PET-CT Imaging in Hypertrophic Cardiomyopathy: A Narrative Review on Risk Stratification and Prognosis
Abstract
1. Introduction
2. PET Imaging of Microvascular Disfunction in Hypertrophic Cardiomyopathy
2.1. Mechanisms of Microvascular Dysfunction
2.2. Predictors of Microvascular Dysfunction
2.3. Clinical Implications
2.4. Limitations
3. 18F-FDG-PET Imaging Assessment of Myocardial Metabolism in Hypertrophic Cardiomyopathy
3.1. Physiopathology of Myocardial Metabolism
3.2. Myocardial Metabolism Patterns
3.3. Myocardial Metabolism and Fibrosis
3.4. Clinical Implications
4. Early-Stage Myocardial Fibrosis Assessed by Radiolabeled Fibroblast Activation Protein Inhibitors (FAPIs)
4.1. Physiopathology of Fibroblast Activation in HCM
4.2. Distribution of Fibroblast Activation in HCM: Predictors and Prognostic Implications
5. Discussion
Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHCM | apical hypertrophic cardiomyopathy |
| BNP | brain natriuretic peptide |
| CAD | coronary artery disease |
| CMD | coronary microvascular dysfunction |
| CMR | cardiac magnetic resonance |
| CV | cardiovascular |
| FAPI | fibroblast activation protein inhibitors |
| FDG | fluorodeoxyglucose |
| HCM | hypertrophic cardiomyopathy |
| hsTnI | high sensitivity troponin I |
| ICD | implantable cardioverter-defibrillator |
| LGE | late gadolinium enhancement |
| LVEF | left ventricular ejection fraction |
| LV | left ventricle |
| LVOT | left ventricular outflow tract |
| MBF | myocardial blood flow |
| MFR | myocardial flow reserve |
| MI | myocardial infarction |
| MVO2 | myocardial oxygen consumption |
| MWT | maximum wall thickness |
| NOHCM | non-obstructive hypertrophic cardiomyopathy |
| NSVT | non-sustained ventricular tachycardia |
| OHCM | obstructive hypertrophic cardiomyopathy |
| PET | positron emission tomography |
| SCD | sudden cardiac death |
| VA | ventricular arrhthymias |
| VT | ventricular tachycardia |
References
- Maron, B.J.; Maron, M.S. Contemporary strategies for risk stratification and prevention of sudden death with the implantable defibrillator in hypertrophic cardiomyopathy. Heart Rhythm. 2016, 13, 1155–1165. [Google Scholar] [CrossRef]
- Task Force Members; Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 76, e159–e240. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.M.; Sharma, S.; Varnava, A.; Poloniecki, J.; Rowland, E.; McKenna, W.J. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1999, 33, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Link, M.S.; Lesser, J.R.; Chan, R.H.; Garberich, R.F.; Udelson, J.E.; Maron, M.S. Hypertrophic Cardiomyopathy in Adulthood Associated With Low Cardiovascular Mortality With Contemporary Management Strategies. J. Am. Coll. Cardiol. 2015, 65, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Todiere, G.; Nugara, C.; Gentile, G.; Negri, F.; Bianco, F.; Falletta, C.; Novo, G.; Di Bella, G.; De Caterina, R.; Zachara, E.; et al. Prognostic Role of Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Low-to-Intermediate Sudden Cardiac Death Risk Score. Am. J. Cardiol. 2019, 124, 1286–1292. [Google Scholar] [CrossRef]
- Maron, B.J.; Olivotto, I.; Spirito, P.; Casey, S.A.; Bellone, P.; Gohman, T.E.; Graham, K.J.; Burton, D.A.; Cecchi, F. Epidemiology of Hypertrophic Cardiomyopathy–Related Death. Circulation 2000, 102, 858–864. [Google Scholar] [CrossRef]
