Shifting Trends in Intensive Cardiovascular Care Unit Admission Patterns: Retrospective Insights and Prospective Implications
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Clinical Data and Study Outcome
2.3. Statistical Analysis
3. Results
3.1. Number of Admissions and Baseline Characteristics
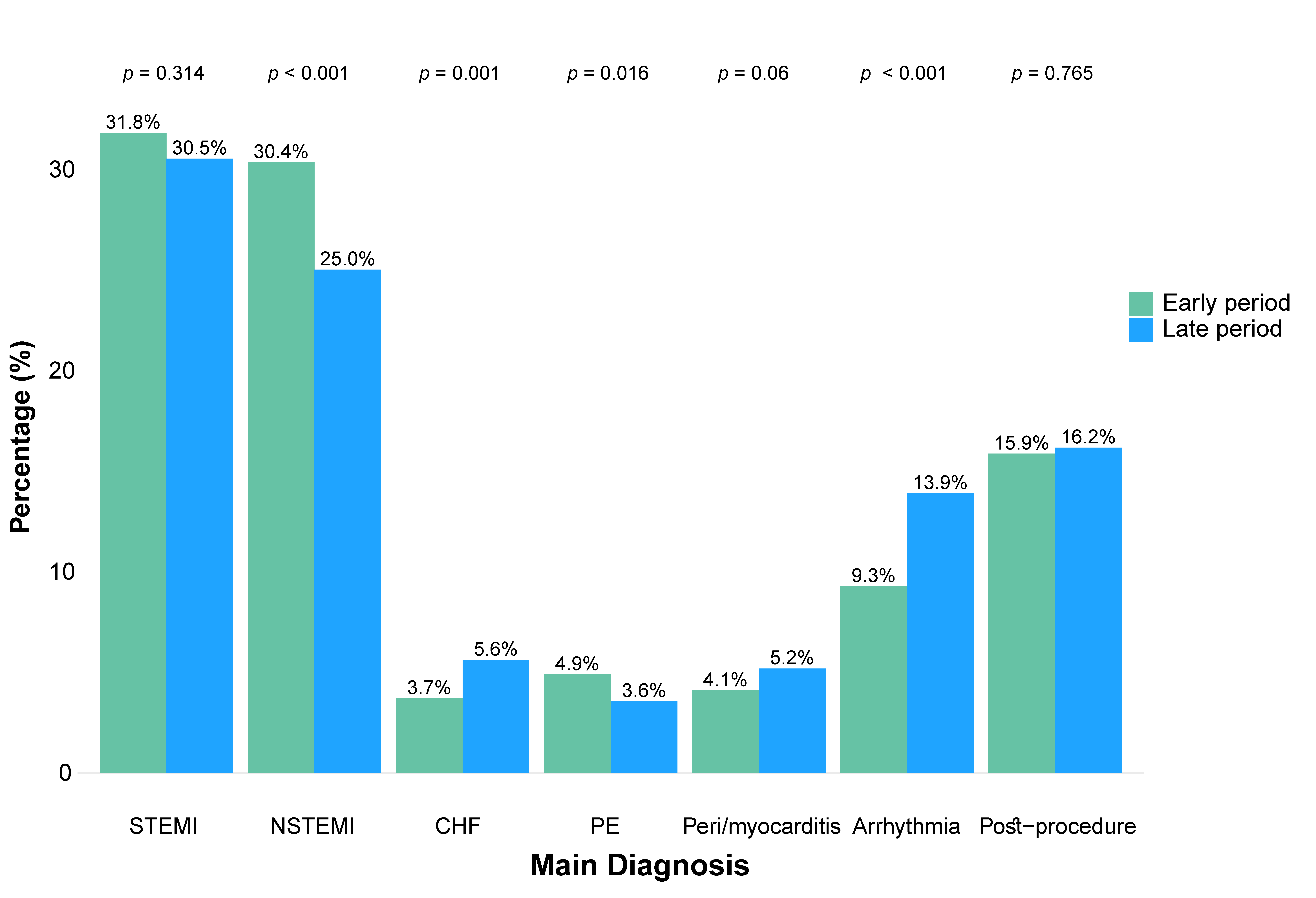
3.2. Main Diagnoses on Admission
3.3. Interventions and Complications
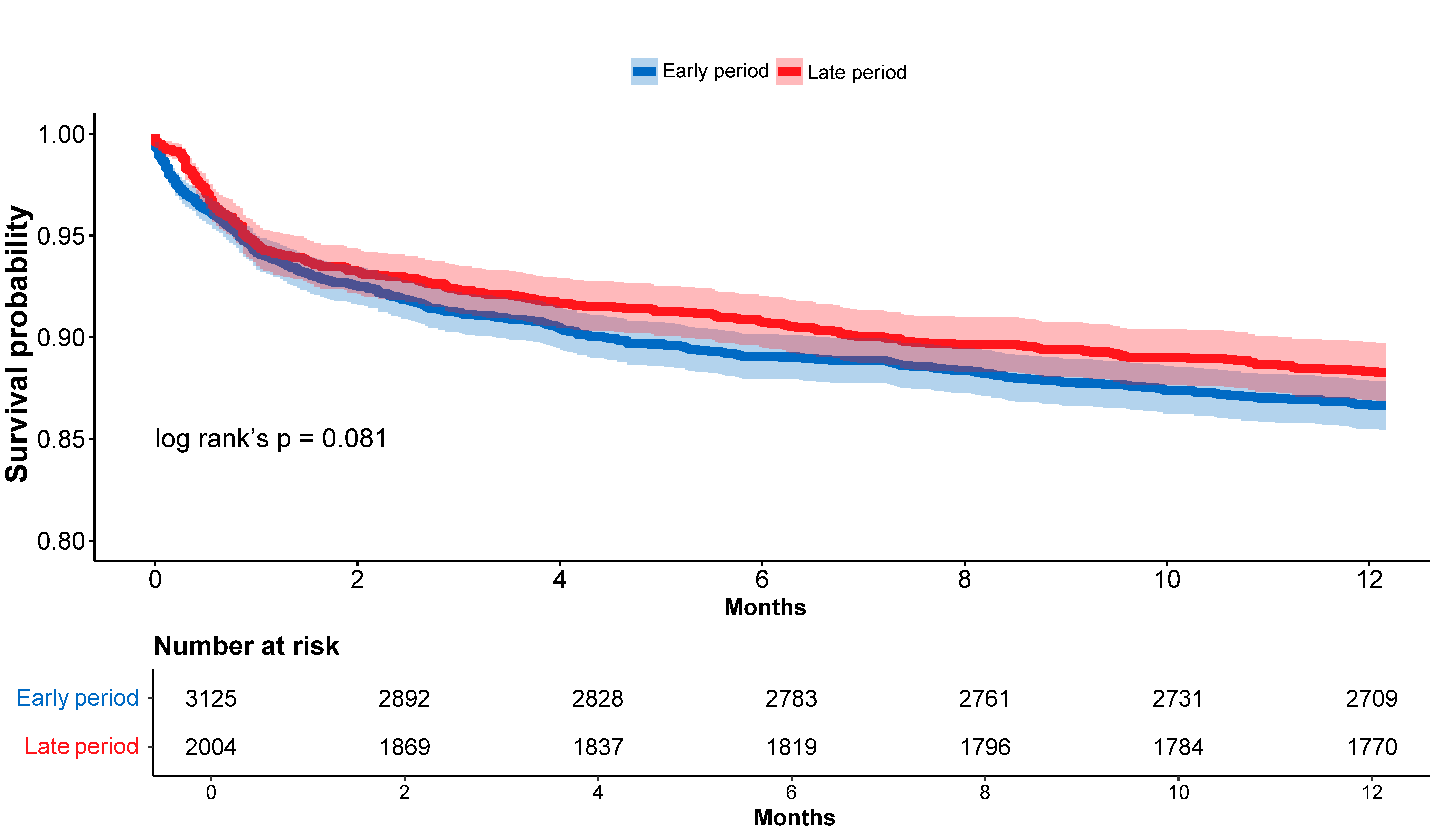
3.4. In-Hospital and One-Year Mortality Rates
4. Discussion
4.1. Study Strengths and Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICCU | Intensive Cardiovascular Care Unit |
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.; Roth, G.A.; Abate, Y.H.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; Abdulah, D.M.; et al. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. JACC 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, Y.; Mubarik, S.; Zhang, Y.; Liu, X.; Cheng, Y.; Yu, C.; Cao, J. Global Burden of Ischemic Heart Disease and Attributable Risk Factors, 1990–2017: A Secondary Analysis Based on the Global Burden of Disease Study 2017. Clin. Epidemiology 2021, 13, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Ahmad, T.; Alexander, K.; Baker, W.L.; Bosak, K.; Breathett, K.; Carter, S.; Drazner, M.H.; Dunlay, S.M.; Fonarow, G.C.; et al. HF STATS 2024: Heart Failure Epidemiology and Outcomes Statistics An Updated 2024 Report from the Heart Failure Society of America. J. Card. Fail. 2025, 31, 66–116. [Google Scholar] [CrossRef]
- Valente, S.; Trambaiolo, P.; Casella, G.; Sacco, A.; Dini, C.S.; Farina, A.; De Luca, L.; Geraci, G.; Tizzani, E.; Lettino, M.; et al. ANMCO Position Paper: Functional Reorganization of Intensive Cardiac Care Units (ICCUs) in Italy. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2025, 27, v205–v215. [Google Scholar] [CrossRef]
