Outcomes of Endoscopic Resection of Circumferential Colorectal Laterally Spreading Lesions: A Western Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria and Data Collection
2.3. Definitions
2.4. Endoscopic Procedures
2.5. Histopathology Assessment
2.6. Study Outcomes
- Incidence of post-ESD stricture in circumferential and near-circumferential colorectal lesions.
- Procedure time and dissection speed (defined as specimen area divided by procedure duration).
- Incidence of adverse events (intra- and post-procedural bleeding, perforation, PECS).
- Identification of clinical and procedural predictors of stricture development.
2.7. Definitions of Adverse Events and Follow-Up
- -
- -
- -
- -
- The presence of abdominal distension at the site of endoscopic resection.
- The presence of fever (>37.5 °C) or increased indices of inflammation (C-reactive protein > 0.5 mg/dL or leukocytosis > 10,000 cells/μL), without evidence of established perforation arising > 6 h after the endoscopic procedure.
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Procedural and Lesion Characteristics
3.3. Stricture Formation and Treatment
3.4. Adverse Events
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESD | Endoscopic Submucosal Dissection |
| EMR | Endoscopic Mucosal Resection |
| LST | Laterally Spreading Tumor |
| JNET | Japan Narrow Band Imaging Expert Team |
| BLI | Blu Light Imaging |
| ASGE | American Society of Gastrointestinal Endoscopy |
| SM | Submucosal |
| AE | Adverse Event |
| PECS | Post-ESD Electrocoagulation Syndrome |
| ASA | American Society of Anesthesiology |
| IQR | Interquartile Range |
| EPMR | Endoscopic Piecemeal Mucosal Resection |
| TEM | Trans-Anal Endoscopic Microsurgery (TEM) |
| LAMS | Lumen-Apposing Metal Stent |
| TTS | Through the Scope |
| OTSC | Over-the-Scope Clip |
References
- Yahagi, N.; Fujishiro, M.; Imagawa, A.; Kakushima, N.; Iguchi, M.; Omata, M. Endoscopic submucosal dissection for the reliable en bloc resection of colorectal mucosal tumors. Dig. Endosc. 2004, 16 (Suppl. S1), S89–S92. [Google Scholar] [CrossRef]
