The Clinical Features and Prognosis of Idiopathic and Infection-Triggered Acute Exacerbation of Idiopathic Inflammatory Myopathy-Associated Interstitial Lung Disease: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Evaluation of HRCT Characteristics
2.4. Statistical Analyses
3. Results
3.1. Characteristics at AE Onset
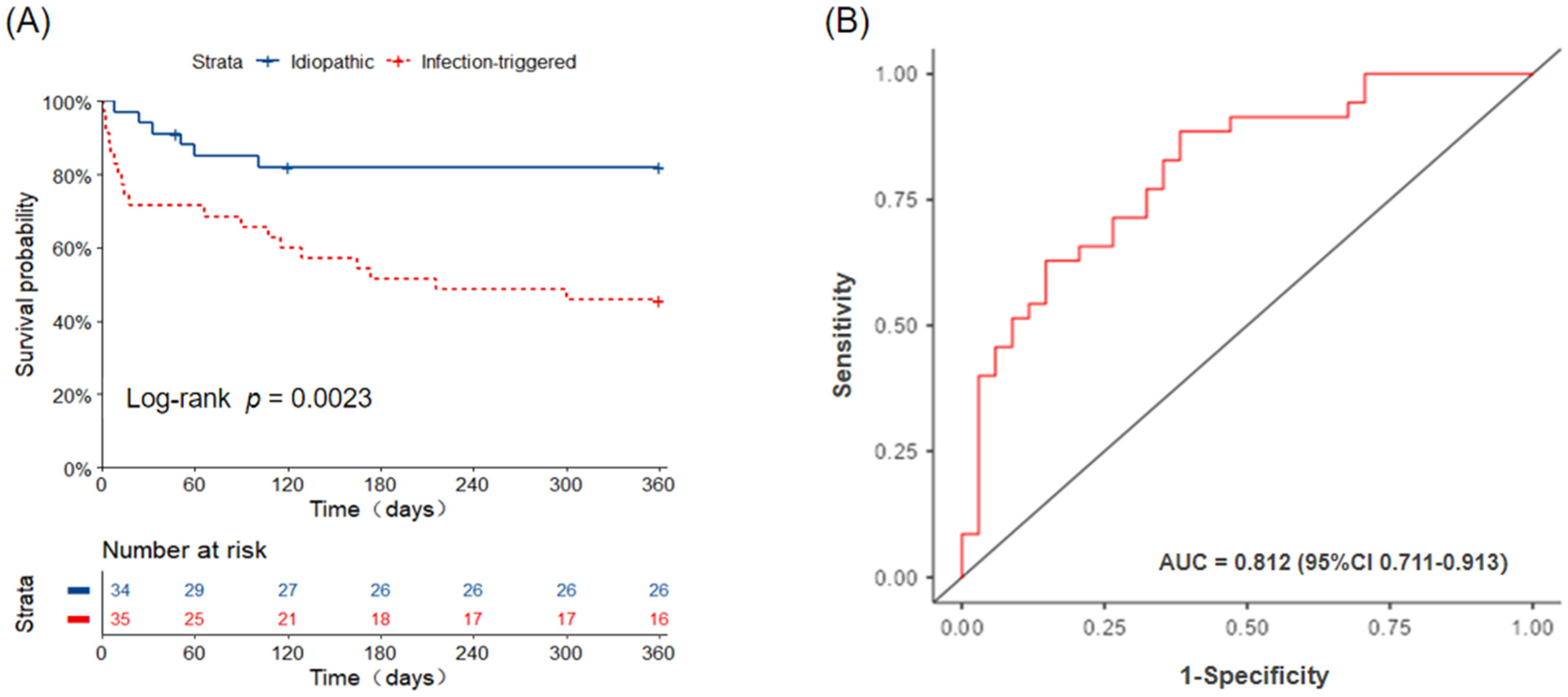
3.2. Mortality
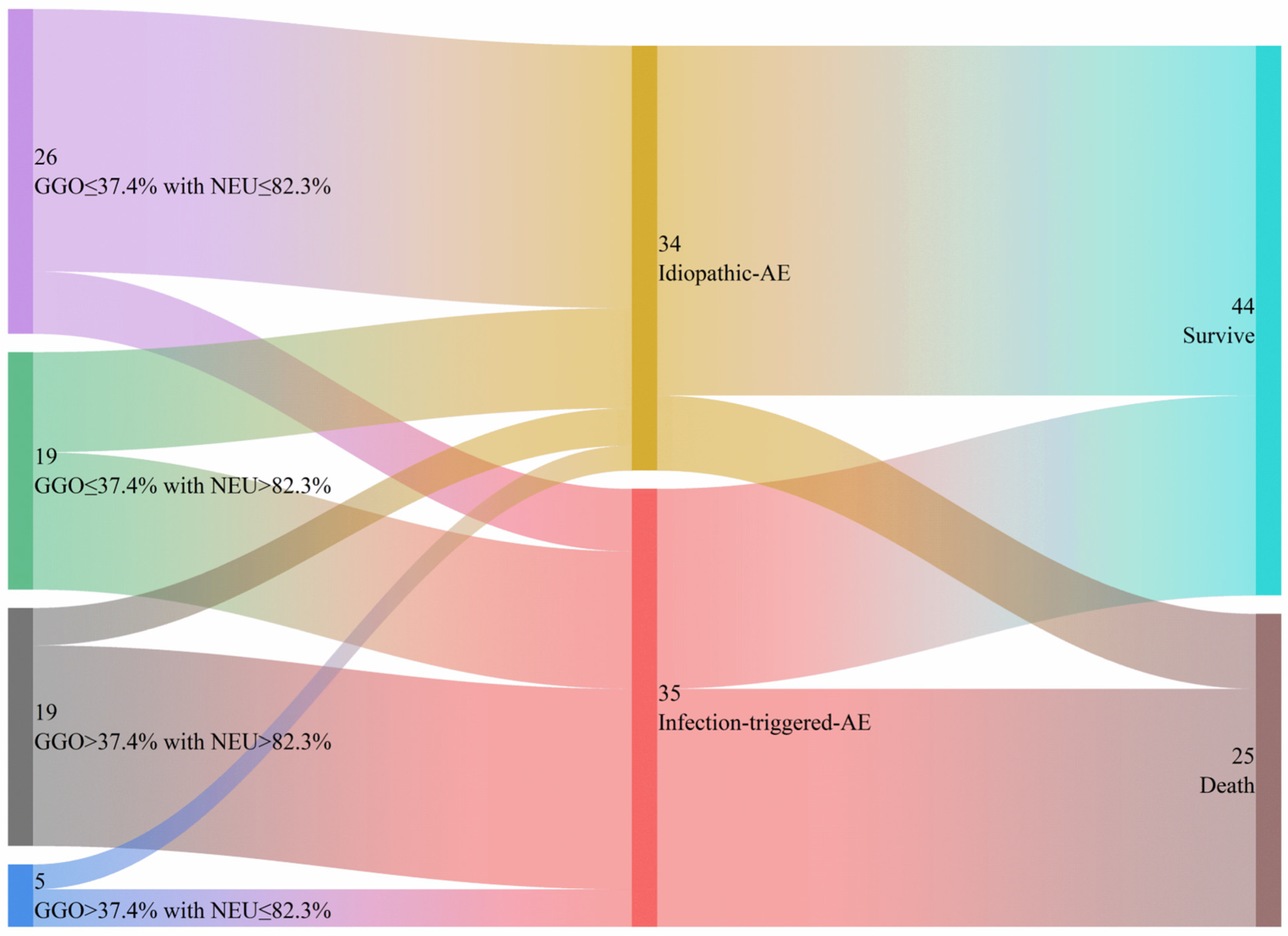
3.3. Factors for Differentiation Between I-AE and iT-AE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Acute exacerbation |
| IIMs | idiopathic inflammatory myopathies |
| ILD | Interstitial lung disease |
| HRCT | High-resolution computed tomography |
| GGO | Ground glass opacity |
| NEU | Neutrophil percentage |
Appendix A
| HRCT Findings | Average Extents a | Interobserver Agreement d | Interobserver Correlation e | ||
|---|---|---|---|---|---|
| ICC Value | p Value | Spearman r | p Value | ||
| GGO | 27.37 [18.75, 43.17] | 0.986 | <0.001 | 0.978 | <0.001 |
| Reticulation | 10.08 [4.67, 18.00] | 0.959 | <0.001 | 0.948 | <0.001 |
| Honeycombing b | 0.00 [0.00, 0.00] | 0.996 | <0.001 | 1.000 | <0.001 |
| Consolidation | 10.62 [2.08, 17.50] | 0.986 | <0.001 | 0.986 | <0.001 |
| Emphysema c | 0.00 [0.00, 0.00] | 0.911 | <0.001 | 1.000 | <0.001 |
References
- Hallowell, R.W.; Paik, J.J. Myositis-associated interstitial lung disease: A comprehensive approach to diagnosis and management. Clin. Exp. Rheumatol. 2022, 40, 373–383. [Google Scholar] [CrossRef]
- Alhamad, E.H.; Cal, J.G.; Alrajhi, N.N.; AlBoukai, A.A. Acute exacerbation in interstitial lung disease. Ann. Thorac. Med. 2021, 16, 178–187. [Google Scholar] [CrossRef]
- Kamiya, H.; Panlaqui, O.M. A systematic review of the incidence, risk factors and prognosis of acute exacerbation of systemic autoimmune disease-associated interstitial lung disease. BMC Pulm. Med. 2021, 21, 150. [Google Scholar] [CrossRef]
- Luppi, F.; Sebastiani, M.; Salvarani, C.; Bendstrup, E.; Manfredi, A. Acute exacerbation of interstitial lung disease associated with rheumatic disease. Nat. Rev. Rheumatol. 2022, 18, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Moore, B.B.; Flaherty, K.R.; Brown, K.K.; Kaner, R.J.; King, T.E., Jr.; Lasky, J.A.; Loyd, J.E.; Noth, I.; Olman, M.A.; et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Azadeh, N.; Limper, A.H.; Carmona, E.M.; Ryu, J.H. The Role of Infection in Interstitial Lung Diseases: A Review. Chest 2017, 152, 842–852. [Google Scholar] [CrossRef]