- Mentias, A.; Raeisi-Giglou, P.; Smedira, N.G.; Feng, K.; Sato, K.; Wazni, O.; Kanj, M.; Flamm, S.D.; Thamilarasan, M.; Popovic, Z.B.; et al. Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Preserved Systolic Function. J. Am. Coll. Cardiol. 2018, 72, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Arbelo, E.; Barriales-Villa, R.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Maron, B.J.; Ommen, S.R.; Semsarian, C.; Spirito, P.; Olivotto, I.; Maron, M.S. Hypertrophic Cardiomyopathy: Present and Future, With Translation Into Contemporary Cardiovascular Medicine. J. Am. Coll. Cardiol. 2014, 64, 83–99. [Google Scholar] [CrossRef]
- Christiaans, I.; van Engelen, K.; van Langen, I.M.; Birnie, E.; Bonsel, G.J.; Elliott, P.M.; Wilde, A.A. Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: Systematic review of clinical risk markers. EP Eur. 2010, 12, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; McKenna, W.J.; Danielson, G.K.; Kappenberger, L.J.; Kuhn, H.J.; Seidman, C.E.; Shah, P.M.; Spencer, W.H.; Spirito, P.; Ten Cate, F.J.; et al. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European S. J. Am. Coll. Cardiol. 2003, 42, 1687–1713. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Maron, M.S. Hypertrophic cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Haas, T.S.; Chan, R.H.; Udelson, J.E.; Garberich, R.F.; Lesser, J.R.; Appelbaum, E.; Manning, W.J.; et al. Risk stratification and outcome of patients with hypertrophic cardiomyopathy >=60 years of age. Circulation. 2013, 127, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Maron, M.S.; Adabag, A.S.; Casey, S.A.; Vargiu, D.; Link, M.S.; Udelson, J.E.; Cecchi, F.; Maron, B.J. Gender-Related Differences in the Clinical Presentation and Outcome of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, S.; Kammoun, I.; Bennour, E.; Laroussi, L.; Kachboura, S. Risk stratification in hypertrophic cardiomyopathy. Herz. 2020, 45, 50–64. [Google Scholar] [CrossRef]
- Maron, B.J.; Casey, S.A.; Olivotto, I.; Sherrid, M.V.; Semsarian, C.; Autore, C.; Ahmed, A.; Boriani, G.; Francia, P.; Winters, S.L.; et al. Clinical Course and Quality of Life in High-Risk Patients with Hypertrophic Cardiomyopathy and Implantable Cardioverter-Defibrillators. Circ. Arrhythmia Electrophysiol. 2018, 11, e005820. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, H. Late gadolinium enhancement and prognosis of hypertrophic cardiomyopathy. Circ. J. 2014, 78, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Panovsky, R.; Pleva, M.; Feitova, V.; Kruzliak, P.; Meluzin, J.; Kincl, V. The prognostic impact of myocardial late gadolinium enhancement. Cardiol. Rev. 2014, 22, 128–139. [Google Scholar] [CrossRef]
- Kay, G.N. Can positron emission tomography help stratify the risk of sudden cardiac death in patients with hypertrophic cardiomyopathy? J. Nucl. Cardiol. 2019, 26, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Girolami, F.; Sciagrà, R.; Ackerman, M.J.; Sotgia, B.; Bos, J.M.; Nistri, S.; Sgalambro, A.; Grifoni, C.; Torricelli, F.; et al. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations. J. Am. Coll. Cardiol. 2011, 58, 839–848. [Google Scholar] [CrossRef]
