Korean Thyroid Imaging Reporting and Data System. FNAC and CNB results, classified using the Bethesda system for reporting thyroid cytopathology and diagnostic categories for thyroid CNB, were categorized as either “conclusive” or “inconclusive.” The rates of conclusive results in the primary examination and nodules transitioning from inconclusive to conclusive results during the secondary examination were analyzed. Finally, the diagnostic yields of FNAC and CNB were assessed using histopathological findings from surgically excised nodules. Results: The rate of nodules classified as “conclusive” was significantly higher in the CNB group than that in the FNAC group. Among nodules subjected to secondary examination, only the group with FNAC followed by CNB demonstrated a significant improvement in the rate of transition from inconclusive to conclusive results. Although FNAC and CNB showed comparable sensitivity and accuracy, the specificity of CNB was greater than that of FNAC. Conclusions: This study confirms the clinical utility of CNB by demonstrating its higher rate of conclusive results than FNAC. Future prospective studies, including cost–benefit analyses, are warranted to further define the indications for CNB.
1. Introduction
Fine-needle aspiration cytology (FNAC) is widely recommended for diagnosing thyroid nodules owing to its simplicity and cost-effectiveness [1,2,3]. However, the incidence of inconclusive cytology results, such as non-diagnostic outcomes or atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), is regarded as its main shortcoming [4]. In such cases, repeated FNAC is often required to establish a definitive diagnosis [5]. Unfortunately, the rates of non-diagnostic or AUS/FLUS results in consecutive FNAC examinations remain substantial, ranging from 17% to 47% overall [6,7,8,9] and reaching up to 67% in nodules with initially inconclusive findings [10,11,12,13].
To address the limitations of FNAC, core needle biopsy (CNB) has been introduced as an alternative diagnostic approach for thyroid nodules. Numerous studies have demonstrated that CNB is more effective than repeated FNAC in achieving definitive diagnoses for nodules initially classified as non-diagnostic by FNAC [14,15,16,17,18]. Additionally, recent studies have validated the efficacy of CNB as a primary diagnostic procedure for thyroid nodules. Despite these findings, most studies have [19,20,21] focused either on CNB’s utility as a primary diagnostic method or on its effectiveness as a secondary diagnostic test for nodules deemed non-diagnostic by FNAC.
In this study, we aimed to evaluate the efficacy of CNB by comparing the diagnostic accuracy of FNAC and CNB when used as primary and secondary diagnostic tools, respectively.
2. Materials and Methods
2.1. Patients
This retrospective study, based on the analysis of medical records, was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital (SCHCA 2024-07-023, The date of approval was 9 August 2024). The study included 289 patients who visited the Department of Otorhinolaryngology-Head and Neck Surgery at Soonchunhyang University Cheonan Hospital and underwent FNAC or CNB for thyroid nodule evaluation between January 2021 and August 2023. All patients were informed of the potential complications, costs, and respective advantages and disadvantages of FNAC and CNB before selecting their preferred diagnostic method and providing informed consent. Before undergoing FNAC or CNB, all patients received high-resolution ultrasonography (Alpinion E-CUBE 15, Alpinion Medical Systems®, Seoul, Republic of Korea) to evaluate the number, size, margins, and internal echogenicity of each nodule. The risk of malignancy in the thyroid nodules was assessed according to the revised 2021 guidelines of the Korean Society of Thyroid Radiology (K-TIRADS). FNAC or CNB was performed when pathological confirmation was required [3,22]. Two experienced otorhinolaryngologists, each with over seven years of experience in ultrasonography, conducted all procedures. FNAC was performed for large cystic nodules, small nodules located lateral or posterior to major vessels, and in cases where needle or biopsy device access was restricted to the transverse plane of the ultrasound probe. In all other cases, the type of diagnostic test was determined based on patient preference. During the study period, FNAC was performed on 248 thyroid nodules, and CNB was performed on 111 thyroid nodules.
2.2. Evaluation of Ultrasonographic Findings
All thyroid nodules were retrospectively analyzed according to the revised 2021 K-TIRADS, regardless of the examination period. The analysis assessed the composition, echogenicity, orientation, margin, and echogenic foci of the nodules. Ultrasonographic features suggestive of malignancy, including non-parallel orientation, irregular margins, and punctate echogenic foci within solid components smaller than 1 mm, were categorized into four K-TIRADS categories ranging from 2 to 5 (Figure 1) [22]. Nodules classified as K-TIRADS 2 were excluded from biopsy recommendations per the guidelines. Consequently, 20 thyroid nodules initially evaluated by FNAC and one nodule evaluated by CNB due to a recent size increase were excluded from the analysis.