- Molyneaux, P.L.; Cox, M.J.; Wells, A.U.; Kim, H.C.; Ji, W.; Cookson, W.O.; Moffatt, M.F.; Kim, D.S.; Maher, T.M. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir. Res. 2017, 18, 29. [Google Scholar] [CrossRef]
- Moghoofei, M.; Mostafaei, S.; Kondori, N.; Armstrong, M.E.; Babaei, F. Bacterial and viral coinfection in idiopathic pulmonary fibrosis patients: The prevalence and possible role in disease progression. BMC Pulm. Med. 2022, 22, 60. [Google Scholar] [CrossRef]
- Yamazoe, M.; Tomioka, H. Acute exacerbation of idiopathic pulmonary fibrosis: A 10-year single-centre retrospective study. BMJ Open Respir. Res. 2018, 5, e000342. [Google Scholar] [CrossRef]
- Izuka, S.; Yamashita, H.; Iba, A.; Takahashi, Y.; Kaneko, H. Acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: Clinical features and prognosis. Rheumatology 2021, 60, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, J.; Yoshizawa, S.; Kudo, K.; Osoreda, H.; Ishimatsu, A.; Taguchi, K.; Moriwaki, A.; Wakamatsu, K.; Iwanaga, T.; Yoshida, M. Clinical features of acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: Comparison with idiopathic pulmonary fibrosis. Respir. Med. 2022, 200, 106898. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cao, H.; Ke, Y.; Sun, C.; Chen, W.; Lin, J. Acute Exacerbation of Interstitial Lung Disease in Adult Patients With Idiopathic Inflammatory Myopathies: A Retrospective Case-Control Study. Front. Med. 2020, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huan, C.; Wang, Q.; Xu, G.; Lin, J.; Zhou, J. Predicting Survival Across Acute Exacerbation of Interstitial Lung Disease in Patients with Idiopathic Inflammatory Myositis: The GAP-ILD Model. Rheumatol. Ther. 2020, 7, 967–978. [Google Scholar] [CrossRef]
- Lee, H.; Chung, S.J.; Kim, S.H.; Choi, H.; Kim, Y.; Park, T.S.; Park, D.W.; Moon, J.Y.; Kim, S.H.; Kim, T.H.; et al. Treatment Outcomes of Infectious and Non-infectious Acute Exacerbation of Myositis-Related Interstitial Lung Disease. Front. Med. 2021, 8, 801206. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef]
