HMGB1 and Kallistatin: Novel Serological Markers for Differentiating Peritonsillar Cellulitis and Abscess
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of PTA and PTC
2.2. Measurement of Serum Kallistatin and HMGB1 Levels
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTA | Peritonsillar Abscess |
| PTC | Peritonsillar Cellulitis |
| HMGB1 | High Mobility Group Box 1 |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| AUC | Area Under the Curve |
| ROC | Receiver Operating Characteristic |
| CI | Confidence Interval |
| OR | Odds Ratio |
| WBC | White Blood Cell |
| DCA | Decision Curve Analysis |
| EPV | Events per Value |
References
- Ketterer, M.C.; Maier, M.; Burkhardt, V.; Mansour, N.; Knopf, A.; Becker, C. The peritonsillar abscess and its management—Is incision and drainage only a makeshift to the tonsillectomy or a permanent solution? Front. Med. 2023, 10, 1282040. [Google Scholar] [CrossRef] [PubMed]
- Galioto, N.J. Peritonsillar Abscess. Am. Fam. Physician 2017, 95, 501–506. [Google Scholar]
- Powell, J.; Wilson, J.A. An evidence-based review of peritonsillar abscess. Clin. Otolaryngol. 2012, 37, 136–145. [Google Scholar] [CrossRef]
- Herzon, F.S.; Martin, A.D. Medical and surgical treatment of peritonsillar, retropharyngeal, and parapharyngeal abscesses. Curr. Infect. Dis. Rep. 2006, 8, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Zhang, Q.; Li, S.; Yu, Z.; Wan, L.; Wu, B.; Wu, R.; Li, S. Tissue kallikrein exacerbating sepsis-induced endothelial hyperpermeability is highly predictive of severity and mortality in