Figure 1.
Illustration of the 2021 Korean Thyroid Imaging Reporting and Data System (2021 K-TIRADS) [22].
2.3. Fine Needle Aspiration Cytology
FNAC was performed with the patient in a supine position, with the neck extended using a pillow placed under the shoulders. Under high-resolution ultrasound guidance, a 23-gauge needle was inserted into the lesion, and cellular material was aspirated through repeated back-and-forth motions. The aspirated samples were smeared onto glass slides and fixed in 95% alcohol. The procedure was repeated 1 to 3 times to ensure adequate sample collection. For pure cystic nodules, the needle was inserted, and the contents were aspirated to the maximum extent possible in a single attempt. These samples were fixed using liquid-based cytology. The fixed specimens were stained with Papanicolaou stain and examined under a light microscope. Diagnoses were made according to the Bethesda system for reporting thyroid cytopathology, which classifies samples into six diagnostic categories (Figure 2) [5].

Figure 2.
The Bethesda System for Reporting Thyroid Cytopathology (Bethesda system) [5].
2.4. Core Needle Biopsy
Similar to the FNAC procedure, an ultrasound examination was conducted, followed by the administration of local anesthesia using 2% lidocaine with 1:100,000 epinephrine at the skin and anticipated needle path. A TSK Ace-cut device (Create Medic®, Yokohama, Japan) with an 11 mm advance length and 18-gauge thickness was used for the biopsy. Under ultrasound guidance, the biopsy device was inserted parallel to the transducer’s transverse axis. The tip of the device was positioned at the boundary of the lesion or 2–3 mm away. The stylet and cutting cannulas were fired sequentially to collect the sample, with only one pass per lesion to minimize the risk of bleeding. The obtained samples were fixed in 10% formalin and classified into six diagnostic categories based on the 2019 Practice Guidelines for Thyroid Core Needle Biopsy by the Korean Thyroid Association (Figure 3) [23].

Figure 3.
Diagnostic categories of thyroid core needle biopsy suggested by the 2019 Practice Guidelines of the Korean Thyroid Association [23].
2.5. Classification of Primary Examination Results
The diagnostic categories outlined in the Bethesda system and the 2019 practice guidelines for thyroid core needle biopsy of the Korean Thyroid Association were unified, as both systems classify results into non-diagnostic (Category I), benign (Category II), AUS/FLUS (Category III), follicular neoplasm/suspicious for follicular neoplasm (FN/SFN) (Category IV), suspicious for malignancy (Category V), and malignancy (Category VI). The outcomes of primary FNAC and CNB were categorized as either “conclusive” (Categories II, IV, V, and VI), which could determine further management without additional testing, or “inconclusive” (Categories I and III), which required further diagnostic procedures. The diagnostic yield of FNAC and CNB was compared by analyzing the frequency of “conclusive” and “inconclusive” results for each test type. Among patients with conclusive diagnoses from the initial tests, those confirmed with malignancy or follicular neoplasm, as well as those with compressive symptoms due to the size of the masses, were recommended for surgical intervention.
2.6. Classification of Secondary Examination Results
Among the nodules that underwent primary FNAC, those with inconclusive results or those confirmed as benign but classified as K-TIRADS 5 on ultrasound were subjected to secondary FNAC or CNB. Based on the secondary test performed, the nodules were divided into two groups: FNAC/FNAC (primary FNAC followed by secondary FNAC) and FNAC/CNB (primary FNAC followed by secondary CNB). The diagnostic outcomes for each group were categorized as ‘conclusive’ or ‘inconclusive’ according to the Bethesda System and the 2019 Practice Guidelines for Thyroid Core Needle Biopsy of the Korean Thyroid Association. The conversion rates from inconclusive primary results to conclusive secondary results were compared between groups. Nodules that underwent multiple tests following primary FNAC were classified according to the final test performed.
