Echoes from Within: Mapping Gastrointestinal Obstruction with Ultrasound
Abstract
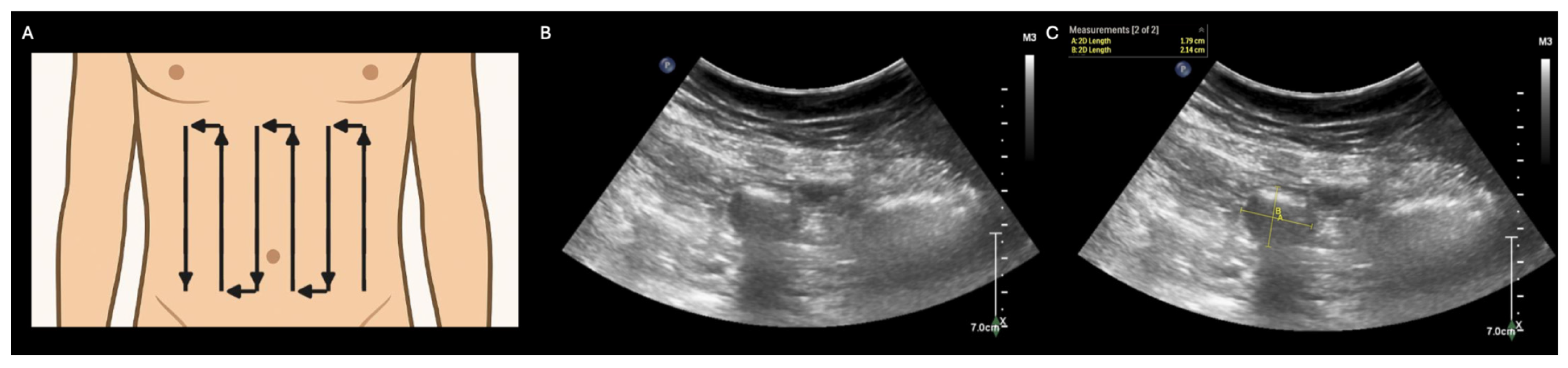

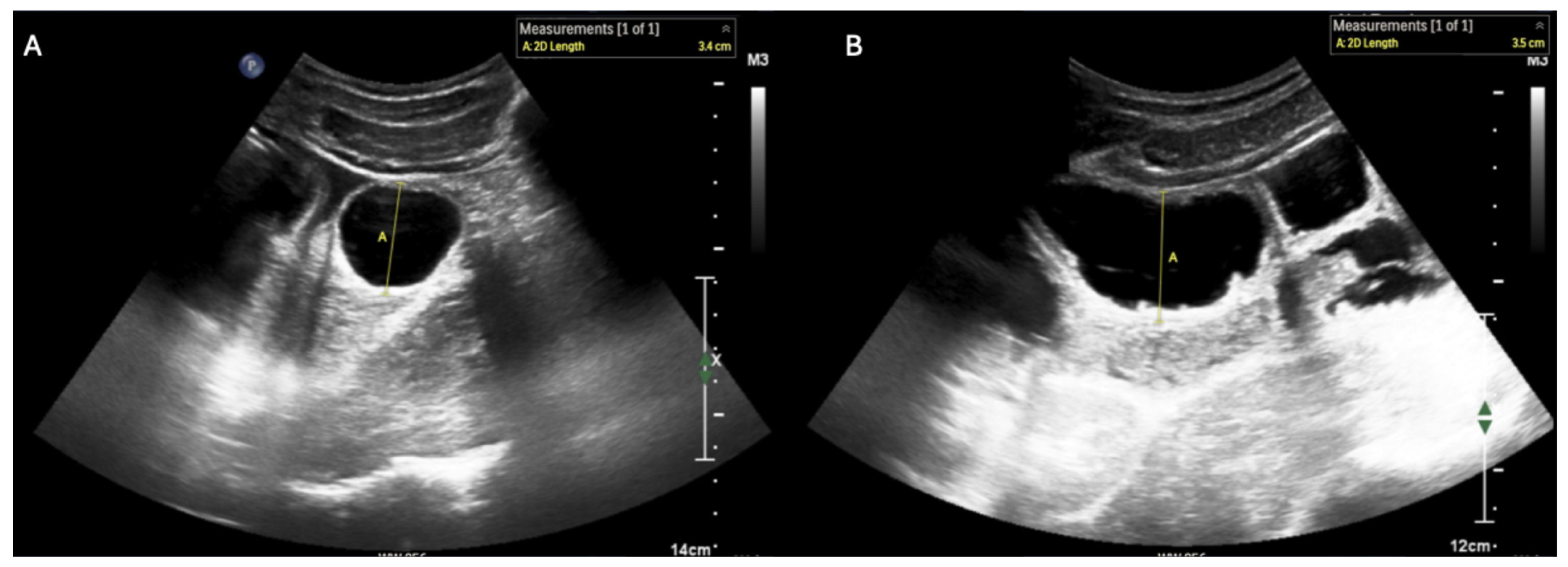
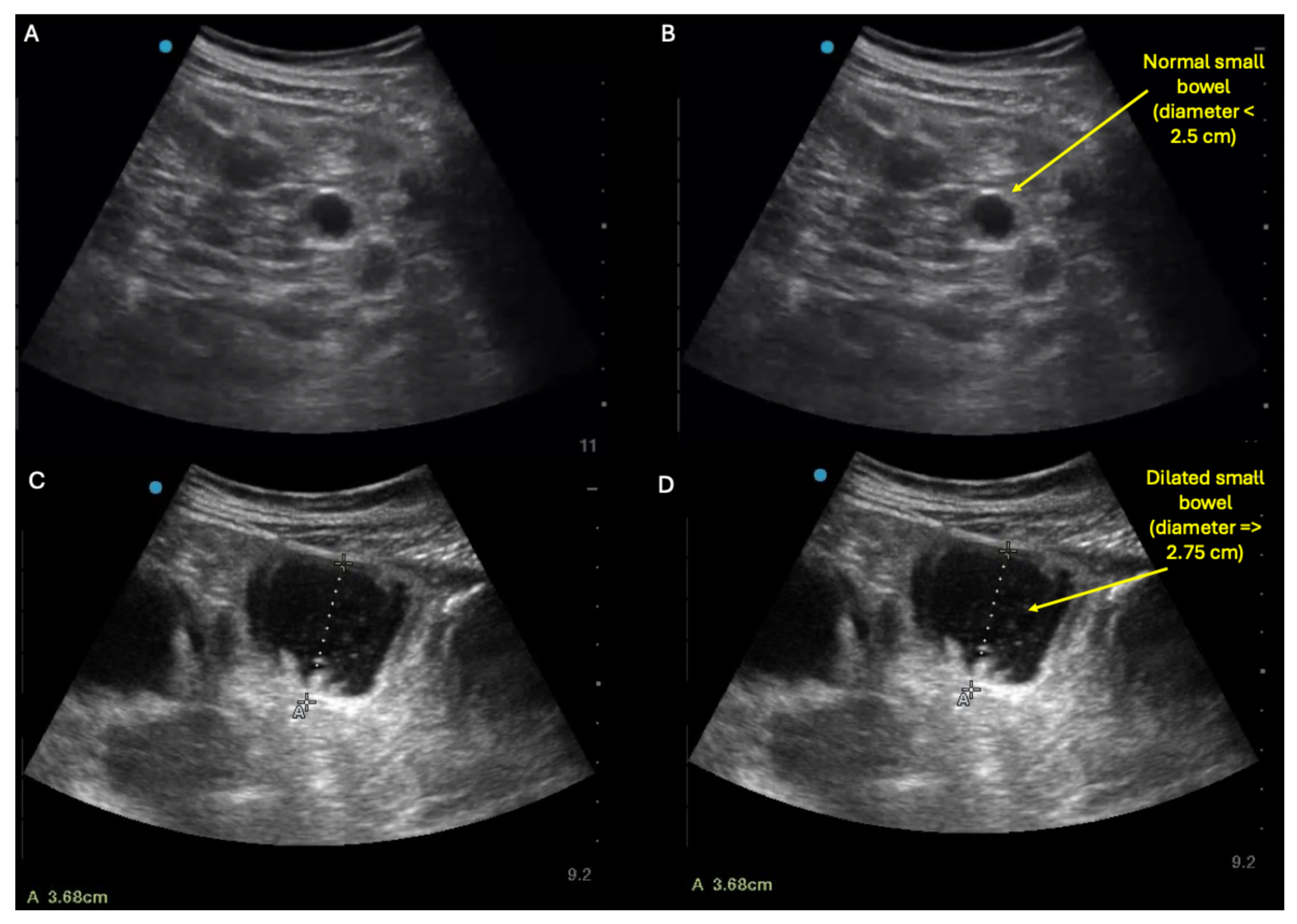
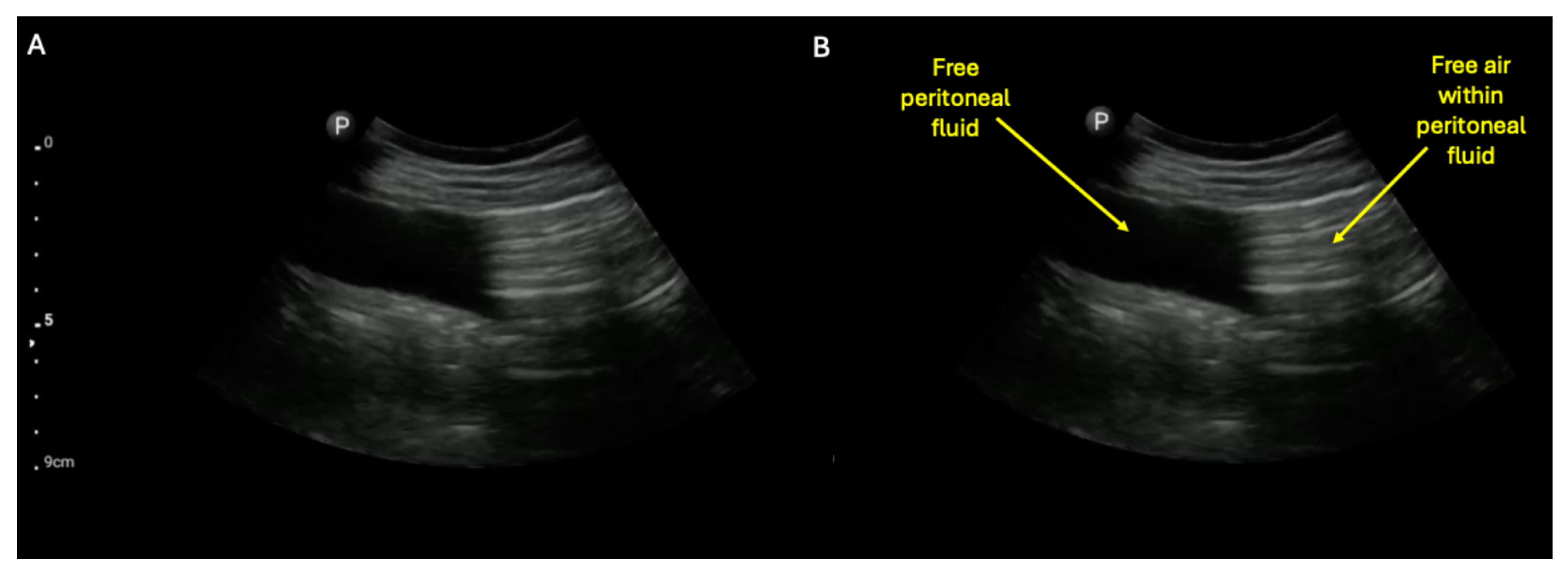

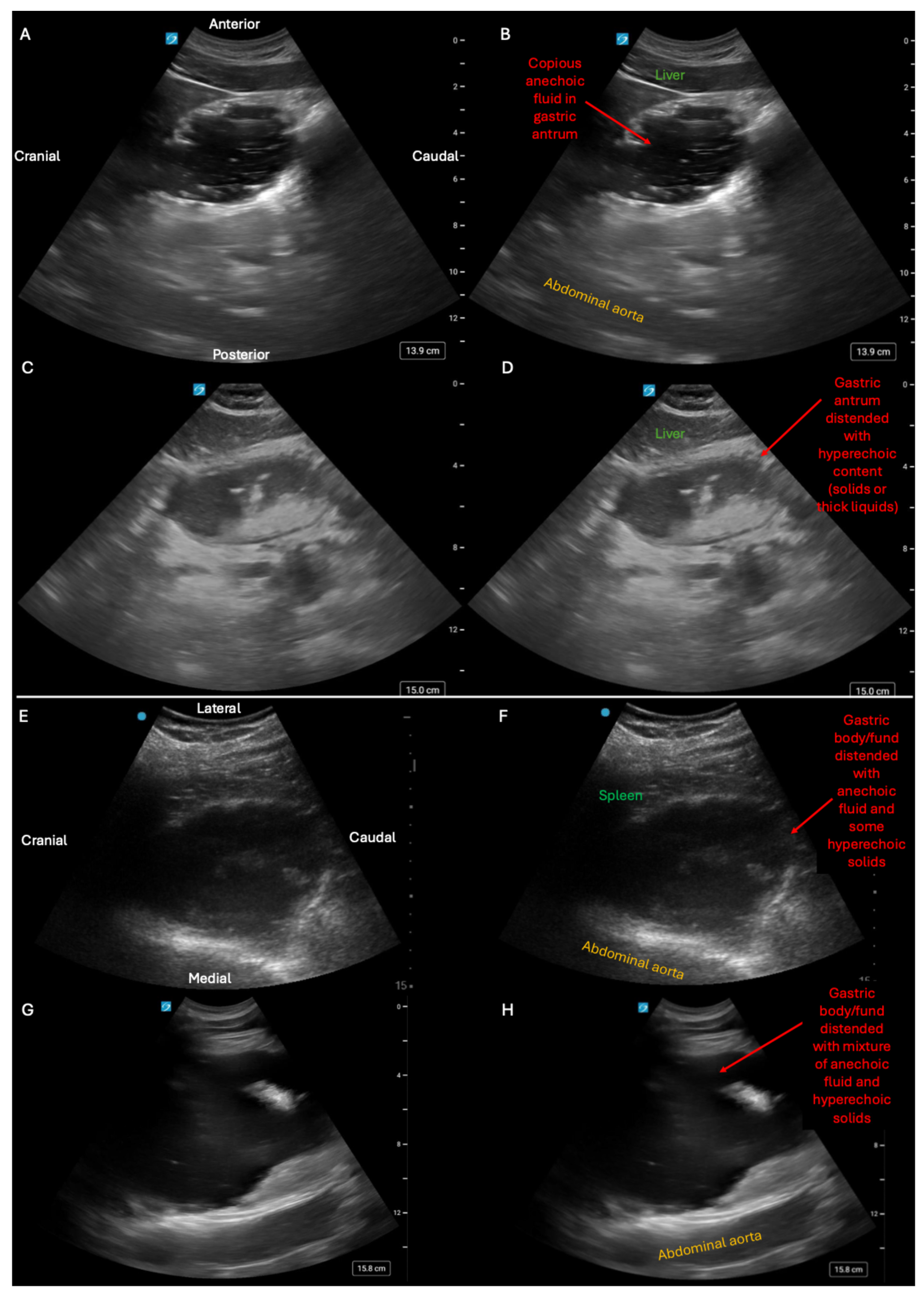
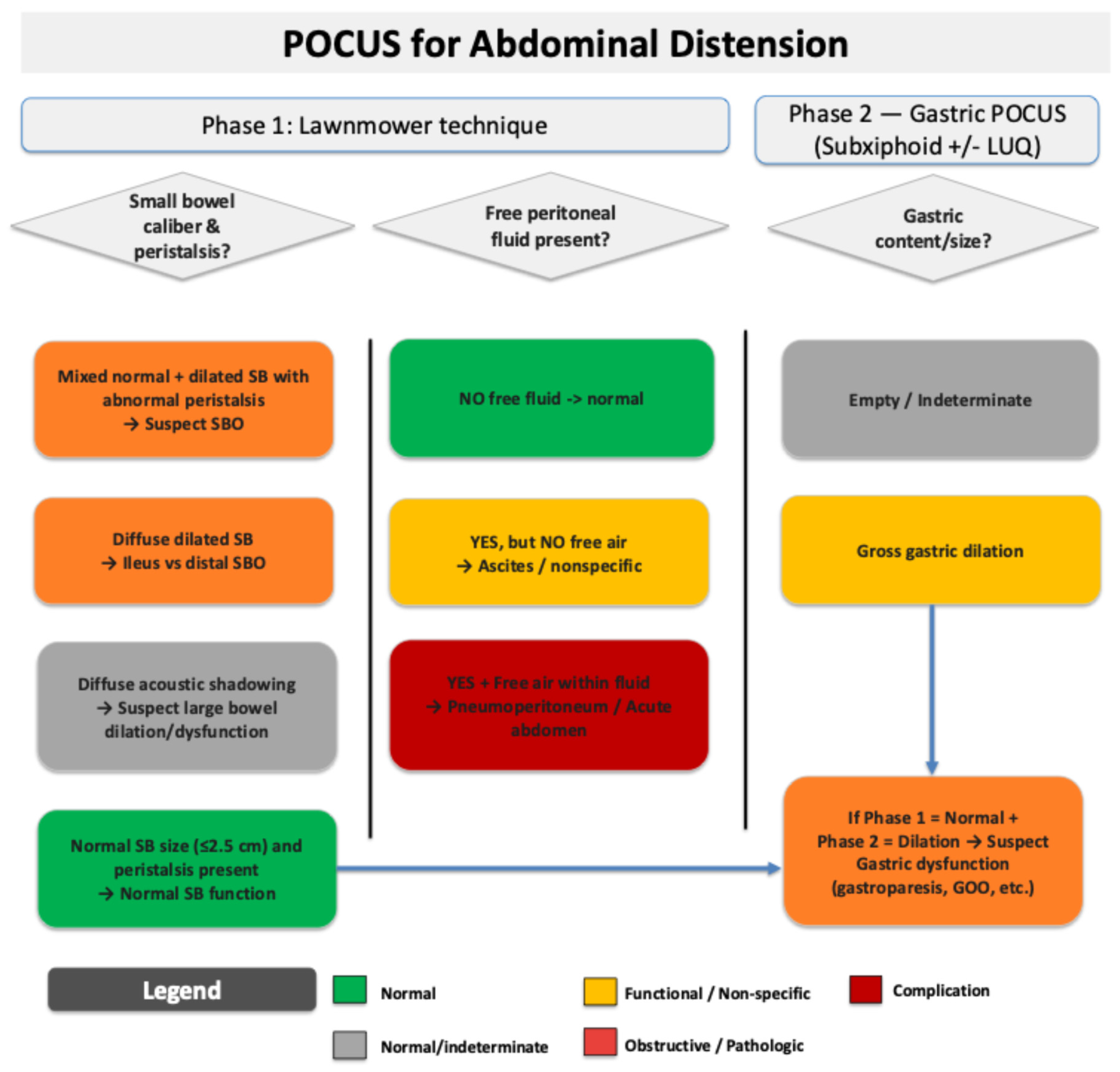
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peery, A.F.; Murphy, C.C.; Anderson, C.; Jensen, E.T.; Deutsch-Link, S.; Egberg, M.D.; Lund, J.L.; Subramaniam, D.; Dellon, E.S.; Sperber, A.D.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2024. Gastroenterology 2025, 168, 1000–1024. [Google Scholar] [CrossRef]
- Cartwright, S.L.; Knudson, M.P. Evaluation of acute abdominal pain in adults. Am. Fam. Physician 2008, 77, 971–978. [Google Scholar]
- Chernock, B.; Hwang, F.; Berlin, A.; Pentakota, S.R.; Singh, R.; Singh, R.; Mosenthal, A.C. Emergency abdominal surgery in patients presenting from skilled nursing facilities: Opportunities for palliative care. Am. J. Surg. 2020, 219, 1076–1082. [Google Scholar] [CrossRef]
- Kopitnik, N.L.; Kashyap, S.; Dominique, E. Acute Abdomen. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Abramson, L.; Olive, J.K.; Lefler, B.; Wu, L.; Bowman, A.; Simpson, T.; Vatsaas, C.; Yanamadala, M.; Bronshteyn, Y.S. Point-of-care Ultrasound to Screen for Gastrointestinal Dysfunction: Image Acquisition and Interpretation. J. Vis. Exp. 2025, e68603. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Chu, P.W.; Azman Firdaus, H.; Stewart, C.; Malekhedayat, M.; Alber, S.; Bolch, W.E.; Mahendra, M.; Berrington de González, A.; Miglioretti, D.L. Projected Lifetime Cancer Risks From Current Computed Tomography Imaging. JAMA Intern. Med. 2025, 185, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Alber, S.A.; Kwan, M.L.; Pequeno, P.; Bolch, W.E.; Bowles, E.J.A.; Greenlee, R.T.; Stout, N.K.; Weinmann, S.; Moy, L.M.; et al. Medical Imaging and Pediatric and Adolescent Hematologic Cancer Risk. N. Engl. J. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, H.; Mayes, K.D.; Peksa, G.D.; Loesche, M.A.; Becker, B.A.; Boniface, K.S.; Lahham, S.; Jang, T.B.; Secko, M.; Gottlieb, M. Multi-center analysis of point-of-care ultrasound for small bowel obstruction: A systematic review and individual patient-level meta-analysis. Am. J. Emerg. Med. 2023, 70, 144–150. [Google Scholar] [CrossRef]
- Haskins, S.C.; Bronshteyn, Y.S.; Ledbetter, L.; Arzola, C.; Kalagara, H.; Hardman, D.; Panzer, O.; Weber, M.M.; Heinz, E.R.; Boublik, J.; et al. ASRA pain medicine narrative review and expert practice recommendations for gastric point-of-care ultrasound to assess aspiration risk in medically complex patients undergoing regional anesthesia and pain procedures. Reg. Anesth. Pain Med. 2025. [Google Scholar] [CrossRef]