- Castagnoli, H.; Ferrantini, C.; Coppini, R.; Passeri, A.; Baldini, K.; Berti, V.; Cecchi, F.; Olivotto, I.; Sciagrà, R. Role of quantitative myocardial positron emission tomography for risk stratification in patients with hypertrophic cardiomyopathy: A 2016 reappraisal. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E.; Pinheiro, A.; Higuchi, T.; Rischpler, C.; Merrill, J.; Santaularia-Tomas, M.; Abraham, M.R.; Wahl, R.L.; Abraham, T.P.; Bengel, F.M. PET/CT assessment of symptomatic individuals with obstructive and nonobstructive hypertrophic cardiomyopathy. J. Nucl. Med. 2012, 53, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, P.; Nordström, J.; Harms, H.J.; Lubberink, M.; Gadler, F.; Sörensen, J.; Mörner, S. Positron emission tomography (15O-water, 11C-acetate, 11C-HED) risk markers and nonsustained ventricular tachycardia in hypertrophic cardiomyopathy. IJC Heart Vasc. 2020, 26, 100452. [Google Scholar] [CrossRef]
- Bravo, P.E. Is there a role for cardiac positron emission tomography in hypertrophic cardiomyopathy? J. Nucl. Cardiol. 2019, 26, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Caobelli, F.; Zwisler, D.; Haaf, P.; Zellweger, M.J. 82Rb myocardial perfusion PET/CT after anterior/antero-septal wall myectomy. J. Nucl. Cardiol. 2019, 26, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.J.; Lee, S.H.; Cho, I.H. Myocardial Fibrosis in Hypertrophic Cardiomyopathy Demonstrated by Integrated Cardiac F-18 FDG PET/MR. Nucl. Med. Mol. Imaging. 2013, 47, 196–200. [Google Scholar] [CrossRef]
- Uehara, T.; Ishida, Y.; Hayashida, K.; Shimonagata, T.; Miyake, Y.; Sago, M.; Oka, H.; Nagata, S.; Miyatake, K.; Nishimura, T. Myocardial glucose metabolism in patients with hypertrophic cardiomyopathy: Assessment by F-18-FDG PET study. Ann. Nucl. Med. 1998, 12, 95–103. [Google Scholar] [CrossRef]
- Takeishi, Y.; Masuda, A.; Kubo, H.; Tominaga, H.; Oriuchi, N.; Takenoshita, S. Cardiac imaging with 18F-fluorodeoxyglucose PET/MRI in hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2017, 24, 1827–1828. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Wang, J.; Xiao, M.; Xi, X.-Y.; Chen, B.-X.; Su, Y.; Zhang, Y.; Xie, B.; Dong, Z.; et al. Myocardial Activity at 18F-FAPI PET/CT and Risk for Sudden Cardiac Death in Hypertrophic Cardiomyopathy. Radiology 2023, 306, e221052. [Google Scholar] [CrossRef]
- Klein, R.; Celiker-Guler, E.; Rotstein, B.H.; deKemp, R.A. PET and SPECT Tracers for Myocardial Perfusion Imaging. Semin. Nucl. Med. 2020, 50, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Maaniitty, T.; Knuuti, J.; Saraste, A. 15O-Water PET MPI: Current Status and Future Perspectives. Semin. Nucl. Med. 2020, 50, 238–247. [Google Scholar] [CrossRef]
- Timmer, S.A.J.; Lubberink, M.; Germans, T.; Götte, M.J.W.; Berg, J.M.T.; Cate, F.J.T.; van Rossum, A.C.; Lammertsma, A.A.; Knaapen, P. Potential of [11C]acetate for measuring myocardial blood flow: Studies in normal subjects and patients with hypertrophic cardiomyopathy. J Nucl Cardiol Off. Publ. Am. Soc. Nucl. Cardiol. 