sepsis. J. Inflamm. Res. 2021, 14, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Chen, C.W.; Chao, L.; Chao, J.; Lin, Y.S. Plasma kallistatin in critically ill patients with severe sepsis and septic shock. PLoS ONE 2017, 12, e0178387. [Google Scholar] [CrossRef]
- Magna, M.; Pisetsky, D.S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 2014, 20, 138–146. [Google Scholar] [CrossRef]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef]
- Rawlins, K.W.; Allen, D.Z.; Onwuka, A.J.; Elmaraghy, C.A. Computed tomography use patterns for pediatric patients with peritonsillar abscess. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 22–25. [Google Scholar] [CrossRef]
- Yurtkal, A.; Canday, M. Kallistatin as a Potential Biomarker in Polycystic Ovary Syndrome: A Prospective Cohort Study. Diagnostics 2024, 14, 1553. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstem, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Voruz, F.; Revol, R.; Combescure, C.; Monnier, Y.; Becker, M.; Dulguerov, N. Diagnosis of Peritonsillar Abscess—A Prospective Study Comparing Clinical with CT Findings in 133 Consecutive Patients. Diagnostics 2025, 15, 228. [Google Scholar] [CrossRef]
- Eliason, M.J.; Wang, A.S.; Lim, J.; Beegle, R.D.; Seidman, M.D. Are Computed Tomography Scans Necessary for the Diagnosis of Peritonsillar Abscess? Cureus 2023, 15, e34820. [Google Scholar] [CrossRef]