- Grassi, R.; Romano, S.; D’Amario, F.; Giorgio Rossi, A.; Romano, L.; Pinto, F.; Di Mizio, R. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur. J. Radiol. 2004, 50, 5–14. [Google Scholar] [CrossRef]
- Rosano, N.; Gallo, L.; Mercogliano, G.; Quassone, P.; Picascia, O.; Catalano, M.; Pesce, A.; Fiorini, V.; Pelella, I.; Vespere, G.; et al. Ultrasound of Small Bowel Obstruction: A Pictorial Review. Diagnostics 2021, 11, 617. [Google Scholar] [CrossRef]
- Tamburrini, S.; Serra, N.; Lugara, M.; Mercogliano, G.; Liguori, C.; Toro, G.; Somma, F.; Mandato, Y.; Guerra, M.V.; Sarti, G.; et al. Ultrasound Signs in the Diagnosis and Staging of Small Bowel Obstruction. Diagnostics 2020, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, H.; Al Jalbout, N.; Peksa, G.D.; Mayes, K.D.; Becker, B.A.; Boniface, K.S.; Lahham, S.; Secko, M.; Chavoshzadeh, M.; Jang, T.; et al. Optimal bowel diameter thresholds for diagnosing small bowel obstruction and surgical intervention with point-of-care ultrasound. Am. J. Emerg. Med. 2024, 84, 1–6. [Google Scholar] [CrossRef]