- Claeys, M.; Roubille, F.; Casella, G.; Zukermann, R.; Nikolaou, N.; De Luca, L.; Gierlotkaa, M.; Iakobishvili, Z.; Thiele, H.; Koutouzis, M.; et al. Organization of Intensive Cardiac Care Units in Europe: Results of a Multinational Survey. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 993–1001. [Google Scholar] [CrossRef]
- Bruoha, S.; Maller, T.; Loutati, R.; Perel, N.; Tabi, M.; Taha, L.; Yosefy, C.; Jafari, J.; Braver, O.; Amsalem, I.; et al. Nonagenarians Admission and Prognosis in a Tertiary Center Intensive Coronary Care Unit—A Prospective Study. BMC Geriatr. 2023, 23, 152. [Google Scholar] [CrossRef]
- Sinha, S.S.; Geller, B.J.; Katz, J.N.; Arslanian-Engoren, C.; Barnett, C.F.; Bohula, E.A.; Damluji, A.A.; Menon, V.; Roswell, R.O.; Vallabhajosyula, S.; et al. Evolution of Critical Care Cardiology: An Update on Structure, Care Delivery, Training, and Research Paradigms: A Scientific Statement from the American Heart Association. JACC 2025, 86, 291–313. [Google Scholar] [CrossRef]
- Bouchlarhem, A.; Bazid, Z.; Ismaili, N.; El Ouafi, N. Cardiac Intensive Care Unit: Where We Are in 2023. Front. Cardiovasc. Med. 2023, 10, 1201414. [Google Scholar] [CrossRef]
- Loutati, R.; Bruoha, S.; Taha, L.; Karmi, M.; Perel, N.; Maller, T.; Sabouret, P.; Galli, M.; Zoccai, G.B.; De Rosa, S.; et al. Association between Peak Troponin Level and Prognosis among Patients Admitted to Intensive Cardiovascular Care Unit. Int. J. Cardiol. 2024, 417, 132556. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; Fang, J.C.; Fintel, D.J.; Granger, C.B.; Katz, J.N.; Kushner, F.G.; Kuvin, J.T.; Lopez-Sendon, J.; McAreavey, D.; Nallamothu, B.; et al. on behalf of the American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Council on Quality of Care and Outcomes Research. Evolution of critical care cardiology: Transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: A scientific statement from the American Heart Association. Circulation 2012, 126, 1408–1428. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Katz, J.N.; van Diepen, S.; Alviar, C.L.; Baird-Zars, V.M.; Park, J.-G.; Barnett, C.F.; Bhattal, G.; Barsness, G.W.; Burke, J.A.; et al. Critical Care Cardiology Trials Network. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: The Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol. 2019, 4, 928–935. [Google Scholar] [CrossRef]
- Hernandez-Montfort, J.; Sinha, S.S.; Thayer, K.L.; Whitehead, E.H.; Pahuja, M.; Garan, A.R.; Mahr, C.; Haywood, J.L.; Harwani, N.M.; Schaeffer, A.; et al. Clinical outcomes associated with acute mechanical circulatory support utilization in heart failure related cardiogenic shock. Circ. Heart Fail. 2021, 14, e007924. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Goßling, A.; Savarese, G.; Dabboura, S.; Yan, I.; Beer, B.; Söffker, G.; Seiffert, M.; Kluge, S.; et al. Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart Fail. 2021, 8, 1295–1303. [Google Scholar] [CrossRef]
- Katz, J.N.; Shah, B.R.M.; Volz, E.M.; Horton, J.R.; Shaw, L.K.; Newby, L.K.M.; Granger, C.B.M.; Mark, D.B.M.; Califf, R.M.M.; Becker, R.C.M. Evolution of the coronary care unit: Clinical characteristics and temporal trends in healthcare delivery and outcomes. Crit. Care Med. 2010, 38, 375–381. [Google Scholar] [CrossRef]
- Sinha, S.S.; Sjoding, M.W.; Sukul, D.; Prescott, H.C.; Iwashyna, T.J.; Gurm, H.S.; Cooke, C.R.; Nallamothu, B.K. Changes in primary noncardiac diagnoses over time among elderly cardiac intensive care unit patients in the United States. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003616. [Google Scholar] [CrossRef]