- Kuroki, Y.; Hoteya, S.; Mitani, T.; Yamashita, S.; Kikuchi, D.; Fujimoto, A.; Matsui, A.; Nakamura, M.; Nishida, N.; Iizuka, T.; et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J. Gastroenterol. Hepatol. 2010, 25, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Hassan, C.; Dewitt, J.M. Colorectal endoscopic submucosal dissection in the United States: Why do we hear so much about it and do so little of it? Gastrointest. Endosc. 2017, 85, 554–558, Erratum in Gastrointest. Endosc. 2017, 86, 756. https://doi.org/10.1016/j.gie.2017.03.003. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kashida, H.; Saito, Y.; Yahagi, N.; Yamano, H.; Saito, S.; Hisabe, T.; Yao, T.; Watanabe, M.; Yoshida, M.; et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig. Endosc. 2020, 32, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Toyonaga, T.; Man-i, M.; East, J.E.; Nishino, E.; Ono, W.; Hirooka, T.; Ueda, C.; Iwata, Y.; Sugiyama, T.; Dozaiku, T.; et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: Complication rates and long-term outcomes. Surg. Endosc. 2013, 27, 1000–1008. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Igarashi, A.; Mizuguchi, Y.; Takamaru, H.; Yamada, M.; Sakamoto, T.; Maltzman, H.; Falkén, Y.; Esaki, M.; Matsuda, T.; et al. Cost-effectiveness analysis of endoscopic resection for colorectal laterally spreading tumors: Endoscopic submucosal dissection versus piecemeal endoscopic mucosal resection. Dig. Endosc. 2022, 34, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Sakamoto, T.; Takamaru, H.; Yamada, M.; Nakajima, T.; Matsuda, T.; Saito, Y. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg. Endosc. 2016, 30, 5459–5464. [Google Scholar] [CrossRef]
- Ohara, Y.; Toyonaga, T.; Tanaka, S.; Ishida, T.; Hoshi, N.; Yoshizaki, T.; Azuma, T. Risk of stricture after endoscopic submucosal dissection for large rectal neoplasms. Endoscopy 2016, 48, 62–70. [Google Scholar] [CrossRef]
- Hayashi, T.; Kudo, S.E.; Miyachi, H.; Sakurai, T.; Ishigaki, T.; Yagawa, Y.; Ishida, F. Management and risk factor of stenosis after endoscopic submucosal dissection for colorectal neoplasms. Gastrointest. Endosc. 2017, 86, 358–369. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Hendrix, J.M.; Garmon, E.H. American Society of Anesthesiologists Physical Status Classification System. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon. Gastrointest. Endosc. 2003, 58 (Suppl. S6), S3–S43. [Google Scholar] [CrossRef]