2010, 17, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Jerosch-Herold, M.; Hudsmith, L.E.; Robson, M.D.; Francis, J.M.; Doll, H.A.; Selvanayagam, J.B.; Neubauer, S.; Watkins, H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: New insights from multiparametric magnetic resonance imaging. Circulation 2007, 115, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, B.; Sciagrà, R.; Olivotto, I.; Casolo, G.; Rega, L.; Betti, I.; Pupi, A.; Camici, P.G.; Cecchi, F. Spatial relationship between coronary microvascular dysfunction and delayed contrast enhancement in patients with hypertrophic cardiomyopathy. J. Nucl. Med. 2008, 49, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Knaapen, P.; Germans, T.; Camici, P.G.; Rimoldi, O.E.; Cate, F.J.T.; Berg, J.M.T.; Dijkmans, P.A.; Boellaard, R.; van Dockum, W.G.; Götte, M.J.W.; et al. Determinants of coronary microvascular dysfunction in symptomatic hypertrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H986–H993. [Google Scholar] [CrossRef] [PubMed]
- Krams, R.; Kofflard, M.J.M.; Duncker, D.J.; Von Birgelen, C.; Carlier, S.; Kliffen, M.; Cate, F.J.T.; Serruys, P.W. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation 1998, 97, 230–233. [Google Scholar] [CrossRef]
- Tanaka, M.; Fujiwara, H.; Onodera, T.; Wu, D.J.; Matsuda, M.; Hamashima, Y.; Kawai, C. Quantitative analysis of narrowings of intramyocardial small arteries in normal hearts, hypertensive hearts, and hearts with hypertrophic cardiomyopathy. Circulation 1987, 75, 1130–1139. [Google Scholar] [CrossRef]
- Lama von Buchwald, C.; Sanghani, R. PET myocardial perfusion imaging in the diagnosis of apical hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2022, 29, 1452–1456. [Google Scholar] [CrossRef]
- II Soliman, O.; Knaapen, P.; Geleijnse, M.L.; A Dijkmans, P.; Anwar, A.M.; Nemes, A.; Michels, M.; Vletter, W.B.; A Lammertsma, A.; Cate, F.J.T. Assessment of intravascular and extravascular mechanisms of myocardial perfusion abnormalities in obstructive hypertrophic cardiomyopathy by myocardial contrast echocardiography. Heart 2007, 93, 1204–1212. [Google Scholar] [CrossRef]
- Bravo, P.E.; Tahari, A.; Pozios, I.; Luo, H.-C.; Bengel, F.M.; Wahl, R.L.; Abraham, M.R.; Abraham, T.P. Apparent left ventricular cavity dilatation during PET/CT in hypertrophic cardiomyopathy: Clinical predictors and potential mechanisms. J. Nucl. Cardiol. 2016, 23, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E.; Zimmerman, S.L.; Luo, H.C.; Pozios, I.; Rajaram, M.; Pinheiro, A.; Steenbergen, C.; Kamel, I.R.; Wahl, R.L.; Bluemke, D.A.; et al. The Relationship of Delayed Enhancement by Magnetic Resonance to Myocardial Perfusion by Positron Emission Tomography in Hypertrophic Cardiomyopathy Paco. Circ. Cardiovasc. Imaging 2013, 6, 210–217. [Google Scholar] [CrossRef]
- Kwon, D.H.; Smedira, N.G.; Rodriguez, E.R.; Tan, C.; Setser, R.; Thamilarasan, M.; Lytle, B.W.; Lever, H.M.; Desai, M.Y. Cardiac Magnetic Resonance Detection of Myocardial Scarring in Hypertrophic Cardiomyopathy: Correlation With Histopathology and Prevalence of Ventricular Tachycardia. J. Am. Coll. Cardiol. 2009, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Akar, F.G.; Tomaselli, G.F. Molecular basis of arrhythmias. Circulation 2005, 112, 2517–2529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Solhjoo, S.; Millare, B.; Plank, G.; Abraham, M.R.; Cortassa, S.; Trayanova, N.; O’Rourke, B. Effects of regional mitochondrial depolarization on electrical propagation: Implications for arrhythmogenesis. Circ. Arrhythm. Electrophysiol. 2014, 7, 143–151. [Google Scholar] [CrossRef]