Similarly, nodules that initially underwent CNB and yielded inconclusive results or those confirmed as benign but classified as K-TIRADS 5 on ultrasound were subjected to secondary testing and categorized into the CNB/CNB group. CNB results were classified as ‘conclusive’ or ‘inconclusive’ according to the 2019 Practice Guidelines for Thyroid Core Needle Biopsy by the Korean Thyroid Association. If the results remained in Category III after two CNB tests, surgical excision was recommended. Other surgical indications remained consistent with those following the primary test (Figure 4).

Figure 4.
Study flowchart. (a) Reasons for no further examination included loss to follow-up, routine ultrasonographic evaluation only, and referral to another hospital owing to patient preferences. A conclusive result is defined as Category II (benign), Category IV (follicular neoplasm/suspicious for follicular neoplasm), Category V (suspicious for malignancy), and Category VI (malignancy) according to the Bethesda system and 2019 Practice Guidelines for Thyroid Core Needle Biopsy of the Korean Thyroid Association. An inconclusive result is defined as Category I (non-diagnostic) and Category III (atypia of undetermined significance/follicular lesion of undetermined significance) according to the Bethesda system and 2019 Practice Guidelines for Thyroid Core Needle Biopsy of the Korean Thyroid Association. FNAC: Fine-needle aspiration cytology; CNB: Core needle biopsy. Of the nodules included, 19 underwent both FNAC and CNB simultaneously; therefore, the total number of unique nodules is 319.
2.7. Comparison of Diagnostic Yield Between FNAC and CNB Based on Postoperative Definitive Histopathological Evaluation
The diagnostic yield of the final preoperative FNAC and CNB samples was analyzed using the histopathological diagnosis obtained after surgery. Based on the Bethesda System and the 2019 Practice Guidelines for Thyroid Core Needle Biopsy by the Korean Thyroid Association, Category I was classified as non-diagnostic, Category II as benign, Categories III and IV as indeterminate, and Categories V and VI as malignant. The final preoperative results were reclassified into these four categories, and the sensitivity, specificity, and accuracy of FNAC and CNB were calculated (Figure 5) [24].
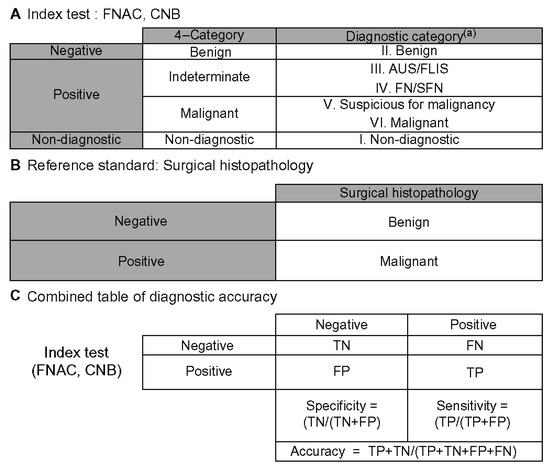
Figure 5.
Schematic for calculating diagnostic accuracy [24]. (A) Benign fine-needle aspiration cytology (FNAC) or core needle biopsy (CNB) results were considered negative. All FNAC and CNB results that were neither benign nor inconclusive were classified as positive FNAC or CNB results. (B) Reference standard consisted solely of surgical histopathology. (C) A 2 × 2 table of diagnostic accuracy was constructed using the above definitions. (a) Diagnoses were categorized according to the six categories of the Bethesda system and the 2019 Practice Guidelines for Thyroid Core Needle Biopsy of the Korean Thyroid Association. FNAC: Fine-needle aspiration cytology; CNB: Core needle biopsy; AUS/FLUS: atypia of undetermined significance/follicular lesion of undetermined significance; FN/SFN: follicular neoplasm/suspicious for follicular neoplasm; FN: false-negative; FP: false-positive; TN: true-negative.
2.8. Statistical Analysis
The evaluation of primary diagnostic tests was performed using the chi-square test or Fisher’s exact test for categorical data and Student’s t-test for continuous data. The McNemar test was employed to compare the conversion rates of inconclusive results to conclusive results in secondary tests across different groups. Statistical analyses were conducted using IBM SPSS 23.0 statistical software (IBM Corp., Armonk, NY, USA), with a p-value of less than 0.05 considered statistically significant.