- Sano, Y.; Tanaka, S.; Kudo, S.E.; Saito, S.; Matsuda, T.; Wada, Y.; Fujii, T.; Ikematsu, H.; Uraoka, T.; Kobayashi, N.; et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig. Endosc. 2016, 28, 526–533. [Google Scholar] [CrossRef]
- Li, M.; Ali, S.M.; Umm-a-OmarahGilani, S.; Liu, J.; Li, Y.Q.; Zuo, X.L. Kudo’s pit pattern classification for colorectal neoplasms: A meta-analysis. World J. Gastroenterol. 2014, 20, 12649–12656. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Tanaka, S.; Oba, S.; Kanao, H.; Oka, S.; Yoshihara, M.; Chayama, K. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand. J. Gastroenterol. 2010, 45, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, G.; Fasulo, E.; Cecinato, P.; Barbara, G.; Barchi, A.; Viale, E.; Esposito, D.; Grillo, S.; Sassatelli, R.; Malesci, A.; et al. Endoscopic Submucosal Dissection (ESD) for the Management of Fibrotic Non-Lifting Colorectal Lesions (NLCLs): Results from a Large Multicenter Retrospective Study. Cancers 2025, 17, 1242. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Pioche, M.; Albéniz, E.; Berr, F.; Deprez, P.; Ebigbo, A.; Dewint, P.; Haji, A.; Panarese, A.; Weusten, B.L.A.M.; et al. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2019, 51, 980–992. [Google Scholar] [CrossRef]
- Kobara, H.; Mori, H.; Rafiq, K.; Fujihara, S.; Nishiyama, N.; Ayaki, M.; Yachida, T.; Matsunaga, T.; Tani, J.; Miyoshi, H.; et al. Submucosal tunneling techniques: Current perspectives. Clin. Exp. Gastroenterol. 2014, 7, 67–74. [Google Scholar] [CrossRef]
- Inayat, F.; Weissman, S.; Malik, A.; Munir, B.; Iqbal, S. Endoscopic Submucosal Tunnel Dissection as a Novel Therapeutic Technique in Patients with Barrett’s Esophagus. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620941318. [Google Scholar] [CrossRef]
- Sakamoto, H.; Hayashi, Y.; Miura, Y.; Shinozaki, S.; Takahashi, H.; Fukuda, H.; Okada, M.; Ino, Y.; Takezawa, T.; Sunada, K.; et al. Pocket-creation method facilitates endoscopic submucosal dissection of colorectal laterally spreading tumors, non-granular type. Endosc. Int. Open 2017, 5, E123–E129. [Google Scholar] [CrossRef]