- Ulloa, L.; Messmer, D. High-mobility group box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev. 2006, 17, 189–201. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Guo, L.; Ma, J.; Zhang, H.; Xiao, M.; Li, N.; Gong, H.; Yan, M. HMGB1 as an extracellular pro-inflammatory cytokine: Implications for drug-induced organic damage. Cell Biol. Toxicol. 2024, 40, 55. [Google Scholar] [CrossRef] [PubMed]
- Sundén-Cullberg, J.; Norrby-Teglund, A.; Rouhiainen, A.; Rauvala, H.; Herman, G.; Tracey, K.J.; Lee, M.L.; Andersson, J.; Tokics, L.; Treutiger, C.J. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit. Care Med. 2005, 33, 564–573. [Google Scholar] [CrossRef]
- Deng, C.; Zhao, L.; Yang, Z.; Shang, J.J.; Wang, C.Y.; Shen, M.Z.; Jiang, S.; Li, T.; Di, W.C.; Chen, Y.; et al. Targeting HMGB1 for the treatment of sepsis and sepsis-induced organ injury. Acta Pharmacol. Sin. 2022, 43, 520–528. [Google Scholar] [CrossRef]
- Chao, J.; Li, P.; Chao, L. Kallistatin suppresses cancer development by multi-factorial actions. Crit. Rev. Oncol. Hematol. 2017, 113, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zou, J.; Yu, X.; Yin, S.; Tang, C. The antiatherogenic function of kallistatin and its potential mechanism. Acta Biochim. Biophys. Sin. 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Schmaier, A.; Chen, L.M.; Yang, Z.; Chao, L. Kallistatin, a novel human tissue kallikrein inhibitor: Levels in body fluids, blood cells, and tissues in health and disease. J. Lab. Clin. Med. 1996, 127, 612–620. [Google Scholar] [CrossRef]