- Malaviya, A.N. 2017 EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: Little emphasis on autoantibodies, why? Ann. Rheum. Dis. 2018, 77, e77. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Sverzellati, N.; Lynch, D.A.; Hansell, D.M.; Johkoh, T.; King, T.E., Jr.; Travis, W.D. American Thoracic Society-European Respiratory Society Classification of the Idiopathic Interstitial Pneumonias: Advances in Knowledge since 2002. Radiographics 2015, 35, 1849–1871. [Google Scholar] [CrossRef]
- Kadoch, M.A.; Cham, M.D.; Beasley, M.B.; Ward, T.J.; Jacobi, A.H.; Eber, C.D.; Padilla, M.L. Idiopathic interstitial pneumonias: A radiology-pathology correlation based on the revised 2013 American Thoracic Society-European Respiratory Society classification system. Curr. Probl. Diagn. Radiol. 2015, 44, 15–25. [Google Scholar] [CrossRef]
- Kishaba, T.; Nei, Y.; Momose, M.; Nagano, H.; Yamashiro, S. Clinical Characteristics Based on the New Criteria of Acute Exacerbation in Patients with Idiopathic Pulmonary Fibrosis. Eurasian J. Med. 2018, 50, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, L.; Han, T.; Tong, J.; Ren, J.; Pu, J.; Zhang, M.; Guo, Y.; Jin, C. HRCT findings predict 1-year mortality in patients with acute exacerbation of idiopathic inflammatory myopathies-associated interstitial lung disease. Heliyon 2024, 10, e31510. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Muller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Sverzellati, N.; Devaraj, A.; Keir, G.J.; Wells, A.U.; Hansell, D.M. Connective tissue disease related fibrotic lung disease: High resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax 2014, 69, 216–222. [Google Scholar] [CrossRef]
- Popovic, Z.B.; Thomas, J.D. Assessing observer variability: A user’s guide. Cardiovasc. Diagn. Ther. 2017, 7, 317–324. [Google Scholar] [CrossRef]
- Bai, Z.; Shen, G.; Dong, L. Analysis of risk factors of interstitial lung disease and mortality rates in Chinese patients with idiopathic inflammatory myopathy. Int. J. Rheum. Dis. 2021, 24, 815–827. [Google Scholar] [CrossRef]
- Jang, H.J.; Yong, S.H.; Leem, A.Y.; Lee, S.H.; Kim, S.Y.; Lee, S.H.; Kim, E.Y.; Chung, K.S.; Jung, J.Y.; Kang, Y.A.; et al. Corticosteroid responsiveness in patients with acute exacerbation of interstitial lung disease admitted to the emergency department. Sci. Rep. 2021, 11, 5762. [Google Scholar] [CrossRef]