3. Results
3.1. Patient Demographic and Ultrasonographic Findings
A total of 228 nodules from 196 patients underwent primary FNAC, and 110 nodules from 93 patients underwent primary CNB. Among the patients in the primary FNAC group, 64 (32.7%) were men and 132 (67.3%) were women. In the primary CNB group, 28 (30.1%) patients were men and 65 (69.9%) were women, with no statistically significant difference in sex distribution between the two groups (p > 0.05). The mean ages of the patients in the primary FNAC and CNB groups were 54.3 ± 15.4 and 56.9 ± 15.2 years, respectively, showing no statistically significant difference (p > 0.05).
The mean size of nodules on ultrasound prior to biopsy was 1.80 ± 0.61 cm in the primary FNAC group and 1.72 ± 1.10 cm in the primary CNB group, with no statistically significant difference between the groups (p > 0.05). When analyzing the K-TIRADS results of the thyroid nodules categorized as low-, intermediate-, and high-suspicion, 99 (43.4%), 84 (36.9%), and 45 (19.7%) nodules in the primary FNAC group were identified as low-, intermediate-, and high-suspicion, respectively. In the primary CNB group, 50 nodules (45.5%) were classified as low suspicion, 28 (25.5%) as intermediate suspicion, and 32 (29.0%) as high suspicion. There was no statistically significant difference in the overall distribution between the groups (p = 0.054) (Table 1).

Table 1.
Patient demographic and ultrasonographic findings.
3.2. Primary Examination Results
A total of 228 nodules underwent FNAC, and 110 nodules underwent CNB during the study period. Nineteen nodules were evaluated by both methods simultaneously, making the total number of eligible nodules 319.
The number of nodules diagnosed as Category I (non-diagnostic), Category II (benign), Category III (AUS/FLUS), Category IV (FN/SFN), Category V (suspicious for malignancy), and Category VI (malignancy) after primary testing were as follows: in the primary FNAC group, there were 68 (29.8%), 92 (40.4%), 45 (19.7%), 1 (0.4%), 19 (8.3%), and 3 (1.3%) nodules, respectively. In the primary CNB group, there were 3 (2.7%), 58 (52.7%), 28 (25.5%), 2 (1.8%), 9 (8.2%), and 10 (9.1%) nodules, respectively. When classified as ‘conclusive’ and ‘inconclusive,’ the primary FNAC group had 115 (50.4%) conclusive and 113 (49.6%) inconclusive results, whereas the primary CNB group had 79 (71.8%) conclusive and 31 (28.2%) inconclusive results. The difference in the proportion of conclusive and inconclusive results between the two groups was statistically significant (p < 0.05) (Table 2).

Table 2.
Primary examination results.
Among the primary tests, 19 nodules underwent both CNB and FNAC simultaneously, with eight showing discrepant results between the two tests. Of these, FNAC results were inconclusive for seven nodules. Surgery was performed on five of these seven nodules, revealing two benign nodules and three cases of papillary thyroid carcinoma (Table 3) (Figure 6).

Table 3.
Cases of patients with concurrent FNAC and CNB as an initial diagnostic procedure.
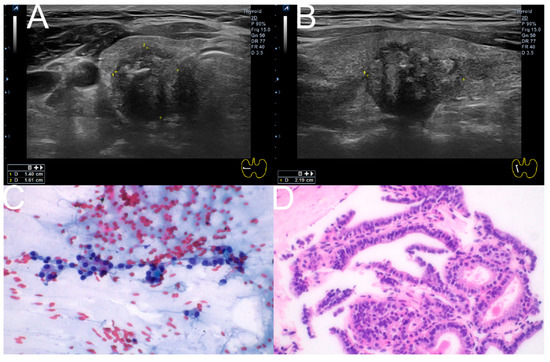
Figure 6.
Composite illustration of the diagnostic pathway for a representative case. (A,B) Ultrasonographic images (Transverse, longitudinal) of the right thyroid showing a 1.40 × 1.61 × 2.19 cm hypoechoic nodule with irregular margins and macrocalcification, classified as K-TIRADS 5. (C) FNAC smear (Papanicolaou stain, ×400) demonstrating features corresponding to Bethesda Category III (AUS/FLUS). (D) CNB specimen (hematoxylin and eosin stain, ×400) from the same nodule, classified as Category V according to the 2019 Practice Guidelines for Thyroid Core Needle Biopsy of the Korean Thyroid Association. FNAC: Fine-needle aspiration cytology; CNB: Core needle biopsy; AUS/FLUS: atypia of undetermined significance/follicular lesion of undetermined significance.