- Khor, M.; Cutten, J.; Lim, J.; Weerakkody, Y. Sonographic detection of pneumoperitoneum. BJR Case Rep. 2017, 3, 20160146. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.S.S.; Bryant, R.V.; Dong, Y.; Maaser, C.; Kucharzik, T.; Maconi, G.; Asthana, A.K.; Blaivas, M.; Goudie, A.; Gilja, O.H.; et al. How to perform gastrointestinal ultrasound: Anatomy and normal findings. World J. Gastroenterol. 2017, 23, 6931–6941. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Imai, S.; Hosotani, R.; Aoyama, H.; Hayashi, M.; Ishikawa, T. Abdominal sonography for the diagnosis of large bowel obstruction. Surg. Today 1994, 24, 791–794. [Google Scholar] [CrossRef]
- Huang, D.; Al-Bassam, M.; Leon, L.N.; Ganti, L. Emergency Department Point-of-Care Ultrasound Diagnosis of a Large Bowel Obstruction Due to Metastatic Rectal Cancer: A Case Report. Cureus 2022, 14, e28817. [Google Scholar] [CrossRef]
- Li, R.T.; Zhao, Y.; Zou, X.J.; Shu, H.Q.; Zhou, T.; Pan, S.W.; Gao, X.H.; Huang, H.Y.; Liu, H.; Shang, Y. Overview of point-of-care ultrasound in diagnosing intestinal obstruction. World J. Emerg. Med. 2022, 13, 135–140. [Google Scholar] [CrossRef]
- Di Gioia, C.C.; Alame, A.; Orso, D. The Impact of Point-of-Care Ultrasound on the Diagnosis and Management of Small Bowel Obstruction in the Emergency Department: A Retrospective Observational Single-Center Study. Medicina 2024, 60, 2006. [Google Scholar] [CrossRef]
- Goder, N.; Gabay, S.; Tome, J.; Nizri, E.; Lichter, Y.; Zemel, M. Point of care ultrasound of small intestine in patients undergoing laparoscopic bowel surgery: A prospective observational study. Minerva Surg. 2025, 80, 115–120. [Google Scholar] [CrossRef]