- Gall, E.; Pezel, T.; Lattuca, B.; Hamzi, K.; Puymirat, E.; Piliero, N.; Deney, A.; Fauvel, C.; Aboyans, V.; Schurtz, G.; et al. Profile of Patients Hospitalized in Intensive Cardiac Care Units in France: ADDICT-ICCU Registry. Arch. Cardiovasc. Dis. 2024, 117, 195–203. [Google Scholar] [CrossRef]
- Ferrer, M.; García-García, C.; El Ouaddi, N.; Rueda, F.; Serra, J.; Oliveras, T.; Labata, C.; Dégano, I.R.; Montero, S.; De Diego, O.; et al. Transitioning from a Coronary to a Critical Cardiovascular Care Unit: Trends over the Past Three Decades. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 437–444. [Google Scholar] [CrossRef]
- Guillermou, H.; Mercier, G.; Bonnefoy-Cudraz, E.; Delmas, C.; Roubille, F. Evolution of Intensive Cardiac Care Units over the Past Decade: A France Nationwide Observational Database Study Comparing Recent Trends (2014–2023). Arch. Cardiovasc. Dis. 2025, 118, 457–463. [Google Scholar] [CrossRef]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, K.; Verceles, A.C.; Netzer, G.; Chaudhry, A.; Bolgiano, M.; Devabhakthuni, S.; Ludmir, J.; Pollock, J.S.; Ramani, G.V.; McCurdy, M.T. A Collaborative Cardiologist-Intensivist Management Model Improves Cardiac Intensive Care Unit Outcomes. JACC 2017, 70, 1422–1423. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Goldenberg, I.; Schleede, S.; McNitt, S.; Gosev, I.; Elbadawi, A.; Pietropaoli, A.; Barrus, B.; Chen, Y.L.; Mazzillo, J.; et al. Effect of a Multidisciplinary Pulmonary Embolism Response Team on Patient Mortality. Am. J. Cardiol. 2021, 161, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Papolos, A.I.; Kenigsberg, B.B.; Berg, D.D.; Alviar, C.L.; Bohula, E.; Burke, J.A.; Carnicelli, A.P.; Chaudhry, S.-P.; Drakos, S.; Gerber, D.A.; et al. Management and Outcomes of Cardiogenic Shock in Cardiac ICUs With Versus Without Shock Teams. JACC 2021, 78, 1309–1317. [Google Scholar] [CrossRef]