- Koyama, Y.; Fukuzawa, M.; Aikawa, H.; Nemoto, D.; Muramatsu, T.; Matsumoto, T.; Uchida, K.; Madarame, A.; Morise, T.; Yamaguchi, H.; et al. Underwater endoscopic submucosal dissection for colorectal tumors decreases the incidence of post-electrocoagulation syndrome. J. Gastroenterol. Hepatol. 2023, 38, 1566–1575. [Google Scholar] [CrossRef]
- de Sire, R.; Capogreco, A.; Massimi, D.; Alfarone, L.; Brandaleone, L.; Vadalà, V.; Minini, F.; Marco, S.; Facciorusso, A.; Alkandari, A.; et al. Underwater endoscopic submucosal dissection for large non-pedunculated colorectal polyps. Gut 2025, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tribonias, G.; Komeda, Y.; Leontidis, N.; Anagnostopoulos, G.; Palatianou, M.; Mpellou, G.; Pantoula, P.; Manola, M.E.; Paspatis, G.; Tzouvala, M.; et al. Endoscopic intermuscular dissection (EID) for removing early rectal cancers and benign fibrotic rectal lesions. Tech. Coloproctology 2023, 27, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Ichimasa, K.; Kudo, S.E.; Misawa, M. Endoscopic intermuscular dissection for rectal cancer: Are we ready to skip surgery? Gut 2025, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Fléjou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef]
- Hurlstone, D.P. Histopathology using the Vienna criteria: Clinical decision making is still adequate. Gut 2004, 53, 1545. [Google Scholar] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Tsuji, Y.; Sakaguchi, Y.; Niimi, K.; Ono, S.; Kodashima, S.; Yamamichi, N.; Fujishiro, M.; Koike, K. Complications related to gastric endoscopic submucosal dissection and their managements. Clin. Endosc. 2014, 47, 398–403. [Google Scholar] [CrossRef]
- Facciorusso, A.; Straus Takahashi, M.; Eyileten Postula, C.; Buccino, V.R.; Muscatiello, N. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig. Liver Dis. 2019, 51, 1633–1640. [Google Scholar] [CrossRef]
- Fuccio, L.; Hassan, C.; Ponchon, T.; Mandolesi, D.; Farioli, A.; Cucchetti, A.; Frazzoni, L.; Bhandari, P.; Bellisario, C.; Bazzoli, F.; et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 86, 74–86.e17. [Google Scholar] [CrossRef]
- Nishizawa, T.; Yahagi, N. Endoscopic mucosal resection and endoscopic submucosal dissection: Technique and new directions. Curr. Opin. Gastroenterol. 2017, 33, 315–319. [Google Scholar] [CrossRef]
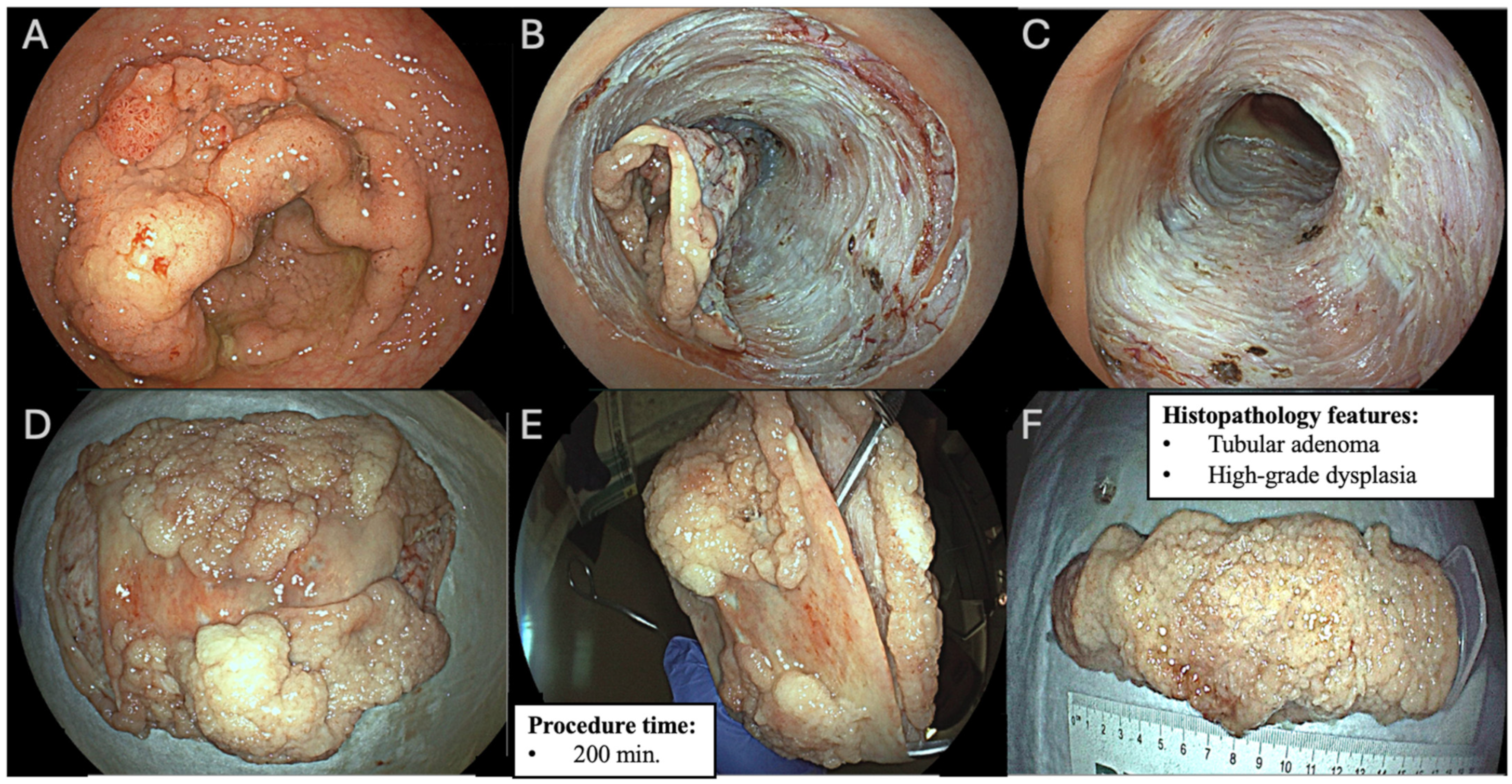