- Lorenzoni, R.; Gistri, R.; Cecchi, F.; Olivotto, I.; Chiriatti, G.; Elliott, P.; McKenna, W.J.; Camici, P.G. Syncope and ventricular arrhythmias in hypertrophic cardiomyopathy are not related to the derangement of coronary microvascular function. Eur. Heart J. 1997, 18, 1946–1950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, D.-Y.; Yalçin, H.; Yalçin, F.; Zhao, M.; Sivalokanathan, S.; Valenta, I.; Tahari, A.; Pomper, M.G.; Abraham, T.P.; Schindler, T.H.; et al. Stress Myocardial Blood Flow Heterogeneity is a Positron Emission Tomography Biomarker of Ventricular Arrhythmias in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Yalçin, H.; Sivalokanathan, S.; Greenland, G.V.; Vasquez, N.; Yalçin, F.; Zhao, M.; Valenta, I.; Ganz, P.; Pampaloni, M.H.; et al. Higher incidence of vasodilator-induced left ventricular cavity dilation by PET when compared to treadmill exercise-ECHO in hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2020, 27, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Olivotto, I.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Camici, P.G. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003, 349, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Koenders, S.S.; van Dalen, J.A.; van Dijk, J.D. The next step in improving (semi-)quantitative MPI PET. J. Nucl. Cardiol. 2022, 29, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Hoff, C.M.; Sørensen, J.; Christensen, N.L.; Bouchelouche, K.; Tolbod, L. Activity regimes for 82Rb cardiac PET: Effects on absolute MBF and MPI. J. Nucl. Cardiol. 2022, 29, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, M.; Nakahara, T.; Murata, M.; Ogata, Y.; Matsusaka, Y.; Iwabuchi, Y.; Yamada, Y.; Fukuda, K.; Jinzaki, M. Incidental spade-shaped FDG uptake in the left ventricular apex suggests apical hypertrophic cardiomyopathy. Ann. Nucl. Med. 2017, 31, 399–406. [Google Scholar] [CrossRef]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; DaSilva, J.; Birnie, D.; et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Nakamura, K.; Sasaki, T.; Goto, K.; Take, Y.; Naito, S. Hypertrophic Cardiomyopathy Complicated by Cardiac Sarcoidosis Diagnosed by Both the Morphological Abnormalities and the Time Course of the Disease. Int. Heart J. 2021, 62, 201–206. [Google Scholar] [CrossRef]
- Norikane, T.; Yamamoto, Y.; Takami, Y.; Mitamura, K.; Tani, R.; Nishiyama, Y. Occasionally increased 18F-FDG uptake in apical hypertrophic cardiomyopathy on serial follow-up PET/CT. J. Nucl. Cardiol. 2019, 26, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, Y.; Ishide, N.; Takeyama, D.; Kanno, Y.; Yamane, Y.; Shirato, K.; Maruyama, Y.; Itoh, M.; Ido, T.; Matsuzawa, T.; et al. Differences in myocardial fluoro-18 2-deoxyglucose uptake in young versus older patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 1992, 69, 242–246. [Google Scholar] [CrossRef]