- Birsen, M.B.; Erturk, D.; Onder, D.; Eryilmaz, A.I.; Kaba, M.; Ellidag, H.Y.; Inal, H.A. Practicability of Serum Kallistatin Levels as a Biomarker in the Diagnosis of Tubo-Ovarian Abscess. Surg. Infect. 2024, 25, 668–673. [Google Scholar] [CrossRef]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- Sanmark, E.; Wikstén, J.; Välimaa, H.; Aaltonen, L.M.; Ilmarinen, T.; Blomgren, K. Peritonsillar abscess may not always be a complication of acute tonsillitis: A prospective cohort study. PLoS ONE 2020, 15, e0228122. [Google Scholar] [CrossRef]
- Righini, M.; Van Es, J.; Den Exter, P.L.; Roy, P.M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. JAMA 2014, 311, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Body, R.; Carley, S.; McDowell, G.; Pemberton, P.; Burrows, G.; Cook, G.; Lewis, P.S.; Smith, A.; Mackway-Jones, K. The Manchester Acute Coronary Syndromes (MACS) decision rule for suspected cardiac chest pain: Derivation and external validation. Heart 2014, 100, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
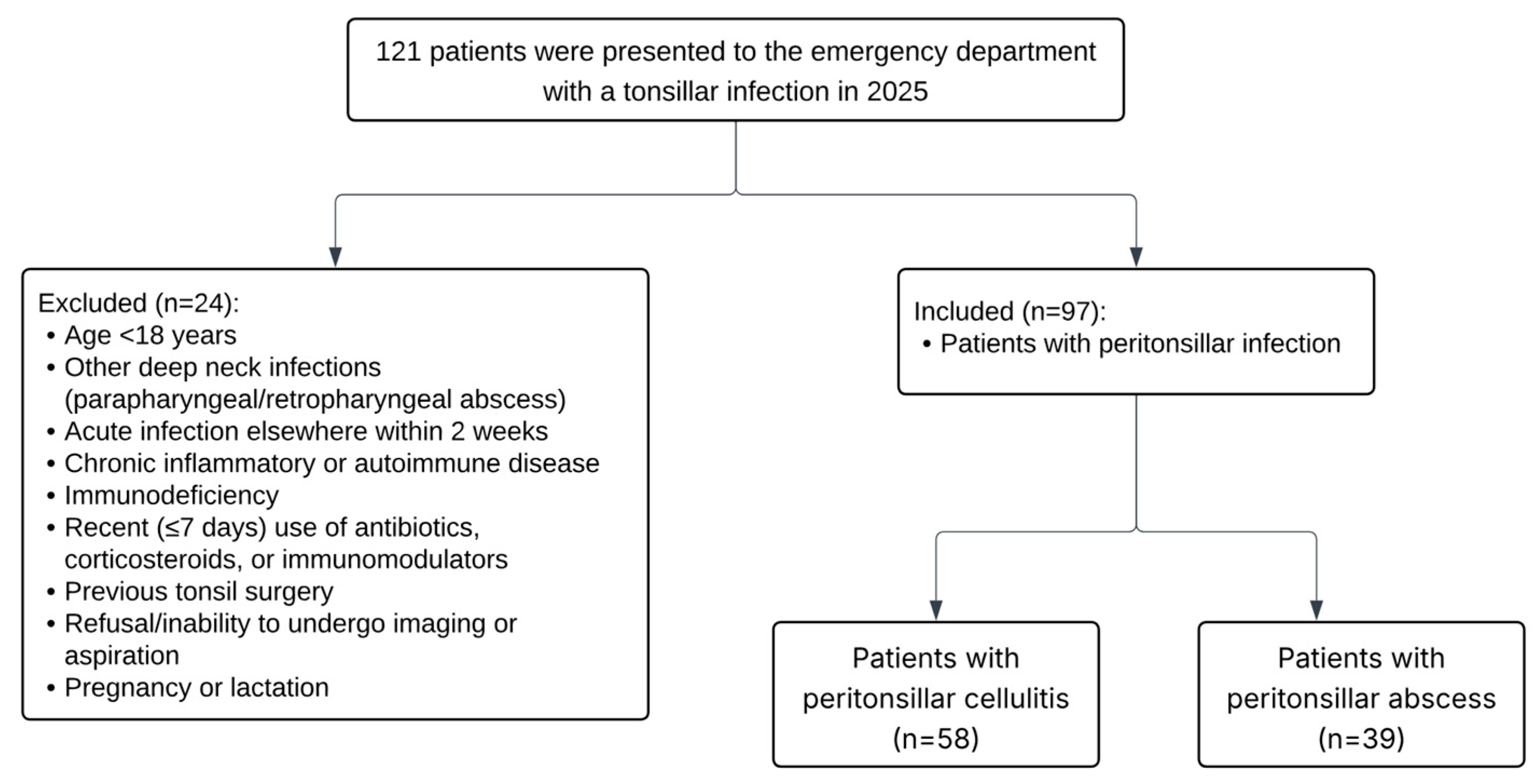

| Peritonsillar Infection Group (n = 97) | Control Group (n = 60) | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value | |
| Age, y | 37.74 ± 12.47 | 40.93 ± 12.50 | 0.564 |
| WBC, 103/μL | 16.08 ± 3.02 | 6.84 ± 1.47 | <0.001 |
| Neutrophil, 103/μL | 12.71 ± 3.06 | 3.97 ± 1.05 | <0.001 |
| Lymphocyte, 103/μL | 2.03 ± 0.98 | 2.18 ± 0.51 | 0.262 |
| CRP, mg/L | 100.23 ± 85.24 | 14.60 ± 8.03 | <0.001 |
| Procalcitonin, ng/mL | 0.15 ± 0.20 | 0.09 ± 0.06 | 0.038 |
| Albumin, g/L | 45.42 ± 3.66 | 45.20 ± 3.23 | 0.713 |
| HMGB1, ng/mL | 21.02 ± 7.61 | 16.12 ± 4.10 | <0.001 |
| Kallistatin, ng/mL | 3.65 ± 2.02 | 5.95 ± 2.50 | <0.001 |