- Heinz, E.R.; Al-Qudsi, O.; Convissar, D.L.; David, M.D.; Dominguez, J.E.; Haskins, S.; Jelly, C.; Perlas, A.; Vincent, A.N.; Bronshteyn, Y.S. Gastric Point of Care Ultrasound in Adults: Image Acquisition and Interpretation. J. Vis. Exp. 2023, e65707. [Google Scholar] [CrossRef]
- Pal, P.; Mateen, M.A.; Pooja, K.; Marri, U.K.; Gupta, R.; Tandan, M.; Reddy, D.N. Leveraging existing mid-end ultrasound machine for point-of-care intestinal ultrasound in low-resource settings: Prospective, real-world impact on clinical decision-making. Aliment. Pharmacol. Ther. 2024, 60, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Arzola, C.; Perlas, A.; Siddiqui, N.T.; Downey, K.; Ye, X.Y.; Carvalho, J.C.A. Gastric ultrasound in the third trimester of pregnancy: A randomised controlled trial to develop a predictive model of volume assessment. Anaesthesia 2018, 73, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hollerweger, A.; Maconi, G.; Ripolles, T.; Nylund, K.; Higginson, A.; Serra, C.; Dietrich, C.F.; Dirks, K.; Gilja, O.H. Gastrointestinal Ultrasound (GIUS) in Intestinal Emergencies—An EFSUMB Position Paper. Ultraschall Med. 2020, 41, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wu, J.; Feng, X. The value of ultrasound in diagnosis of pneumoperitoneum in emergent or critical conditions: A meta-analysis. Hong Kong J. Emerg. Med. 2019, 26, 111–117. [Google Scholar] [CrossRef]