3.3. Secondary Examination Results
Among the 144 nodules initially diagnosed as inconclusive, 40 underwent secondary testing. Additionally, out of 14 nodules initially diagnosed as conclusive but meeting the criteria for secondary testing, 7 underwent secondary testing, resulting in a total of 47 nodules. Of the seven nodules with conclusive results that underwent repeat testing, three were benign nodules with K-TIRADS 5 features on ultrasound, and the remaining four were repeated for other reasons. Of these, 37 nodules underwent FNAC and 10 underwent CNB as secondary tests. In the FNAC/FNAC group, 14 (77.8%) of the 18 were initially diagnosed as inconclusive, and 6 (33.3%) converted to conclusive upon secondary testing. Conversely, two of the four nodules (11.1%) initially diagnosed as conclusive were reclassified as inconclusive in the secondary test. Overall, there was no statistically significant difference in the conversion rates between the initial and secondary test results (p > 0.05) (Table 4). Among the eight nodules (44.4%) that remained inconclusive in both tests, three underwent surgery, revealing two cases of papillary thyroid carcinoma and one benign nodule.

Table 4.
Results of nodules subjected to FNAC as primary and secondary examinations.
In the FNAC/CNB group, 16 of 19 nodules (84.2%) were initially diagnosed as inconclusive, with eight nodules (42.1%) converting to conclusive upon secondary testing. None of the three nodules initially diagnosed as conclusive were reclassified as inconclusive in the secondary test. The conversion rates between the initial and secondary test results showed a statistically significant difference (p < 0.05) (Table 5). Among the eight nodules (42.1%) that remained inconclusive in both tests, three underwent surgery, revealing one case of papillary thyroid carcinoma, one case of Hashimoto’s thyroiditis, and one benign nodule.

Table 5.
Results of nodules subjected to FNAC as primary and CNB as secondary examinations.
In the CNB/CNB group, all 10 nodules were initially diagnosed as inconclusive, with four nodules (40%) converting to conclusive results upon secondary testing. Owing to the small sample size, the analysis of conversion rates between the initial and secondary tests was limited. Among the six nodules (60%) that remained inconclusive in both tests, two underwent surgery, revealing two benign nodules.
3.4. Comparison of Diagnostic Yields Between FNAC and CNB Based on Post-Surgical Definitive Histopathological Results
Thyroidectomy was performed on 62 nodules based on the results of the primary and secondary tests. Histopathological analysis revealed 36 cases of papillary thyroid carcinoma, 22 benign nodules, 1 follicular adenoma, 1 case of Hashimoto’s thyroiditis, 1 oncocytic adenoma, and 1 case of anaplastic thyroid carcinoma (Table 6).

Table 6.
Final histopathological results.
When comparing the diagnostic yields of FNAC and CNB, the sensitivity, specificity, and accuracy of FNAC were 100% (95% confidence interval [CI], 86.7–100%), 42.9% (95% CI, 1.0–81.6%), and 87.9% (95% CI, 71.8–96.6%), respectively. For CNB, the sensitivity, specificity, and accuracy were 100% (95% CI, 78.2–100%), 70.0% (95% CI, 34.8–93.3%), and 88.0% (95% CI, 68.8–97.5%), respectively, indicating a higher specificity in the CNB group (Table 7).

Table 7.
Comparison of diagnostic yields between fine needle aspiration cytology and core needle biopsy based on post-surgical definitive histopathological results.
3.5. Post-Procedural Complications
No post-procedural complications, such as hematoma due to vascular injury, voice changes due to recurrent laryngeal nerve damage, infection, subcutaneous emphysema, or hemoptysis due to tracheal injury, were reported for both FNAC and CNB.