- Bhagat, A.A.; Greene, S.J.; Vaduganathan, M.; Fonarow, G.C.; Butler, J. Initiation, Continuation, Switching, and Withdrawal of Heart Failure Medical Therapies During Hospitalization. JACC Heart Fail. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Baecker, A.; Meyers, M.; Koyama, S.; Taitano, M.; Watson, H.; Machado, M.; Nguyen, H.Q. Evaluation of a Transitional Care Program After Hospitalization for Heart Failure in an Integrated Health Care System. JAMA Netw. Open 2020, 3, e2027410. [Google Scholar] [CrossRef]
- McAlister, F.A.; Chu, L.M. Postdischarge Follow-Up After Cardiac Hospitalizations Via Telehealth or In-Person. JACC Adv. 2025, 4, 101653. [Google Scholar] [CrossRef]
- Zhan, Y.-F.; Li, F.; Wu, L.-C.; Li, J.-M.; Zhu, C.-Y.; Han, M.-S.; Sheng, Y. Role of Charlson Comorbidity Index in Predicting the ICU Admission in Patients with Thoracic Aortic Aneurysm Undergoing Surgery. J. Orthop. Surg. Res. 2023, 18, 870. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Lonati, C.; Rigamonti, E.; Viganò, M.; Nicolosi, G.L.; Proietti, M.; Lombardo, M.; Harari, S. CHA2DS2-VASc Score Stratifies Mortality Risk in Heart Failure Patients Aged 75 Years and Older with and without Atrial Fibrillation. Aging Clin. Exp. Res. 2022, 34, 1707–1720. [Google Scholar] [CrossRef]
- Goldberger, N.; Aburbeh, M.; Haklai, Z. Leading Causes of Death in Israel 2000–2019; Health Information Division of the Ministry of Health: Tel Aviv, Israel, 2022. [Google Scholar]
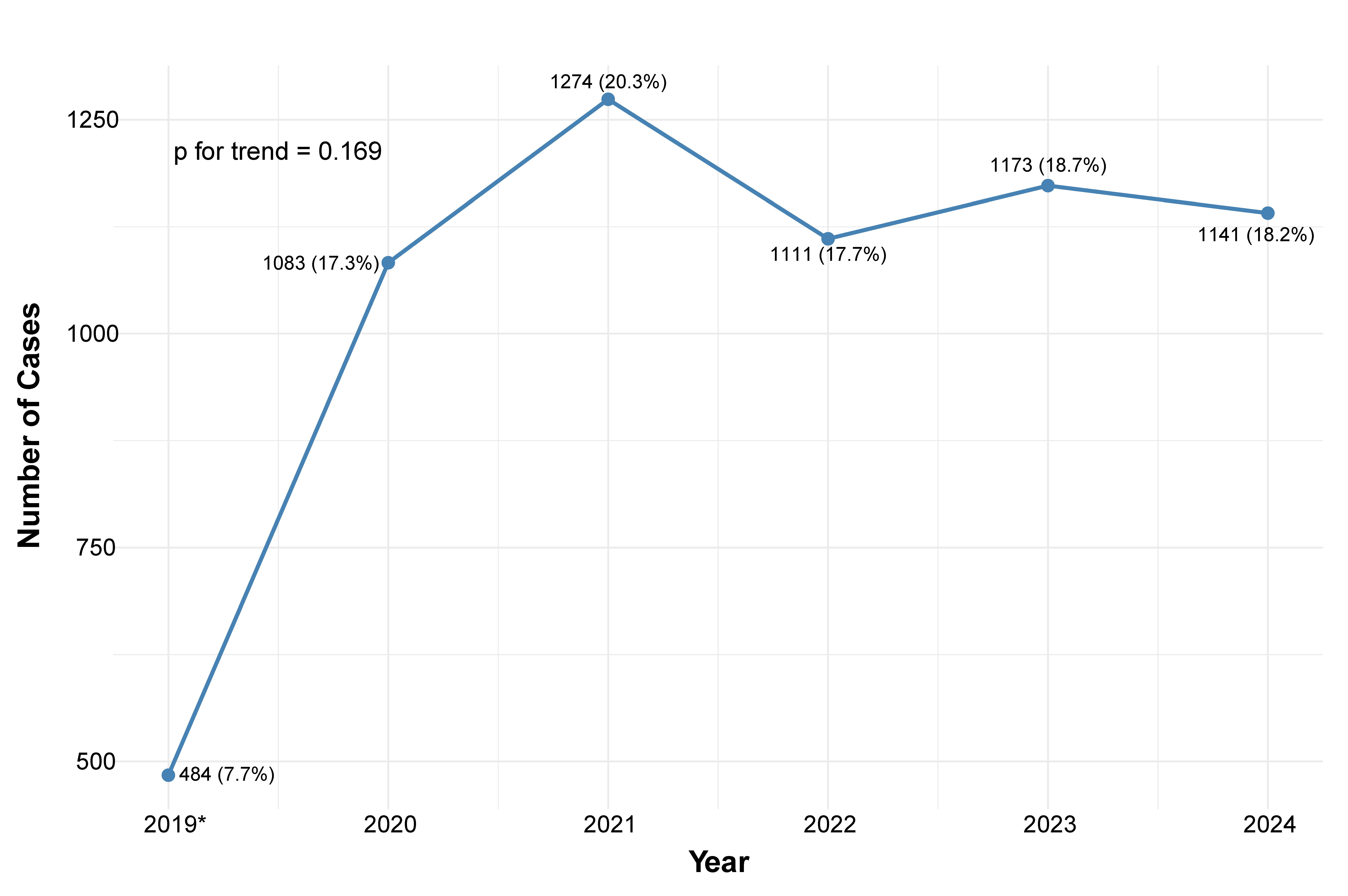

| Variable | Early Period (N = 3125) | Late Period (N = 3141) | p-Value |
|---|---|---|---|
| Age (years) | 69.0 (58.0–79.0) | 69.0 (58.0–79.0) | 0.301 |
| Female sex | 960 (30.7%) | 1030 (32.8%) | 0.082 |
| BMI (kg/m2) | 27.3 (24.2–30.9) | 27.2 (24.3–30.7) | 0.634 |