- Kato, M.; Yamada, T.; Kataoka, S.; Arai, Y.; Miura, K.; Ochi, Y.; Ihara, H.; Koyama, R.; Sasaki, S.; Takahashi, K. Prognostic differences among patients with idiopathic interstitial pneumonias with acute exacerbation of varying pathogenesis: A retrospective study. Respir. Res. 2019, 20, 287. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sapra, L.; Saini, C.; Azam, Z.; Mishra, P.K.; Verma, B.; Mishra, G.C.; Srivastava, R.K. COVID-19: Immunology, Immunopathogenesis and Potential Therapies. Int. Rev. Immunol. 2022, 41, 171–206. [Google Scholar] [CrossRef]
- Kebbe, J.; Abdo, T. Interstitial lung disease: The diagnostic role of bronchoscopy. J. Thorac. Dis. 2017, 9 (Suppl. 10), S996–S1010. [Google Scholar] [CrossRef]
- Ba, C.; Wang, H.; Jiang, C.; Shi, X.; Jin, J.; Fang, Q. Clinical manifestations and prognostic factors analysis of patients hospitalised with acute exacerbation of idiopathic pulmonary fibrosis and other interstitial lung diseases. BMJ Open Respir. Res. 2024, 11, e001997. [Google Scholar] [CrossRef]

| Characteristics | Total (n = 69) | Idiopathic (n = 34) | Infection-Triggered (n = 35) | p Values |
|---|---|---|---|---|
| Sex (female), n (%) | 55(79.7) | 25(73.5) | 30(85.7) | 0.244 |
| Age at AE, y | 50.7(10.0) | 50.9(9.56) | 50.4(10.4) | 0.823 |
| BMI(Kg/m2) | 21.4(3.4) | 21.6(3.0) | 21.3(3.7) | 0.768 |
| SBP (mmHg) | 115.8(17.4) | 117(15.5) | 115(19.2) | 0.618 |
| DBP (mmHg) | 74.5(9.9) | 75.2(10.3) | 73.8(9.58) | 0.559 |
| Pulse | 102(19.7) | 96.1(17.5) | 108(20.3) | 0.013 |
| Body temperature (℃) (M, IQR) | 36.7(1.6) | 36.5(0.8) | 37.5(1.6) | 0.037 |
| IIM subtypes, n (%) | 0.799 * | |||
| DM | 50(72.5) | 24(70.6) | 26(74.3) | |
| PM | 11(15.9) | 5(14.7) | 6(17.1) | |
| Overlap in IIM | 8(11.6) | 5(14.7) | 3(8.6) | |
| Autoantibody #, n (%) | 0.213 * | |||
| Anti-ARS | 18(26.1) | 11(32.4) | 7(20.0) | |
| Anti-Jo-1 | 13(18.8) | 6(17.6) | 7(20.0) | |
| Anti-EJ | 1(1.4) | 1(2.9) | 0(0) | |
| Anti-PL-7 | 4(5.8) | 4(11.8) | 0(0) | |
| Anti-MDA5 | 10(14.5) | 2(5.9) | 8(22.9) | |
| Anti-Ro52 | 20(29) | 9(26.5) | 11(31.4) | |