- Aoyama, R.; Takano, H.; Kobayashi, Y.; Kitamura, M.; Asai, K.; Amano, Y.; Kumita, S.-I.; Shimizu, W. Evaluation of myocardial glucose metabolism in hypertrophic cardiomyopathy using 18F-fluorodeoxyglucose positron emission tomography. PLoS ONE. 2017, 12, e0188479. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kitagawa, T.; Susawa, H.; Hata, R.; Tatsugami, F.; Higaki, T.; Awai, K.; Kihara, Y. Occasionally increased 18F-fluorodeoxyglucose uptake in apical hypertrophic cardiomyopathy in mid-ventricular obstruction. J. Cardiol. Cases 2017, 16, 44–47. [Google Scholar] [CrossRef]
- Miyamoto, K.; Norikane, T.; Ihara-Nishishita, A.; Takami, Y.; Mitamura, K.; Yamamoto, Y.; Noma, T.; Nishiyama, Y. What is this image? 2022 image 5 result: Apical ring uptake on 18F-FDG PET/CT indicating apical hypertrophic cardiomyopathy with apical aneurysm. J. Nucl. Cardiol. 2022, 29, 403–408. [Google Scholar] [CrossRef]
- Funabashi, N.; Nakagawa, K.; Komuro, I. Partial myocardial fibrosis in hypertrophic cardiomyopathy demonstrated by 18F-fluoro-deoxyglucose positron emission tomography and multislice computed tomography [5]. Int. J. Cardiol. 2006, 107, 284–286. [Google Scholar] [CrossRef]
- Matthijs Blankesteijn, W. Has the search for a marker of activated fibroblasts finally come to an end? J. Mol. Cell. Cardiol. 2015, 88, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wang, J.; Xi, X.-Y.; Guo, X.; Chen, B.-X.; Li, L.; Hua, C.; Zhao, S.; Su, P.; Chen, M.; et al. Fibroblast activation protein imaging in reperfused ST-elevation myocardial infarction: Comparison with cardiac magnetic resonance imaging. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2786–2797. [Google Scholar] [CrossRef]
- Shi, X.; Lin, X.; Huo, L.; Li, X. Cardiac fibroblast activation in dilated cardiomyopathy detected by positron emission tomography. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2022, 29, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, Y.H.; Yang, M.F. Heterogeneous Uptake of Al18F-NOTA-FAPI and 18F-FDG in Apical Aneurysm in Apical Hypertrophic Cardiomyopathy. Clin. Nucl. Med. 2024, 49, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Z.; Wang, L.; Wang, Y.-L.; Chen, B.-X.; Su, Y.; Zhao, S.; Yang, M.-F. Functional significance of myocardial activity at (18)F-FAPI PET/CT in hypertrophic cardiomyopathy identified by cardiac magnetic resonance feature-tracking strain analysis. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gu, X.; Chen, Y.; Liu, D. A Systematic Review and Meta-analysis of Efficacy and Safety of Mavacamten for the Treatment of Hypertrophic Cardiomyopathy. Rev. Cardiovasc. Med. 2024, 25, 375. [Google Scholar] [CrossRef] [PubMed]
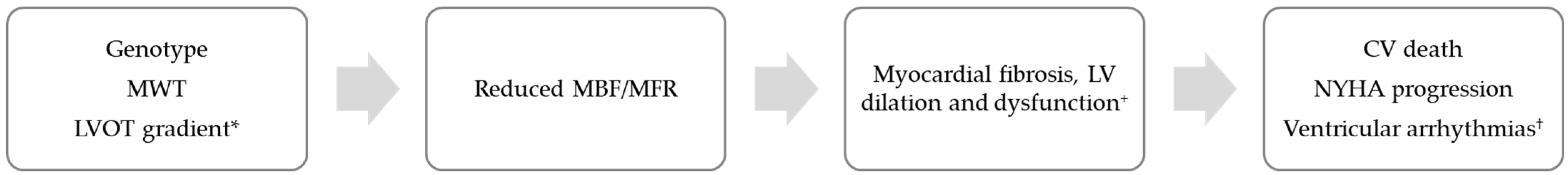
| Predictors of myocardial perfusion impairment in HCM | |||||||
| Author (journal, year) | Pts (N) | Tracer | Predictor | MBF | MFR | Outcome | Ref |
| Olivotto (JACC, 2011) | 61 | 13NH3 | Genotype | <1.5 | LGE | [21] | |
| Knaapen (Am J Physiol Heart Circ Physiol, 2008) | 18 | 13NH3 | MWT | Reduced | [36] | ||
| LVOT gradient | Reduced | ||||||
| Bravo (J Nucl Med, 2012) | 33 | 13NH3 | MWT | Reduced | Lower in NOHCM | [23] | |
| LVOT gradient | No correlation | No correlation | |||||
| Buchwald (J Nucl Cardiol, 2022) | Case report | 13NH3 | MWT (apex) | Reduced (apex) | [39] | ||
| Outcomes resulting from myocardial perfusion impairment in HCM | |||||||
| Author (journal, year) | Pts (N) | Tracer | Predictor | MBF | MFR | Outcome | Ref |
| Castagnoli (Eur J Nucl Med Mol Immaging, 2016) | 100 | 13NH3 | <1.1 | CV death NYHA progression Ischemic stroke VA | [22] | ||