| Hypertension—no. (%) | 1904 (60.9%) | 1909 (60.8%) | 0.923 |
| Dyslipidemia—no. (%) | 1632 (52.2%) | 1651 (52.6%) | 0.808 |
| Diabetes—no. (%) | 1132 (36.2%) | 1201 (38.2%) | 0.105 |
| Smoking—no. (%) | 848 (27.1%) | 793 (25.2%) | 0.094 |
| Family History of CAD—no. (%) | 210 (6.7%) | 259 (8.2%) | 0.024 |
| Prior CAD—no. (%) | 958 (30.7%) | 957 (30.5%) | 0.893 |
| Prior CABG—no. (%) | 198 (6.3%) | 235 (7.5%) | 0.082 |
| CVA/TIA—no. (%) | 230 (7.4%) | 223 (7.1%) | 0.727 |
| PAD—no. (%) | 145 (4.6%) | 140 (4.5%) | 0.774 |
| CHF—no. (%) | 516 (16.5%) | 512 (16.3%) | 0.848 |
| AFIB—no. (%) | 471 (15.1%) | 532 (16.9%) | 0.047 |
| COPD—no. (%) | 263 (8.4%) | 255 (8.1%) | 0.703 |
| Pulmonary Hypertension—no. (%) | 158 (5.1%) | 170 (5.4%) | 0.564 |
| CIED—no. (%) | 184 (5.9%) | 238 (7.6%) | 0.008 |
| Cognitive Decline—no. (%) | 102 (3.3%) | 135 (4.3%) | 0.037 |
| Debilitated—no. (%) | 105 (3.4%) | 131 (4.2%) | 0.105 |
| Malignancy—no. (%) | 272 (8.7%) | 270 (8.6%) | 0.915 |
| Anemia—no. (%) | 157 (5.0%) | 185 (5.9%) | 0.146 |
| CKD—no. (%) | 416 (13.3%) | 408 (13.0%) | 0.734 |
| Dialysis—no. (%) | 61 (2.0%) | 88 (2.8%) | 0.033 |
| Length of stay (days) | 2 (1–3) | 2 (1–3) | 0.151 |
| Intervention | Early Period (N = 3125) | Late Period (N = 3141) | p-Value |
|---|---|---|---|
| Diagnostic Cath.—no. (%) | 354 (11.3%) | 410 (13.1%) | 0.040 |
| Urgent PCI | 765 (24.5%) | 852 (27.1%) | 0.035 |
| PCI—no. (%) | 550 (17.6%) | 504 (16.0%) | 0.107 |
| CABG—no. (%) | 57 (1.8%) | 76 (2.4%) | 0.159 |
| TAVI—no. (%) | 226 (7.2%) | 296 (9.4%) | 0.002 |
| Mitral TEER—no. (%) | 33 (1.0%) | 78 (2.5%) | <0.001 |
| Ablation/CIED implantation—no. (%) | 327 (10.5%) | 335 (10.7%) | 0.827 |
| Pulmonary Thrombolysis/aspiration—no. (%) | 13 (0.4%) | 23 (0.7%) | 0.136 |
| Blood transfusion—no. (%) | 69 (2.2%) | 185 (5.9%) | <0.001 |