| IIM disease duration, m, (M, IQR) | 8(15.7) | 9(15.9) | 8(16.4) | 0.564 |
| Smoking, n (%) | 1.00 * | |||
| Ever-smokers | 9(13.0) | 4(11.8) | 5(14.3) | |
| Never-smokers | 60(87.0) | 30(88.2) | 30(85.7) | |
| Comorbidity, n (%) | 55(79.9) | 26(76.5) | 29(82.9) | 0.561 |
| Treatments before AE, n (%) | ||||
| Maintenance CS therapy | 53(76.8) | 26(76.4) | 27(77.1) | 0.947 |
| Cyclophosphamide | 21(30.4) | 10(29.4) | 11(31.4) | 0.733 |
| Cyclosporin | 4(5.8) | 1(2.9) | 3(8.6) | 1.00 * |
| Mycophenolate mofetil | 7(10.1) | 2(5.9) | 5(14.3) | 0.71 * |
| Hydroxychloroquine | 18(26.1) | 10(29.4) | 8(22.6) | 0.535 |
| Thalidomide | 10(14.5) | 6(17.6) | 4(11.4) | 0.513 * |
| Tofacitinib | 2(2.9) | 1(2.9) | 1(2.9) | 1.00 * |
| Anti-fibrotic therapy | 4(5.8) | 3(11.7) | 1(2.9) | 0.356 * |
| Laboratory data (M, IQR) | ||||
| WBCs (109/L) | 7.8(5.7) | 7.1(5.9) | 8.5(6.4) | 0.071 |
| NEU | 83.1(14.7) | 79.2(18.9) | 87.6(10.1) | <0.001 |
| HGB (g/dL) | 120(18.6) | 125(17.4) | 115(18.7) | 0.029 |
| PLTs (×104/µL) | 230.3(96.5) | 241(117) | 220(71.1) | 0.361 |
| CRP (mg/dL) | 22(45.0) | 10.7(31.8) | 30.9(77.9) | 0.029 |
| ESR (mm/h) | 33(44.5) | 28(37.3) | 49(48) | 0.015 |
| CK(U/L) | 79(579.3) | 67(734.6) | 182(524.5) | 0.384 |
| LDH (U/L) | 443(331) | 353(176.8) | 594(402.4) | <0.001 |
| HBDH (U/L) | 344(210.5) | 289(126.8) | 428(338) | <0.001 |
| PaO2/FiO2 ratio | 218.6(94) | 249(81.7) | 189(96.6) | 0.008 |
| HRCT features, %, (M, IQR) | ||||
| GGO | 27.4(25) | 20.6(22.1) | 38.1(28.8) | 0.001 |
| Reticulation | 10(13.4) | 10.2(15) | 10.1(12.4) | 0.838 |
| Honeycombing | 0(0) | 0(0) | 0(0) | 0.293 |
| Consolidation | 10.6(15.6) | 7.8(16.7) | 11(14.5) | 0.592 |
| Emphysema | 0(0) | 0(0) | 0(0) | 0.647 |
| Treatments after AE, n (%) | ||||
| CS pulse therapy | 22(31.9) | 12(35.3) | 10(28.6) | 0.549 |
| Cyclophosphamide | 19(27.5) | 13(38.2) | 6(17.1) | 0.05 |
| Cyclosporine | 4(5.8) | 3(8.8) | 1(2.9) | 0.356 * |
| Mycophenolate mofetil | 6(8.7) | 4(11.8) | 2(5.7) | 0.428 * |
| Hydroxychloroquine | 10(14.5) | 5(14.7) | 5(14.3) | 1.00 * |
| Thalidomide | 3(4.3) | 2(5.9) | 1(2.9) | 0.538 |
| Tofacitinib | 3(4.3) | 1(2.9) | 2(5.7) | 1.00 * |
| Tocilizumab | 2(2.9) | 0(0) | 2(5.7) | 0.493 * |
| IVIG | 25(36.2) | 10(29.4) | 15(42.9) | 0.245 |
| Antimicrobial therapy | 65(94.2) | 31(91.2) | 34(97.1) | 0.289 |