| Cechi (N Engl J Med, 2003) | 51 | 13NH3 | Reduced | Reduced | CV death | [49] | |
| Sotgia (J Nucl Med, 2008) | 34 | 13NH3 | Reduced | Fibrosis (LGE) | [35] | ||
| Bravo (Circ Cardiovasc Imaging, 2013) | 47 | 13NH3 | Reduced | Reduced | Fibrosis (LGE) (small proportion with normal perfusion) | [42] | |
| Lorenzoni (Eur Heart J, 1997) | 84 | 13NH3 H215O | No variation | No variation | Syncope NSVT | [46] | |
| Lu (Am J Cardiol, 2018) | 133 | 13NH3 | No variation | No variation | NSVT | [24] | |
| Lower gradient | |||||||
| Higher MVO2 consumption | |||||||
| Bravo (J Nucl Cardiol, 2016) | 61 | 13NH3 | Reduced | Reduced | LV dilation | [41] | |
| Lu (J Nucl Cardiol, 2020) | 108 | 13NH3 | Reduced | LV dilation and dysfunction | [48] | ||
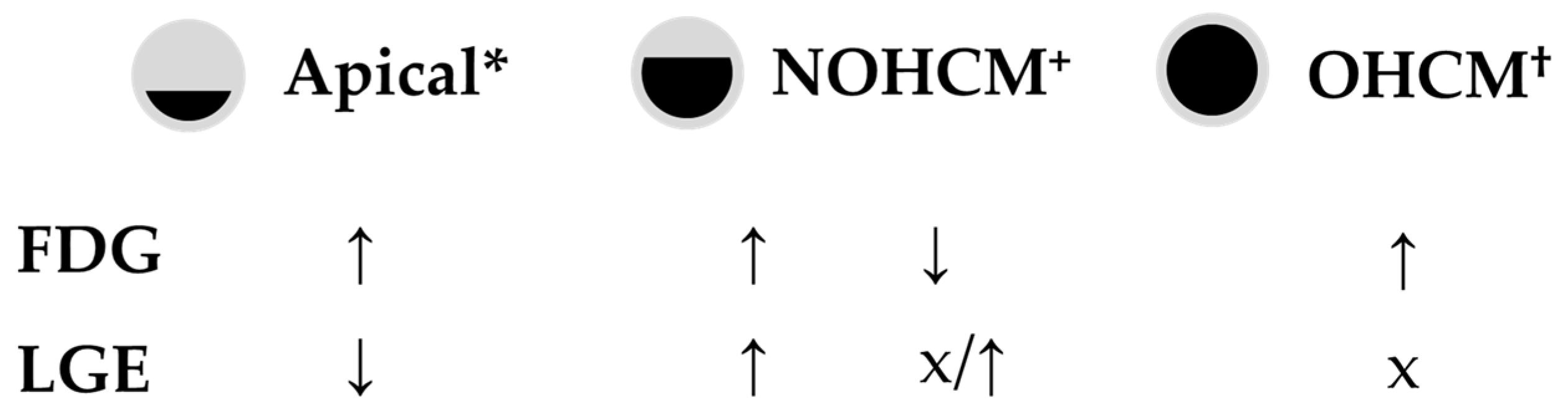
| Author (Journal, Year) | Pts (N) | 18F-FDG Uptake Distribution | 18F-FDG Uptake and LGE | Ref |
|---|---|---|---|---|
| Uehara (Ann Nucl Med, 1998) | 32 | Higher in dilated-phase HCM Lower in AHCM | [28] | |
| Kagaya (Am J Cardiol, 1992) | 16 | Younger pts have non-homogeneous uptake | [56] | |
| Aoyama (PLOS one, 2017) | 30 | NOHCM—limited to the hypertrophied segments OHCM—beyond hypertrophied segments | NOHCM—LGE present in hypertrophied segments OHCM—LGE not commonly observed | [57] |
| NOHCM—related to hsTnI levels OHCM—related to BNP levels | ||||
| OHCM—reduced with septal ablation | ||||
| Kong (Nucl Med Mol Imaging, 2013) | Case report | Reduced in the asymmetrical hypertrophied septum | Lower uptake matched LGE areas (septum) | [27] |
| Funabashi (Int J Cardiol, 2006) | Case report | Reduced in the anterior and apical inter-ventricular septum | [60] | |
| Takeishi (J Nucl Cardiol, 2016) | Case report | Increased in middle walls, reduced in apex | Lower uptake matched LGE areas (apex) | [29] |
| Katagiri (Ann Nucl Med, 2017) | Case series (17) | AHCM—increased in the apex | [52] | |
| Norikane (J Nucl Cardiol, 2019) | Case report | AHCM—increased in the apex | [55] | |
| Yamamoto (Journal of Cardiol Cases, 2017) | Case report | AHCM—increased in the apex | Lower uptake matched LGE areas | [58] |
| Miyamoto (J Nucl Cardiol, 2022) | Case report | AHCM—increased in the apex | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques-Alves, P.; Gonçalves, L.; Ferreira, M.J. PET-CT Imaging in Hypertrophic Cardiomyopathy: A Narrative Review on Risk Stratification and Prognosis. Diagnostics 2025, 15, 133. https://doi.org/10.3390/diagnostics15020133
Marques-Alves P, Gonçalves L, Ferreira MJ. PET-CT Imaging in Hypertrophic Cardiomyopathy: A Narrative Review on Risk Stratification and Prognosis. Diagnostics. 2025; 15(2):133. https://doi.org/10.3390/diagnostics15020133
Chicago/Turabian StyleMarques-Alves, Patrícia, Lino Gonçalves, and Maria João Ferreira. 2025. "PET-CT Imaging in Hypertrophic Cardiomyopathy: A Narrative Review on Risk Stratification and Prognosis" Diagnostics 15, no. 2: 133. https://doi.org/10.3390/diagnostics15020133
APA StyleMarques-Alves, P., Gonçalves, L., & Ferreira, M. J. (2025). PET-CT Imaging in Hypertrophic Cardiomyopathy: A Narrative Review on Risk Stratification and Prognosis. Diagnostics, 15(2), 133. https://doi.org/10.3390/diagnostics15020133