- Kruisselbrink, R.; Gharapetian, A.; Chaparro, L.E.; Ami, N.; Richler, D.; Chan, V.W.S.; Perlas, A. Diagnostic Accuracy of Point-of-Care Gastric Ultrasound. Anesth. Analg. 2019, 128, 89–95. [Google Scholar] [CrossRef]
- Motavaselian, M.; Farrokhi, M.; Jafari Khouzani, P.; Moghadam Fard, A.; Daeizadeh, F.; Pourrahimi, M.; Mehrabani, R.; Amani-Beni, R.; Farrokhi, M.; Jalayer Sarnaghy, F.; et al. Diagnostic Performance of Ultrasonography for Identification of Small Bowel Obstruction; a Systematic Review and Meta-analysis. Arch. Acad. Emerg. Med. 2024, 12, e33. [Google Scholar] [CrossRef]
- Steinsvik, E.K.; Sangnes, D.A.; Søfteland, E.; Biermann, M.; Assmus, J.; Dimcevski, G.; Gilja, O.H.; Hausken, T. Gastric function in diabetic gastroparesis assessed by ultrasound and scintigraphy. Neurogastroenterol. Motil. 2022, 34, e14235. [Google Scholar] [CrossRef]
- Wang, J.; Shuai, Y.; Cheng, Y.; Zhang, Y. Ultrasound assessment of gastric residual volume in patients over 60 years of age undergoing gastroscopy under sedation: A prospective cohort study. Can. J. Anaesth. = J. Can. D’anesth. 2023, 70, 1315–1322. [Google Scholar] [CrossRef]