4. Discussion
In this study, CNB demonstrated a higher frequency of conclusive results than FNAC in primary examinations, consistent with the findings of Suh et al. [21]. Although Bethesda Category IV (follicular neoplasm/suspicious for follicular neoplasm) is pathologically indeterminate and cannot discriminate between a follicular adenoma and a follicular carcinoma without evaluation of the entire tumor capsule, as stated in the 2019 Practice Guidelines for Thyroid Core Needle Biopsy of the Korean Thyroid Association, in surgical departments these findings routinely lead to surgical management for diagnostic or therapeutic purposes. Therefore, in this study, we classified Category IV as ‘conclusive’ to reflect its role in guiding clinical decision-making rather than a confirmed histopathological distinction [23]. This observation was particularly evident in patients who underwent both CNB and FNAC simultaneously, likely because of the greater quantity of tissue obtained with CNB [10]. Furthermore, as indicated by Hong et al., CNB can analyze both cytological and histological features, which may account for the differing results compared to FNAC [25]. The higher rate of conclusive results with CNB in primary examinations highlights its potential clinical utility in guiding decisions for surgery or monitoring.
When comparing nodules that underwent secondary FNAC or CNB following an initially inconclusive FNAC, the rate of conversion to a conclusive diagnosis was significantly higher in the secondary CNB group. This finding aligns with previous studies supporting the utility of CNB as a secondary diagnostic procedure [17]. Although both FNAC and CNB demonstrated similar sensitivity and diagnostic accuracy in this study, CNB exhibited higher specificity. Moreover, no complications were reported for either procedure, suggesting that CNB may offer a higher diagnostic yield than FNAC when performed safely and appropriately.
According to the 2017 guidelines from the Korean Society of Thyroid Radiology, the indications for CNB include: (1) non-diagnostic results from FNAC, (2) indeterminate nodules from FNAC, and (3) suspected lymphoma, anaplastic thyroid carcinoma, medullary thyroid carcinoma, or metastatic cancer to the thyroid [26]. The guidelines currently state that there is insufficient evidence to replace FNAC with CNB as an initial diagnostic procedure for thyroid nodules, emphasizing the need for further research on CNB’s efficacy. Additionally, future studies should consider a cost–benefit analysis of FNAC and CNB, given the increased number of visits, costs, and diagnostic delays associated with indeterminate results.
The strength of this study lies in its design, which concurrently analyzed primary and secondary examination results within the same patient cohort, rather than focusing solely on nodules with initially non-diagnostic FNAC results. This comprehensive approach evaluates the complementarity of FNAC and CNB, provides evidence for selecting diagnostic methods, and aids in planning the diagnosis and treatment of thyroid nodules. These findings may also guide the design of future research.
The limitations of this study include the potential selection bias inherent in its retrospective design, particularly as cases in which CNB was performed following primary FNAC may have had a higher suspicion of malignancy or follicular neoplasm. Moreover, although FNAC was mandated for specific nodule types (e.g., large cystic nodules), in certain cases, the selection of the diagnostic modality was influenced by patient preference. This real-world practice may have introduced additional selection bias beyond what is reflected by baseline demographics. For instance, patients with nodules exhibiting high-suspicion ultrasonographic features or with prior inconclusive FNAC results may have been more inclined to undergo CNB, whereas those with small, low-suspicion nodules may have favored FNAC. Such factors could have altered the baseline characteristics of the two groups and should be considered when interpreting our findings. The relatively small sample size and the presence of some patients without final surgical pathology due to drop-out are also limitations. Additionally, CNB requires operator proficiency, and results for nodules smaller than 1 cm or located posteriorly in the thyroid may vary among practitioners [25]. Therefore, large-scale, multicenter prospective studies are necessary to further validate our findings and establish cost-effectiveness. Although CNB demonstrated a higher frequency of conclusive results than FNAC in our cohort, its application should be carefully tailored to appropriate clinical contexts. During the study period, FNAC and CNB were selected based on both specific clinical circumstances and patient preference. Nevertheless, in our institution, CNB has typically been performed in nodules with repeated inconclusive FNAC results, high-suspicion ultrasonographic features (K-TIRADS 4 or 5), suspected lymphoma or anaplastic thyroid carcinoma, or in cases in which FNAC was technically challenging (e.g., deeply located or posteriorly situated nodules). Clearly defining these indications underscores the appropriate clinical context for CNB use and may help avoid unnecessary procedures.
Author Contributions
Conceptualization: M.J.B., H.K.B. and J.H.P.; Methodology: Y.L.; Data Curation: D.H.K. and J.-Y.K.; Writing—Original Draft: Y.L. and M.J.B.; Writing—Review and Editing: M.J.B., H.K.B. and J.H.P.; Funding Acquisition: J.H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Soonchunhyang University Research Fund. The funding organization had no role in the design of the study, data collection, analysis, interpretation of results, or writing of the manuscript.