| Peritonsillar Abscess Group (n = 39) | Peritonsillar Cellulitis Group (n = 58) | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value | |
| WBC, 103/μL | 16.52 ± 4.05 | 15.79 ± 2.06 | 0.243 |
| Neutrophil, 103/μL | 13.14 ± 4.14 | 12.43 ± 2.03 | 0.263 |
| Lymphocyte, 103/μL | 1.92 ± 0.62 | 2.10 ± 1.16 | 0.387 |
| CRP, mg/L | 122.30 ± 79.08 | 85.39 ± 86.66 | 0.036 |
| Procalcitonin, ng/mL | 0.16 ± 0.27 | 0.14 ± 0.15 | 0.557 |
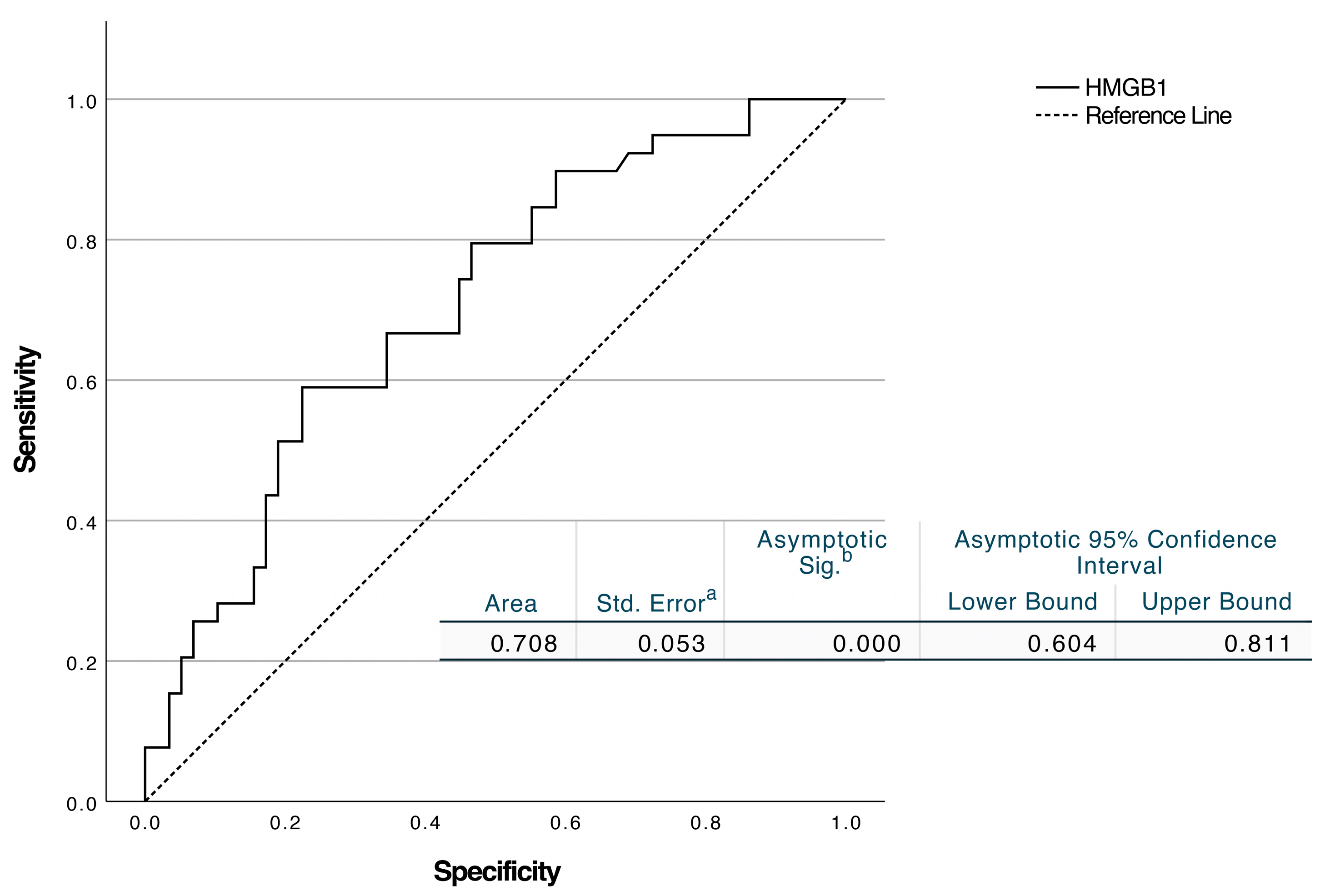
| HMGB1, ng/mL | 23.82 ± 8.40 | 19.13 ± 6.44 | 0.003 |
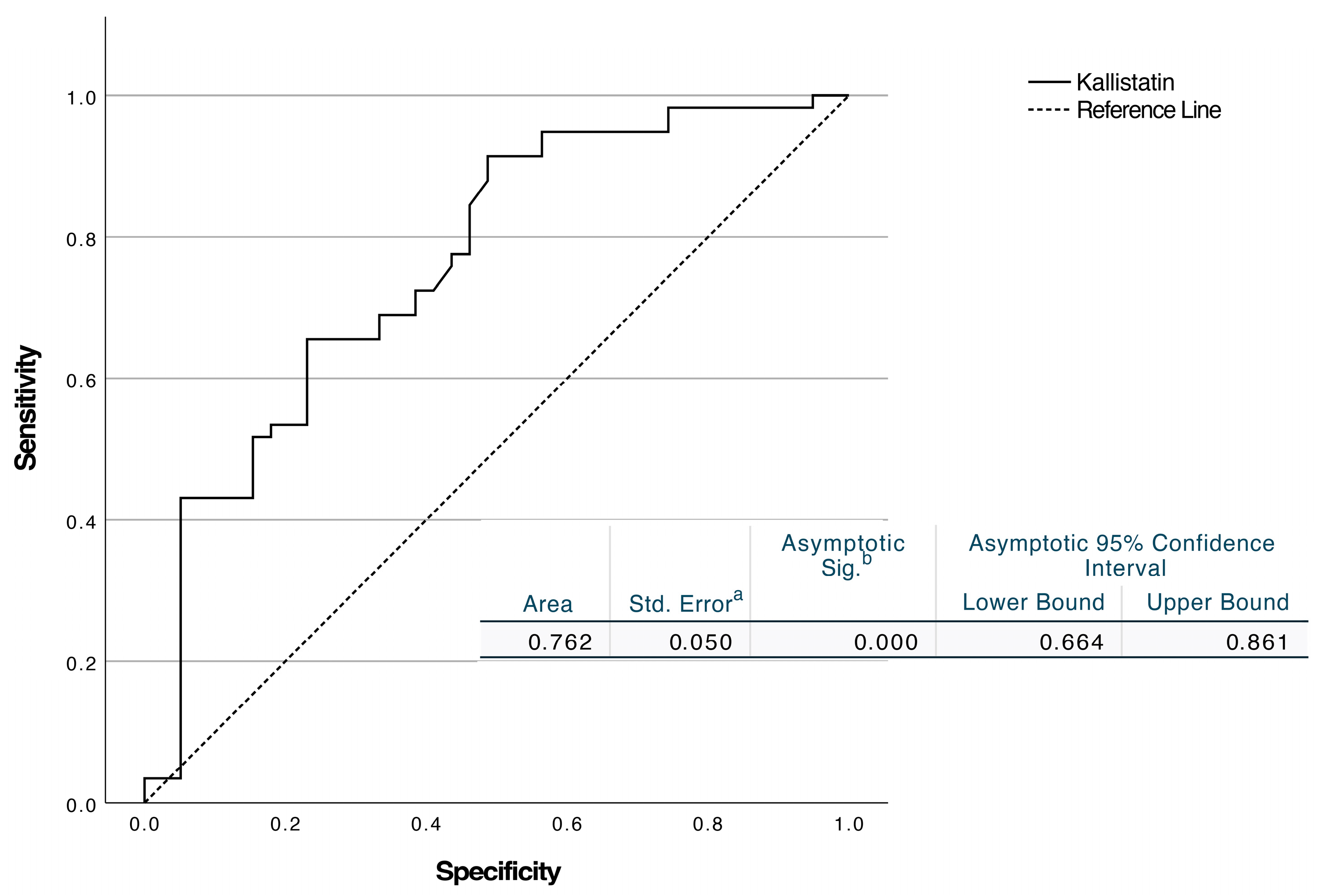
| Kallistatin, ng/mL | 2.76 ± 1.57 | 4.25 ± 2.08 | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% C.I for EXP(B) | 95% C.I for EXP(B) | |||||||||
| B | p | Exp(B) | Lower | Upper | B | p | Exp(B) | Lower | Upper | |
| CRP | 0.005 | 0.042 | 1.005 | 1.00 | 1.01 | 0.004 | 0.162 | 1.004 | 0.998 | 1.01 |
| HMGB1 | 0.088 | 0.006 | 1.092 | 1.026 | 1.162 | 0.194 | <0.001 | 1.21 | 1.09 | 1.35 |
| Kallistatin | −0.533 | 0.001 | 0.587 | 0.423 | 0.815 | −0.930 | <0.001 | 0.395 | 0.24 | 0.648 |
| Biomarker | Prevalences (%) | PPV (%) | NPV (%) |
|---|---|---|---|
| HMGB1 | 20 | 32.6 | 88.7 |
| 30 | 45.3 | 82.1 | |
| 40.2 * | 56.5 | 74.5 | |
| Kallistatin | 20 | 31.5 | 89.9 |
| 30 | 44.1 | 84.0 | |
| 40.2 * | 55.8 | 76.8 |
| PTA (n = 39) | PTC (n = 58) | |
|---|---|---|
| Polymicrobial | 4 | 4 |
| Streptococcus pyogenes | 6 | 4 |
| Streptococcus anginosus | 2 | 1 |
| Streptococcus mitis | 2 | 1 |
| Streptococcus constellatus | 1 | 0 |
| Streptococcus salivarius | 1 | 0 |
| Staphylococcus spp. | 2 | 0 |
| Haemophilus influenzae | 0 | 1 |