- Pan, X.; Chai, J.; Gao, X.; Li, S.; Liu, J.; Li, L.; Li, Y.; Li, Z. Diagnostic performance of ultrasound in the assessment of gastric contents: A meta-analysis and systematic review. Insights Imaging 2024, 15, 98. [Google Scholar] [CrossRef]
- Carter, D.; Albshesh, A.; Shimon, C.; Segal, B.; Yershov, A.; Kopylov, U.; Meyers, A.; Brzezinski, R.Y.; Ben Horin, S.; Hoffer, O. Automatized Detection of Crohn’s Disease in Intestinal Ultrasound Using Convolutional Neural Network. Inflamm. Bowel Dis. 2023, 29, 1901–1906. [Google Scholar] [CrossRef]
- Chen, X.; You, G.; Chen, Q.; Zhang, X.; Wang, N.; He, X.; Zhu, L.; Li, Z.; Liu, C.; Yao, S.; et al. Development and evaluation of an artificial intelligence system for children intussusception diagnosis using ultrasound images. iScience 2023, 26, 106456. [Google Scholar] [CrossRef]
- Cui, X.W.; Goudie, A.; Blaivas, M.; Chai, Y.J.; Chammas, M.C.; Dong, Y.; Stewart, J.; Jiang, T.A.; Liang, P.; Sehgal, C.M.; et al. WFUMB Commentary Paper on Artificial intelligence in Medical Ultrasound Imaging. Ultrasound Med. Biol. 2025, 51, 428–438. [Google Scholar] [CrossRef]
- Kumaralingam, L.; Le May, K.; Dang, V.B.; Alavi, J.; Huynh, H.Q.; Le, L.H. Artificial intelligence-assisted approach to assessing bowel wall thickness in pediatric inflammatory bowel disease using intestinal ultrasound images. J. Crohn’s Colitis 2025, 19, jjaf037. [Google Scholar] [CrossRef]