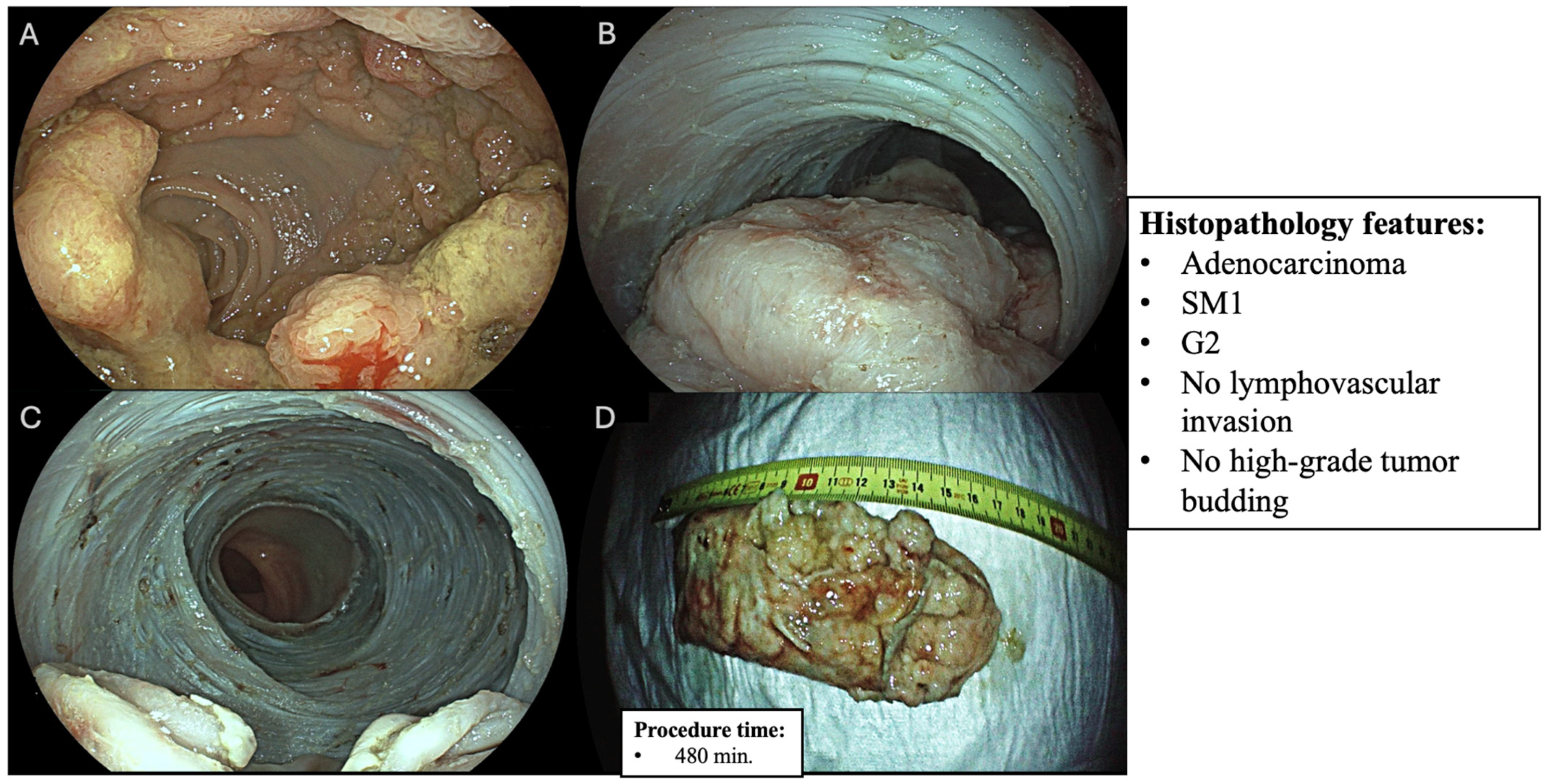
| Variables | n (%) |
|---|---|
| Demographics and clinical data | |
| Number of patients enrolled | 53 (100%) |
| Sex (females) | 26 (49.1%) |
| Mean age (years) | 70.6 ± 9.3 |
| Charlson Comorbidity Index (CCI) | |
| • CCI 0 | 2 (3.8%) |
| • CCI 1–2 | 13 (24.6%) |
| • CCI 3–5 | 37 (69.7%) |
| • CCI > 5 | 1 (1.9%) |
| ASA classification | |
| • ASA-I | 28 (52.8%) |
| • ASA-II | 0 (0%) |
| • ASA-III | 25 (47.2%) |
| Antithrombotic treatment | |
| • Anticoagulant | 6 (11.3%) |
| • Antiplatelet | 14 (26.4%) |
| • Both | 3 (5.6%) |
| Endoscopy: lesion assessment and procedural details | |
| Lesion location | |
| • Rectum | 36 (67.9%) |
| • Rectosigmoid | 3 (5.7%) |
| • Sigmoid | 1 (1.9%) |
| • Descending | 0 (0%) |
| • Transverse | 4 (7.5%) |
| • Ascending | 4 (7.5%) |
| • Caecum | 6 (11.3%) |
| Mean lesion size (mm) | 91.8 ± 25.3 |
| Paris classification | |
| • LST-GM | 48 (90.6%) |
| • LST-GH | 3 (5.7%) |
| • LST-NG-F | 2 (3.8%) |
| JNET classification | |
| • 2A | 17 (32.1%) |
| • 2B | 36 (67.9%) |
| Circumferential involvement | |
| • 90–99% involved | 20 (37.7%) |
| • 100% involved | 33 (62.3%) |
| Fibrosis | |
| • F1 | 14 (26.4%) |
| • F2 | 2 (3.8%) |
| ESD technique | |
| • Underwater | 21 (39.6%) |
| • Tunnel | 49 (92.5%) |
| • Intermuscular | 2 (3.8%) |
| General anesthesia with orotracheal intubation | 22 (41.5%) |
| En bloc resection | 51 (96.2%) |
| Median procedural time (min) | 160.0 (IQR 112.0–200.0) |
| Variables | n (%) |
|---|---|
| Histopathology evaluation | |
| Median lesion size at histology (mm) | 90.0 (IQR 75.0–110.0) |
| High-grade dysplasia | 25 (47.2%) |
| Adenocarcinoma diagnosis | 28 (52.8%) |
| In situ adenocarcinoma | 11 (39.3%) |
| Submucosal (SM) involvement | |
| • SM1 | 12 (42.9%) |
| • SM2 | 5 (17.8%) |
| Lymphovascular invasion | 2 (7.1%) |
| High-grade tumor budding | 7 (25.0%) |
| Grading | |
| • G1 | 13 (46.4%) |
| • G2 | 14 (50.0%) |
| • G3 | 1 (3.6%) |
| Oncologically curative resection (out of adenocarcinoma diagnosis) | 21 (75%) |
| Variables | Recurrence Patient 1 | Recurrence Patient 2 |
|---|---|---|
| Age | 69 | 61 |
| Sex | M | F |
| ASA | 3 | 1 |
| CCI | 4 | 2 |
| Time to recurrence | 14 months | 8 months |
| Lesion location | Transverse colon | Cecum |