| CPR—no. (%) | 127 (4.1%) | 109 (3.5%) | 0.243 |
| Mechanical Ventilation—no. (%) | 276 (8.8%) | 255 (8.1%) | 0.333 |
| IABP—no. (%) | 75 (2.4%) | 29 (0.9%) | <0.001 |
| Impella—no. (%) | 8 (0.3%) | 25 (0.8%) | 0.005 |
| ECMO—no. (%) | 12 (0.4%) | 15 (0.5%) | 0.710 |
| TTM—no. (%) | 33 (1.1%) | 35 (1.1%) | 0.920 |
| Complication | Early Period (N = 3125) | Late Period (N = 3141) | p-Value |
|---|---|---|---|
| Malignant arrhythmia—no. (%) | 77 (2.5%) | 69 (2.2%) | 0.537 |
| Cardiogenic shock *—no. (%) | 287 (9.2%) | 203 (6.5%) | 0.014 |
| Mechanical Complication (VSR/rupture)—no. (%) | 17 (0.5%) | 15 (0.5%) | 0.848 |
| LV Thrombus—no. (%) | 16 (0.5%) | 34 (1.1%) | 0.016 |
| Septic shock *—no. (%) | 58 (1.9%) | 70 (2.2%) | 0.341 |
| Stroke—no. (%) | 36 (1.2%) | 26 (0.8%) | 0.242 |
| AKI—no. (%) | 143 (4.6%) | 98 (3.1%) | 0.003 |
| Significant bleeding—no. (%) | 124 (4.0%) | 116 (3.7%) | 0.616 |
| Vascular complication—no. (%) | 45 (1.4%) | 71 (2.3%) | 0.020 |
| Anoxic brain damage—no. (%) | 10 (0.3%) | 21 (0.7%) | 0.074 |
| (a) | |||
|---|---|---|---|
| Univariable | |||
| Characteristic | HR | 95% CI | p-value |
| Late period | 0.98 | 0.73–1.33 | >0.9 |
| Multivariable | |||
| Characteristic | HR | 95% CI | p-value |
| Late period | 1.06 | 0.75–1.51 | 0.7 |
| Age (per year) | 1.06 | 1.05–1.08 | <0.001 |
| Male gender | 0.53 | 0.36–0.76 | <0.001 |
| Prior MI | 1.61 | 1.11–2.33 | 0.012 |
| AF | 1.31 | 0.87–1.96 | 0.2 |
| CHF | 1.24 | 0.85–1.81 | 0.3 |
| CKD | 1.59 | 0.74–3.44 | 0.2 |
| Cognitive decline | 1.51 | 0.78–2.89 | 0.2 |
| EF (per %) | 0.96 | 0.94–0.98 | <0.001 |
| (b) | |||
| Univariable | |||
| Characteristic | HR | 95% CI | p-value |
| Late period | 0.90 | 0.80–1.02 | 0.11 |
| Multivariable | |||
| Characteristic | HR | 95% CI | p-value |
| Late period | 0.84 | 0.71–0.98 | 0.037 |