- Zou, T.; He, H.; Yang, J.; Wu, Y.; Lv, C.; Zhao, L.; Yin, W. Artificial intelligence real-time automated recognition of the gastric antrum cross-sectional area and motility rhythm via bedside ultrasound: A pilot study. Sci. Rep. 2025, 15, 13883. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramson, L.; Theophanous, R.G.; Lefler, B.; Wu, L.; Bowman, A.L.; Olive, J.K.; Bronshteyn, Y.S. Echoes from Within: Mapping Gastrointestinal Obstruction with Ultrasound. Diagnostics 2025, 15, 2511. https://doi.org/10.3390/diagnostics15192511
Abramson L, Theophanous RG, Lefler B, Wu L, Bowman AL, Olive JK, Bronshteyn YS. Echoes from Within: Mapping Gastrointestinal Obstruction with Ultrasound. Diagnostics. 2025; 15(19):2511. https://doi.org/10.3390/diagnostics15192511
Chicago/Turabian StyleAbramson, Lior, Rebecca G. Theophanous, Brice Lefler, Lindsey Wu, Amber L. Bowman, Jacqueline K. Olive, and Yuriy S. Bronshteyn. 2025. "Echoes from Within: Mapping Gastrointestinal Obstruction with Ultrasound" Diagnostics 15, no. 19: 2511. https://doi.org/10.3390/diagnostics15192511
APA StyleAbramson, L., Theophanous, R. G., Lefler, B., Wu, L., Bowman, A. L., Olive, J. K., & Bronshteyn, Y. S. (2025). Echoes from Within: Mapping Gastrointestinal Obstruction with Ultrasound. Diagnostics, 15(19), 2511. https://doi.org/10.3390/diagnostics15192511