| Mechanical ventilation | 13(18.8) | 4(11.8) | 9(25.7) | 0.138 |
| Status(death), n (%) | 25(36.2) | 6(17.6) | 19(54.3) | 0.002 |
| Variates | Per Unit | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| HGB | 1 g/dL | 0.97 | 0.94–1.00 | 0.035 | 0.97 | 0.93–1.02 | 0.223 |
| NEU | 1% | 1.11 | 1.04–1.18 | 0.001 | 1.10 | 1.01–1.20 | 0.03 |
| ESR | 1 mm/h | 1.02 | 1.00–1.04 | 0.024 | 1.00 | 0.97–1.03 | 0.861 |
| LDH | 1 U/L | 1.00 | 1.00–1.01 | 0.002 | 1.01 | 0.97–1.01 | 0.265 |
| HDBH | 1 U/L | 1.01 | 1.00–1.01 | 0.002 | 1.00 | 0.98–1.01 | 0.551 |
| PaO2/FiO2 | 1 mmHg | 0.99 | 0.99–1.00 | 0.01 | 1.00 | 1.00–1.01 | 0.769 |
| GGO | 10% | 1.59 | 1.17–2.16 | 0.003 | 1.53 | 1.02–2.30 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, K.; Mo, L.; He, L.; Tong, J.; Hei, H.; Zhang, Y.; Sheng, Y.; Kondowe, B.; Jin, C. The Clinical Features and Prognosis of Idiopathic and Infection-Triggered Acute Exacerbation of Idiopathic Inflammatory Myopathy-Associated Interstitial Lung Disease: A Preliminary Study. Diagnostics 2025, 15, 2516. https://doi.org/10.3390/diagnostics15192516
Zhang J, Yang K, Mo L, He L, Tong J, Hei H, Zhang Y, Sheng Y, Kondowe B, Jin C. The Clinical Features and Prognosis of Idiopathic and Infection-Triggered Acute Exacerbation of Idiopathic Inflammatory Myopathy-Associated Interstitial Lung Disease: A Preliminary Study. Diagnostics. 2025; 15(19):2516. https://doi.org/10.3390/diagnostics15192516
Chicago/Turabian StyleZhang, Jingping, Kai Yang, Lingfei Mo, Liyu He, Jiayin Tong, He Hei, Yuting Zhang, Yadan Sheng, Blessed Kondowe, and Chenwang Jin. 2025. "The Clinical Features and Prognosis of Idiopathic and Infection-Triggered Acute Exacerbation of Idiopathic Inflammatory Myopathy-Associated Interstitial Lung Disease: A Preliminary Study" Diagnostics 15, no. 19: 2516. https://doi.org/10.3390/diagnostics15192516
APA StyleZhang, J., Yang, K., Mo, L., He, L., Tong, J., Hei, H., Zhang, Y., Sheng, Y., Kondowe, B., & Jin, C. (2025). The Clinical Features and Prognosis of Idiopathic and Infection-Triggered Acute Exacerbation of Idiopathic Inflammatory Myopathy-Associated Interstitial Lung Disease: A Preliminary Study. Diagnostics, 15(19), 2516. https://doi.org/10.3390/diagnostics15192516