| Lesion size | 90 | 65 |
| Morphology | LST-GM | LST-GM |
| JNET | 2B | 2B |
| Histology | In situ adenocarcinoma | In situ adenocarcinoma |
| Lymphovascular invasion | None | None |
| High-grade tumor budding | None | None |
| Grading | G1–G2 | G1–G2 |
| En bloc resection | Yes | Yes |
| R0 | Yes | Yes |
| Procedure time | 210 | 153 |
| Variables | Group 90% Circumferential Involvement (n = 20) | Group 100% Circumferential Involvement (n = 33) | p-Value |
|---|---|---|---|
| Age (years, mean ± std dev) | 69.9 ± 10.0 | 71 ± 9.1 | 0.682 |
| Gender (n females) | 9 (45%) | 17 (51.5%) | 0.859 |
| ASA class ≥ 3 (n) | 6 (30%) | 19 (57.6%) | 0.095 |
| Long axis size (mm) | 95.2 ± 36.8 | 91.9 ± 25.9 | 0.382 |
| Distal rectum involvement (n) | 6 (30%) | 16 (48.5%) | 0.300 |
| En bloc resection (n) | 1 (5%) | 1 (3%) | 1.000 |
| Procedure time (min, mean ± std dev) | 173.7 ± 93.1 | 156.2 ± 55.6 | 0.876 |
| Orotracheal intubation (n) | 10 (50%) | 12 (36.4%) | 0.490 |
| Adenocarcinoma (n) | 12 (60%) | 16 (48.5%) | 0.596 |
| High-grade tumor budding (n) | 3 (15%) | 4 (12.1%) | 1.000 |
| Lymphovascular invasion (n) | 2 (10%) | 0 (0%) | 0.138 |
| SM+ (n) | 7 (35%) | 10 (30.3%) | 0.959 |
| Recurrence (n) | 1 (5%) | 1 (3%) | 1.000 |
| Stricture development (n) | 5 (25%) | 5 (15.2%) | 0.475 |
| Variable | Stricture (n = 10) | No Stricture (n = 43) | p-Value |
|---|---|---|---|
| Demographics | |||
| Sex (females) | 6 (60%) | 21 (48.8%) | 0.7277 |
| Age (mean yrs ± STD) | 68.9 ± 9.9 | 71.0 ± 9.1 | 0.5332 |
| Endoscopic evaluation and procedural variables | |||
| Long axis size (mm) | 110 (90.5–127.5) | 90 (70–110) | 0.035 |
| Circumferential involvement • 90–100% involved • 100% involved | 5 (50%) 5 (50%) | 15 (34.9%) 28 (65.1%) | 0.475 |
| Morphology (Paris) • LST-GH • LST-GM • LST-NG-F | 0 (0%) 10 (100%) 0 (0%) | 3 (7.0%); 38 (88.4%) 2 (4.7%) | 0.57 |
| JNET class • 2A • 2B | 1 (10%) 9 (90%) | 16 (37.2%) 27 (62.8%) | 0.1405 |
| Fibrosis | 4 (40%) | 12 (27.9%) | 0.4667 |
| Orotracheal intubation | 9 (90%) | 13 (30.2%) | 0.0008 |
| Procedural time (min) | 206 (170.5–236) | 145 (105–182.5) | 0.0061 |
| Intra-procedural major bleeding | 5 (50%) | 5 (11.6%) | 0.0138 |
| Model Variables | Odds Ratio | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| Const | 0.001 | 1.77 × 10−8 | 18.496 | 0.158 |