Institutional Review Board Statement
This retrospective study, based on the analysis of medical records, was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital (SCHCA 2024-07-023; 9 August 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to restrictions on patient privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AUS | atypia of undetermined significance |
| CI | confidence interval |
| CNB | core needle biopsy |
| FLUS | follicular lesion of undetermined significance |
| FN | follicular neoplasm |
| FNAC | Fine-needle aspiration cytology |
| K-TIRADS | Korean Society of Thyroid Radiology |
| SFN | suspicious for follicular neoplasm |
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H.; Papini, E.; Paschke, R.; Duick, D.S.; Valcavi, R.; Hegedüs, L.; Vitti, P. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules: Executive Summary of Recommendations. J. Endocrinol. Investig. 2010, 33, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Spitale, A.; Faquin, W.C.; Mazzucchelli, L.; Baloch, Z.W. The Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis. Acta Cytol. 2012, 56, 333–339. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef]
- Alexander, E.K.; Heering, J.P.; Benson, C.B.; Frates, M.C.; Doubilet, P.M.; Cibas, E.S.; Marqusee, E. Assessment of Nondiagnostic Ultrasound-Guided Fine Needle Aspirations of Thyroid Nodules. J. Clin. Endocrinol. Metab. 2002, 87, 4924–4927. [Google Scholar] [CrossRef]
- Yassa, L.; Cibas, E.S.; Benson, C.B.; Frates, M.C.; Doubilet, P.M.; Gawande, A.A.; Moore, F.D.; Kim, B.W.; Nosé, V.; Marqusee, E.; et al. Long-Term Assessment of a Multidisciplinary Approach to Thyroid Nodule Diagnostic Evaluation. Cancer 2007, 111, 508–516. [Google Scholar] [CrossRef]
- Yang, J.; Schnadig, V.; Logrono, R.; Wasserman, P.G. Fine-Needle Aspiration of Thyroid Nodules: A Study of 4703 Patients with Histologic and Clinical Correlations. Cancer 2007, 111, 306–315. [Google Scholar] [CrossRef]
- Orija, I.B.; Piñeyro, M.; Biscotti, C.; Reddy, S.S.; Hamrahian, A.H. Value of Repeating a Nondiagnostic Thyroid Fine-Needle Aspiration Biopsy. Endocr. Pract. 2007, 13, 735–742. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Krane, J.F.; Cibas, E.S.; Faquin, W.C. The Atypical Thyroid Fine-Needle Aspiration: Past, Present, and Future. Cancer Cytopathol. 2012, 120, 73–86. [Google Scholar] [CrossRef]
- Ho, A.S.; Sarti, E.E.; Jain, K.S.; Wang, H.; Nixon, I.J.; Shaha, A.R.; Shah, J.P.; Kraus, D.H.; Ghossein, R.; Fish, S.A.; et al. Malignancy Rate in Thyroid Nodules Classified as Bethesda Category III (AUS/FLUS). Thyroid 2014, 24, 832–839. [Google Scholar] [CrossRef]
- Hyeon, J.; Ahn, S.; Shin, J.H.; Oh, Y.L. The Prediction of Malignant Risk in the Category ‘Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance’ of the Bethesda System for Reporting thyroid Cytopathology Using Subcategorization and BRAF Mutation Results. Cancer Cytopathol. 2014, 122, 368–376. [Google Scholar] [CrossRef]
- Sullivan, P.S.; Hirschowitz, S.L.; Fung, P.C.; Apple, S.K. The Impact of Atypia/Follicular Lesion of Undetermined Significance and Repeat Fine-Needle Aspiration: 5 Years Before and After Implementation of the Bethesda System. Cancer Cytopathol. 2014, 122, 866–872. [Google Scholar] [CrossRef]
- Na, D.G.; Kim, J.H.; Sung, J.Y.; Baek, J.H.; Jung, K.C.; Lee, H.; Yoo, H. Core-Needle Biopsy is More Useful Than Repeat Fine-Needle Aspiration in Thyroid Nodules Read as Nondiagnostic or Atypia of Undetermined Significance by the Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2012, 22, 468–475. [Google Scholar] [CrossRef]