| No bacterial growth | 14 | 22 |
| Culture not performed | 7 | 25 |
| Model | Mean Net Benefit | Net Benefit 25% | Net Benefit 50% | Net Benefit 75% |
|---|---|---|---|---|
| Kallistatin + HMGB-1 + CRP | 0.183 | 0.275 | 0.113 | 0.124 |
| Kallistatin + HMGB-1 | 0.176 | 0.247 | 0.134 | 0.124 |
| Kallistatin | 0.131 | 0.258 | 0.083 | 0.000 |
| HMGB-1 | 0.111 | 0.216 | 0.041 | −0.031 |
| CRP | 0.099 | 0.203 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulut, K.S.; Gul, F.; Deveci Bulut, T.S.; Celik, B.; Serifler, S.; Babademez, M.A. HMGB1 and Kallistatin: Novel Serological Markers for Differentiating Peritonsillar Cellulitis and Abscess. Diagnostics 2025, 15, 2554. https://doi.org/10.3390/diagnostics15202554
Bulut KS, Gul F, Deveci Bulut TS, Celik B, Serifler S, Babademez MA. HMGB1 and Kallistatin: Novel Serological Markers for Differentiating Peritonsillar Cellulitis and Abscess. Diagnostics. 2025; 15(20):2554. https://doi.org/10.3390/diagnostics15202554
Chicago/Turabian StyleBulut, Kadir Sinasi, Fatih Gul, Tuba Saadet Deveci Bulut, Burak Celik, Serkan Serifler, and Mehmet Ali Babademez. 2025. "HMGB1 and Kallistatin: Novel Serological Markers for Differentiating Peritonsillar Cellulitis and Abscess" Diagnostics 15, no. 20: 2554. https://doi.org/10.3390/diagnostics15202554
APA StyleBulut, K. S., Gul, F., Deveci Bulut, T. S., Celik, B., Serifler, S., & Babademez, M. A. (2025). HMGB1 and Kallistatin: Novel Serological Markers for Differentiating Peritonsillar Cellulitis and Abscess. Diagnostics, 15(20), 2554. https://doi.org/10.3390/diagnostics15202554