| Age | 1.027 | 0.884 | 1.191 | 0.730 |
| ASA class | 0.341 | 0.064 | 1.803 | 0.205 |
| Procedural time | 1.017 | 0.995 | 1.038 | 0.119 |
| Long axis size | 1.008 | 0.966 | 1.050 | 0.726 |
| Circumferential involvement | 1.722 | 0.194 | 15.274 | 0.625 |
| Distal rectum involvement | 1.588 | 0.223 | 11.294 | 0.644 |
| Orotracheal intubation | 9.749 | 0.683 | 139.115 | 0.093 |
| Intra-procedural bleeding | 3.066 | 0.323 | 29.018 | 0.328 |
| Variables | n (%) |
|---|---|
| Intra-procedural, post-procedural, and delayed complications | |
| Intra-procedural major bleeding | 10 (18.9%) |
| Intra-procedural perforation | 3 (5.7%) |
| Post-procedural bleeding | 2 (3.8%) |
| Delayed bleeding | 1 (1.9%) |
| Post-procedural perforation | 0 (0%) |
| Delayed perforation | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrisani, G.; Brigida, M.; Antonelli, G.; Hassan, C.; Taffon, C.; D’Amico, A.; Gregorio, V.; Parente, G.; Cicala, M.; Facciorusso, A.; et al. Outcomes of Endoscopic Resection of Circumferential Colorectal Laterally Spreading Lesions: A Western Experience. Diagnostics 2025, 15, 2534. https://doi.org/10.3390/diagnostics15192534
Andrisani G, Brigida M, Antonelli G, Hassan C, Taffon C, D’Amico A, Gregorio V, Parente G, Cicala M, Facciorusso A, et al. Outcomes of Endoscopic Resection of Circumferential Colorectal Laterally Spreading Lesions: A Western Experience. Diagnostics. 2025; 15(19):2534. https://doi.org/10.3390/diagnostics15192534
Chicago/Turabian StyleAndrisani, Gianluca, Mattia Brigida, Giulio Antonelli, Cesare Hassan, Chiara Taffon, Andrea D’Amico, Virginia Gregorio, Giovanni Parente, Michele Cicala, Antonio Facciorusso, and et al. 2025. "Outcomes of Endoscopic Resection of Circumferential Colorectal Laterally Spreading Lesions: A Western Experience" Diagnostics 15, no. 19: 2534. https://doi.org/10.3390/diagnostics15192534
APA StyleAndrisani, G., Brigida, M., Antonelli, G., Hassan, C., Taffon, C., D’Amico, A., Gregorio, V., Parente, G., Cicala, M., Facciorusso, A., & Di Matteo, F. M. (2025). Outcomes of Endoscopic Resection of Circumferential Colorectal Laterally Spreading Lesions: A Western Experience. Diagnostics, 15(19), 2534. https://doi.org/10.3390/diagnostics15192534