- Samir, A.E.; Vij, A.; Seale, M.K.; Desai, G.; Halpern, E.; Faquin, W.C.; Parangi, S.; Hahn, P.F.; Daniels, G.H. Ultrasound-Guided Percutaneous Thyroid Nodule Core Biopsy: Clinical Utility in Patients with Prior Nondiagnostic Fine-Needle Aspirate. Thyroid 2012, 22, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Shin, J.H.; Oh, Y.L.; Hahn, S.Y. Atypia of Undetermined Significance in Thyroid Fine-Needle Aspiration Cytology: Prediction of Malignancy by US and Comparison of Methods for Further Management. Ann. Surg. Oncol. 2014, 21, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.S.; Sohn, J.H.; Kang, G. Core Needle Biopsy is a More Conclusive Follow-Up Method Than Repeat Fine Needle Aspiration for Thyroid Nodules with Initially Inconclusive Results: A Systematic Review and Meta-Analysis. J. Pathol. Transl. Med. 2016, 50, 217–224. [Google Scholar] [CrossRef]
- Cao, H.; Kao, R.H.; Hsieh, M.C. Comparison of Core-Needle Biopsy and Fine-Needle Aspiration in Screening for Thyroid Malignancy: A Systematic Review and Meta-Analysis. Curr. Med. Res. Opin. 2016, 32, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Nasrollah, N.; Guidobaldi, L.; Taccogna, S.; Cicciarella Modica, D.D.; Amendola, S.; Romanelli, F.; Lenzi, A.; Nigri, G.; Centanni, M.; et al. The Use of Core Needle Biopsy as First-Line in Diagnosis of Thyroid Nodules Reduces False Negative and Inconclusive Data Reported by Fine-Needle Aspiration. World J. Surg. Oncol. 2014, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Fu, S.; Lv, F.; Tang, J. Thyroid Nodules with Suspicious Ultrasound Findings: The Role of Ultrasound-Guided Core Needle Biopsy. Clin. Imaging 2014, 38, 434–438. [Google Scholar] [CrossRef]
- Suh, C.H.; Baek, J.H.; Lee, J.H.; Choi, Y.J.; Kim, J.K.; Sung, T.Y.; Yoon, J.H.; Shong, Y.K. The Role of Core-Needle Biopsy as a First-Line Diagnostic Tool for Initially Detected Thyroid Nodules. Thyroid 2016, 26, 395–403. [Google Scholar] [CrossRef]
- Ha, E.J.; Chung, S.R.; Na, D.G.; Ahn, H.S.; Chung, J.; Lee, J.Y.; Park, J.S.; Yoo, R.E.; Baek, J.H.; Baek, S.M.; et al. 2021 Korean Thyroid Imaging Reporting and Data System and Imaging-Based Management of Thyroid Nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2021, 22, 2094–2123. [Google Scholar] [CrossRef]
- Jung, C.K.; Baek, J.H.; Na, D.G.; Oh, Y.L.; Yi, K.H.; Kang, H.C. 2019 Practice Guidelines for Thyroid Core Needle Biopsy: A Report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association. J. Pathol. Transl. Med. 2020, 54, 64–86. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, V.; Massoud, E.; Jensen, C.; Zhang, Y.; Hanlon, B.M.; Hitchcock, M.; Arroyo, N.; Chiu, A.S.; Fernandes-Taylor, S.; Alagoz, O.; et al. Diagnostic Accuracy of Fine-Needle Biopsy in the Detection of Thyroid Malignancy: A Systematic Review and Meta-Analysis. JAMA Surg. 2022, 157, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.J.; Na, D.G.; Kim, S.J.; Kim, D.S. Role of Core Needle Biopsy as a First-Line Diagnostic Tool for Thyroid Nodules: A Retrospective Cohort Study. Ultrasonography 2018, 37, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Na, D.G.; Baek, J.H.; Jung, S.L.; Kim, J.H.; Sung, J.Y.; Kim, K.S.; Lee, J.H.; Shin, J.H.; Choi, Y.J.; Ha, E.J.; et al. Core Needle Biopsy of the Thyroid: 2016 Consensus Statement and Recommendations from Korean Society of Thyroid Radiology. Korean J. Radiol. 2017, 18, 217–237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).