| Age (per year) | 1.06 | 1.05–1.06 | <0.001 |
| Male gender | 0.78 | 0.68–0.88 | <0.001 |
| Prior MI | 1.26 | 1.12–1.43 | <0.001 |
| AF | 1.42 | 1.17–1.71 | <0.001 |
| CHF | 1.98 | 1.75–2.25 | <0.001 |
| CKD | 3.08 | 2.44–3.90 | <0.001 |
| Cognitive decline | 1.52 | 1.10–2.09 | 0.011 |
| EF (per %) | 0.98 | 0.97–0.98 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loutati, R.; Taha, L.; Karmi, M.; Fink, N.; Sabouret, P.; Mamas, M.A.; Naimark, A.; Tvito, A.; Wiener-Well, Y.; Abu-Salman, A.; et al. Shifting Trends in Intensive Cardiovascular Care Unit Admission Patterns: Retrospective Insights and Prospective Implications. Diagnostics 2025, 15, 2563. https://doi.org/10.3390/diagnostics15202563
Loutati R, Taha L, Karmi M, Fink N, Sabouret P, Mamas MA, Naimark A, Tvito A, Wiener-Well Y, Abu-Salman A, et al. Shifting Trends in Intensive Cardiovascular Care Unit Admission Patterns: Retrospective Insights and Prospective Implications. Diagnostics. 2025; 15(20):2563. https://doi.org/10.3390/diagnostics15202563
Chicago/Turabian StyleLoutati, Ranel, Louay Taha, Mohammad Karmi, Noam Fink, Pierre Sabouret, Mamas A. Mamas, Ari Naimark, Ariella Tvito, Yonit Wiener-Well, Amjad Abu-Salman, and et al. 2025. "Shifting Trends in Intensive Cardiovascular Care Unit Admission Patterns: Retrospective Insights and Prospective Implications" Diagnostics 15, no. 20: 2563. https://doi.org/10.3390/diagnostics15202563
APA StyleLoutati, R., Taha, L., Karmi, M., Fink, N., Sabouret, P., Mamas, M. A., Naimark, A., Tvito, A., Wiener-Well, Y., Abu-Salman, A., Shuvy, M., Merin, O., Glikson, M., & Asher, E. (2025). Shifting Trends in Intensive Cardiovascular Care Unit Admission Patterns: Retrospective Insights and Prospective Implications. Diagnostics, 15(20), 2563. https://doi.org/10.3390/diagnostics15202563







